Abstract
Background:
Cognitive function impairment is one of the most common complications in elderly patients after surgery, and an ideal nonpharmacological therapy has not yet been identified. Thus, we hypothesized that remote ischemic preconditioning could improve cognitive functions in elderly patients after surgery and investigated the mechanism underlying this effect.
Methods:
Ninety patients classified as American Society of Anaesthesiologists (ASA) physical status of 2 or 3 and aged 65 to 75 years who were scheduled for elective colon surgery under general anesthesia were randomly allocated to either a remote ischemic preconditioning group (Group R, n = 45) or a control group (Group C, n = 45). Remote ischemic preconditioning was performed by applying a static pressure of 200 mm Hg with a blood pressure cuff wrapped around the right upper limb for 3 ischemia cycles of 5 minutes each.
Results:
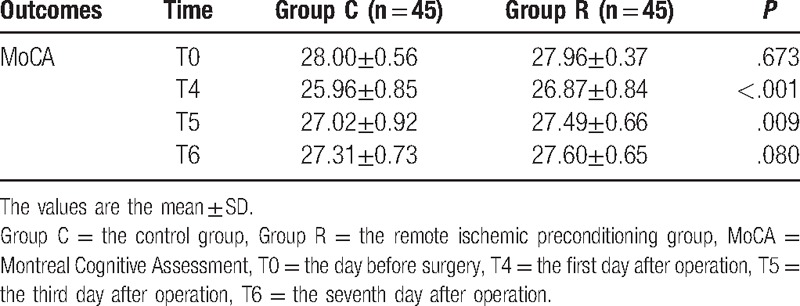
The Montreal Cognitive Assessment (MoCA) scores between the 2 groups were not significantly different on the day before surgery or the seventh day after surgery, but the scores on the first day after surgery (26.87 ± 0.84 vs 25.96 ± 0.85, P < .001) and third day after surgery (27.49 ± 0.66 vs 27.02 ± 0.92, P = .009) were significantly higher for Group R than those for Group C. Moreover, remote ischemic preconditioning markedly decreased the serum concentrations of the interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and S100B proteins compared with the control group (P < .001).
Conclusion:
Remote ischemic preconditioning improves postoperative cognitive function in elderly patients following colon surgery. The cognitive protective effects of remote ischemic preconditioning are partially related to the inhibition of inflammation.
Keywords: cognitive function, colon surgery, elderly patients, inflammation, Montreal Cognitive Assessment, remote ischemic preconditioning
1. Introduction
The elderly are prone to cognitive dysfunction after surgery.[1] Postoperative cognitive dysfunction prolongs hospital stays and increases perioperative costs, surgical morbidity, and mortality.[2] Thus, the effective prevention of postoperative cognitive dysfunction in elderly patients is very important.
The pathophysiology of postoperative cognitive dysfunction (POCD) is not fully understood, but its occurrence is highly related to the inflammatory response.[3] Previous experiments have examined the relationship between inflammation and postoperative cognitive dysfunction and found that inflammation can cause cognitive dysfunction after surgery, whereas anti-inflammatory treatment ameliorates cognitive function in patients undergoing surgery.[4] However, physiological changes due to ageing affect the pharmacokinetic and pharmacodynamic properties of many drugs.[5] The elderly are at increased risk of adverse effects during drug use due to their declines in liver and kidney functions; therefore, the development of a safe and effective nonpharmaceutical method to prevent postoperative cognitive dysfunction is an important goal.
Remote ischemic preconditioning (RIPC) is noninvasive and easy to perform. Studies have shown that RIPC can improve tolerance of the brain to ischemic injury in animal models.[6] This method has been demonstrated to be safe in humans[7] and can induce tolerance by raising the threshold of tissue vulnerability in the human brain.[8] RIPC confers neuroprotection following asphyxial cardiac arrest or transient focal ischemia[9] and has been found to significantly decrease the incidence of recurrent stroke in patients with intracranial arterial stenosis.[10] The mechanism of RIPC is still unclear but is thought to be related to inhibition of the inflammatory reaction.[11,12] The evidence presented above prompted us to hypothesize that RIPC might improve cognitive functions in elderly patients after surgery. Therefore, we designed the following clinical trial to investigate the effect of RIPC on the cognitive function of elderly patients following surgery.
2. Materials and methods
2.1. Study design
This study was a randomized, placebo-controlled, parallel-armed pilot clinical trial that assessed the superiority of remote ischemia preconditioning. Ethical approval for this study (Ethical Committee YLXJS No. 004) was provided by the Ethical Committee of the Fourth Affiliated Hospital of Harbin Medical University, Heilongjiang Province, China (Chairperson, Professor Chang-jiu Zhao) on October 20, 2015, and this study is registered at http://www.chictr.org.cn/registry.aspx (ChiCTR-IPR-15007287.; registration: 27 October, 2015). This trial was conducted from November 2015 to June 2016 at the Fourth Affiliated Hospital of Harbin Medical University, Heilongjiang Province, China.
2.2. Patient recruitment
After obtaining written informed consent from each patient or an authorized person if the patient could not provide informed consent, 90 Chinese patients aged 65 to 75 years and classified as American Society of Anaesthesiologists (ASA) physical status 2 to 3 who were scheduled for laparotomy colon carcinoma surgery were included in this study. Patients were excluded if they met any of the following criteria: Montreal Cognitive Assessment (MoCA) score < 26 before surgery; history of schizophrenia, tristimania, dementia, epilepsy, parkinsonism, or Alzheimer disease; brain injury or neurosurgery; serious hepatic dysfunction (Child–Pugh class C) or serious renal dysfunction (receiving dialysis before the operation); serious cardiac dysfunction (preoperative New York Heart Association classification > III); diabetes mellitus; right upper limb vascular anomalies (including thrombus, angiostenosis, or vascular malformation examined by ultrasound); systolic pressure > 170 mm Hg before ischemic preconditioning; body mass index (BMI) < 18.5 kg/m2 or > 28 kg/m2; and an inability to speak or read Chinese. Detailed information, including baseline demographic data, type of tumor and cancer staging, type of operation, and perioperative variables, was obtained after recruitment.
2.3. Randomization, intervention method, and procedures
Simple randomization was performed. The patients were randomly and equally divided into an RIPC group (Group R, n = 45) and a control group (Group C, n = 45) using random numbers generated with the SAS 9.4 software (SAS Institute, Raleigh). The allocation results were sealed in opaque envelopes. One envelope per patient was handed to a nurse in the anesthesia preparation room who helped perform RIPC. None of the patients received premedication. Upon arrival at the anesthesia preparation room, pulse oximetry, electrocardiography, and blood pressure measurement were performed. Then, midazolam (0.02 mg/kg) and sufentanil (0.1 μg/kg) were administered intravenously via a peripheral vein, and after 5 minutes dorsalis pedis artery catheterization was performed under local anesthesia with 1% lidocaine to monitor blood pressure and collect arterial blood samples. The patients in Group R were treated with RIPC by applying a static pressure of 200 mm Hg with a blood pressure cuff wrapped around the right upper limb for 3 cycles of ischemia at 5 minutes each, followed by 5 minutes of reperfusion. The patients in Group C were treated in the same manner as those in Group R, but they did not receive ischemic preconditioning. After 45 minutes, all patients were transferred to an operating room. The personnel involved in the study, including the investigators, anesthesiologists, and patients, were blinded to the group assignments.
The patients underwent pulse oximetry measurement, electrocardiography, and monitoring of the bispectral index (BIS) and invasive blood pressure, measured at the dorsalis pedis artery, in the operating room. General anesthesia was induced with intravenous midazolam (0.02 mg/kg), etomidate (0.15–0.2 mg/kg), sufentanil (0.3–0.4 μg/kg), and cisatracurium (0.15 mg/kg). Anesthesia was maintained with remifentanil (0.1–0. 2 μg/kg/min), sevoflurane (end-tidal concentration of 2%–3%) in air/O2 to maintain the blood pressure within 20% of the preincision value, and cisatracurium (0.08 mg/kg/min). Surgeries were performed on the 90 patients by the same group of doctors. The type of surgery used depended on the cancer stage and nutritional status of the patient. The surgical procedures performed included local resection, intestinal tumor resection, radical resection, and extended radical resection of colon cancer. A bolus of sufentanil (0.1 μg/kg) was administered intravenously to the patients for postoperative analgesia during suturing of the peritoneum, and remifentanil and sevoflurane were discontinued after the last skin suture was applied. Tracheal extubation was performed after the return of adequate spontaneous breathing, responsiveness, and reflexes. The patients were then transferred to the postanesthetic care unit (PACU) for observation for approximately 1 hour. The peripheral oxygen saturation, invasive blood pressure, heart rate, and BIS were recorded before and during surgery. All of the patients were infused with 500 mL hydroxyethyl starch (130/0.4). No patients received transfusion. The volume of lactated Ringer solution, the estimated amount of intraoperative bleeding, and the amount of blood transfused during surgery were recorded by another nurse who was not participating in this study. The end-tidal carbon dioxide tension remained between 35 and 45 mm Hg. Moreover, the depth of anesthesia monitored using a BIS sensor (BIS Model A-2000; Aspect Medical System, Norwood, MA) applied to the forehead was maintained at BIS levels between 40 and 60.
2.4. Blood specimen collection/methods
Arterial blood was drawn (3 mL at each time point) on the day before surgery (T0) and at 30 minutes after surgery (T1), 60 minutes after surgery (T2), the end of surgery (T3), the first day after surgery (T4), the third day after surgery (T5), and the seventh day after surgery (T6). The blood samples were allowed to clot at room temperature and were then immediately centrifuged for 10 minutes at 3000 × g. The serum fractions were removed and stored at −80°C for further analysis. The tumor necrosis factor-α (TNF-a), interleukin-1β (IL-1β), and S100B protein (S100B) levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Xinle Biotechnology Co, Ltd, Shanghai, China) according to the manufacturer's protocols.
2.5. Cognitive function evaluation
Cognitive function was evaluated with the MoCA. The total MoCA score is 30 points, and a MoCA score < 26 is considered abnormal. One trained investigator (NX) was responsible for the MoCA grading and was blinded to the study protocol. The MoCA score was evaluated the day before surgery (T0) and on the first day (T4), third day (T5), and seventh day (T6) postoperatively in a quiet room with only the patient.
2.6. Outcome measures
The primary outcome of the study was MoCA score on the first day after the operation. Secondary outcomes included the serum IL-1β, TNF-α, and S100B concentrations. Postoperative complications included fever, bleeding, anastomotic leakage, and postoperative ileus. The occurrence of these complications was recorded during the first 7 days. The length of stay in hospital after the operation was also documented.
2.7. Statistical analysis
2.7.1. Sample size calculation
In our preliminary experiment, we calculated the MoCA scores for patients who received RIPC and found that their scores were 2.98 points higher on average on the first day after surgery compared with the control subjects, with a standard deviation of σ = 1.47; a 2-point increase in the postoperative MoCA score was considered clinically significant in patients.[13] We determined that 36 patients had to be included in each group to reach an α level of 0.05 and 80% statistical power. Considering a dropout rate of 25%, we determined that a sample size of 45 patients per group was needed; therefore, a total of 90 patients were enrolled in the study.
Continuous variables were presented as the mean ± SD and were analyzed with the t test if the assumptions of a normal distribution and homogeneity of variance were met; otherwise, the variables were analyzed using the 2 independent sample Wilcoxon rank sum test. In addition, qualitative variables are presented as numbers and were analyzed using the χ2 test. Moreover, post hoc adjustments were performed for any baseline or perioperative factors that differed between the 2 groups (R and C); then, the factors were reanalyzed using a multivariate linear regression analysis to identify potential confounding factors (if any) with effects on the MoCA score. Repeated measures data were analyzed using a mixed effects model with SAS 9.4 software. All tests were 2 sided, and a P value of less than.05 was considered significant in all tests.
3. Results
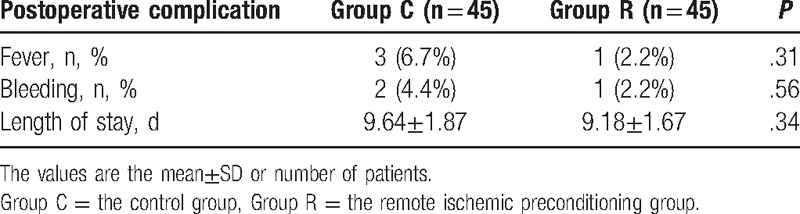
From November 2015 to June 2016, 106 elderly patients were screened for this study, and 90 patients were recruited. Among the 13 patients who were excluded, 1 had a history of epilepsy, 1 had a history of parkinsonism, 3 had a history of cerebral ischemia, 2 had undergone neurosurgery, 2 had a systolic pressure of >170 mm Hg before ischemic preconditioning, 2 had a history of diabetes mellitus, 1 had a BMI < 18.5 kg/m2, and 1 had right upper limb vascular anomalies; in addition, 3 patients refused to participate. The remaining 90 recruited patients were included in the randomization procedure and data analysis, as shown in Fig. 1. All participants in Group R received RIPC. The demographic characteristics of the patients were comparable between the 2 groups, with no significant or clinically meaningful differences (Table 1). No patient in either group developed postoperative anastomotic leakage or postoperative ileus, and the incidences of fever, bleeding, and the length of stay in the 2 groups were comparable (Table 2).
Figure 1.
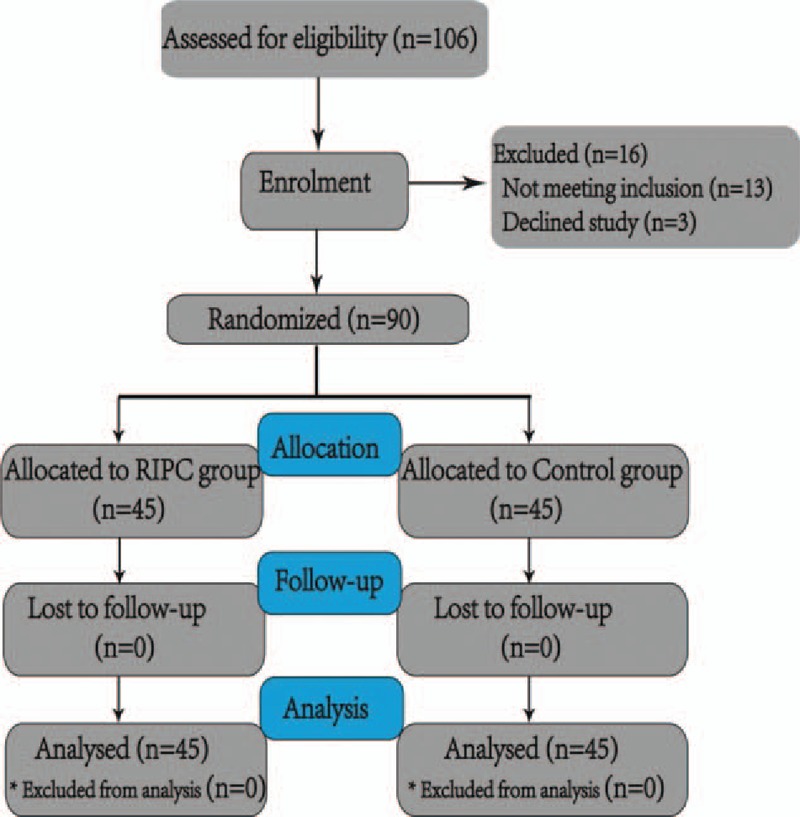
Flow chart of the study inclusion process. RIPC = remote ischemic preconditioning.
Table 1.
Characteristics and intraoperative data for patients in the Group C and Group R.
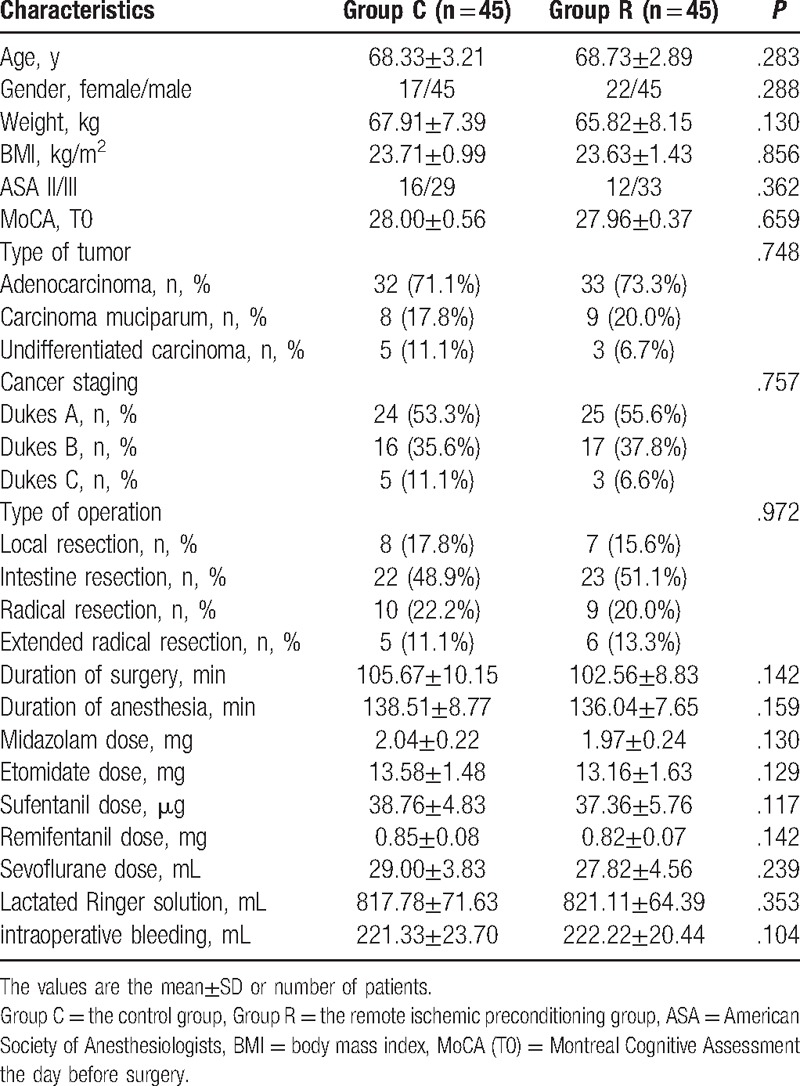
Table 2.
The postoperative complications for patients in the Group C and Group R.
The MoCA scores of the 2 groups were not significantly different on the day before surgery or the seventh day after surgery, but the scores on the first (26.87 ± 0.84 vs 25.96 ± 0.85, P < .001) and third days after surgery (27.49 ± 0.66 vs 27.02 ± 0.92, P = .009) were significantly higher for Group R than those for Group C (Table 3).
Table 3.
MoCA at different times for patients in the Group C and Group R.
The serum S100B concentrations were similar, and no significant differences were observed between the 2 groups on the day before surgery or the seventh day after surgery. Conversely, the serum S100B concentrations measured 30 minutes after surgery (43.39 ± 0.77 μg/mL vs 44.57 ± 1.14 μg/mL), 60 minutes after surgery (44.52 ± 0.98 μg/mL vs 49.70 ± 2.48 μg/mL), at the end of surgery (45.61 ± 1.36 μg/mL vs 54.66 ± 4.46 μg/mL), on the first day after surgery (49.45 ± 1.52 μg/mL vs 60.87 ± 4.20 μg/mL) and on the third day after surgery (46.87 ± 1.16 μg/mL vs 53.24 ± 2.76 μg/mL) were significantly reduced in the patients who received RIPC compared with the control group (P < .001) (Table 4).
Table 4.
Serum concentration of IL-β, TNF-α, and S100B at different times for patients in the Group C and Group R.
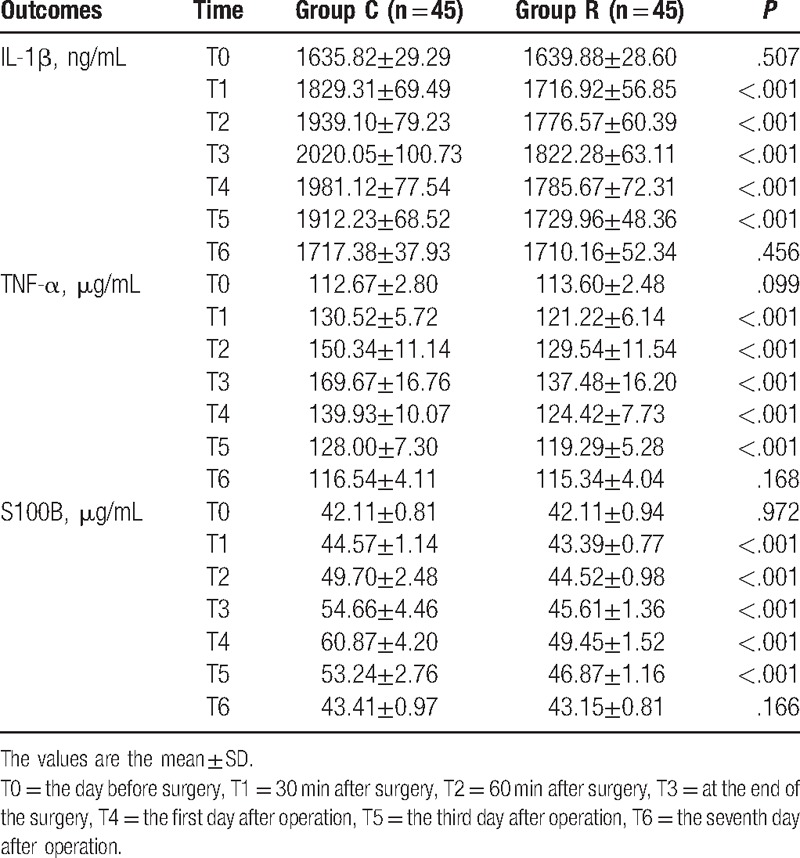
The serum IL-1β and TNF-α concentrations were similar, and no significant differences were observed between the 2 groups on the day before surgery or the seventh day after surgery. Conversely, the serum IL-1β concentrations measured 30 minutes after surgery (1716.92 ± 56.85 ng/mL vs 1829.31 ± 69.49 ng/mL), 60 minutes after surgery (1776.57 ± 60.39 ng/mL vs 1939.10 ± 79.23 ng/mL), at the end of surgery (1822.28 ± 63.11 ng/mL vs 2020.05 ± 100.73 ng/mL), on the first day after surgery (1785.67 ± 72.31 ng/mL vs 1981.12 ± 77.54 ng/mL), and on the third day after surgery (1729.96 ± 48.36 ng/mL vs 1912.23 ± 68.52 ng/mL) were significantly reduced in the patients who received RIPC compared with the control group (P < .001) (Table 4). The serum TNF-α concentrations measured 30 minutes after surgery (121.22 ± 6.14 μg/mL vs 130.52 ± 5.72 μg/mL), 60 minutes after surgery (129.54 ± 11.54 μg/mL vs 150.34 ± 11.14 μg/mL), at the end of surgery (137.48 ± 16.20 μg/mL vs 169.67 ± 16.76 μg/mL), on the first day after surgery (124.42 ± 7.73 μg/mL vs 139.93 ± 10.07 μg/mL), and on the third day after surgery (119.29 ± 5.28 μg/mL vs 128.00 ± 7.30 μg/mL) were significantly reduced in the patients who received RIPC compared with the control group (P < .001) (Table 4).
The concentrations of S100B, IL-1β, and TNF-α began increasing 30 minutes after operation. IL-1β and TNF-α levels peaked at the end of surgery in both groups, whereas the peak S100B concentration was observed later than the peak IL-1β and TNF-α concentrations; the S100B levels peaked on the first day after surgery (Fig. 2).
Figure 2.
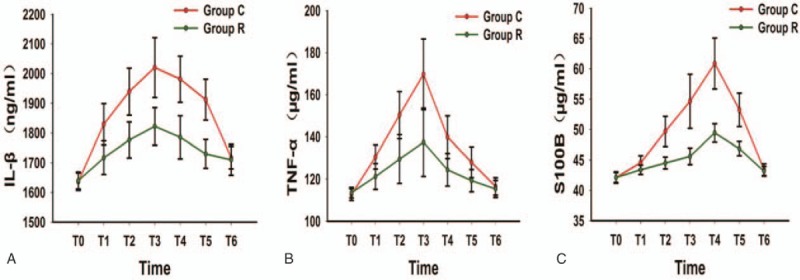
Trend graphs for IL-1β, TNF-α, and S100B. IL-1β = interleukin-1β, S100B = S100B protein.
4. Discussion
To the best of our knowledge, this study is the first to assess the effects of RIPC on the postoperative cognitive function of elderly patients following colon surgery. We found that RIPC improved the early postoperative MoCA score and reduced the serum S100B, IL-1β, and TNF-α concentrations. The effects of RIPC on cognitive function were related to inhibition of the inflammatory response triggered by surgery.
POCD in elderly individuals has gained increasing attention, but no effective nonpharmacological therapy to prevent this condition has been identified. RIPC is an inexpensive and noninvasive technique used to reduce organ injury. Several clinical trials have shown that RIPC has protective effects on the lungs, kidneys, and heart[14,15]; in contrast, 2 recent, multicenter, randomized trials performed by Meybohm et al[16] and Hausenloy et al[17] have revealed that RIPC does not confer cardioprotection. These contradictory findings may be attributed to the use of propofol, which inhibits the organ-protective properties of RIPC,http://www.nejm.org/doi/full/10.1056/NEJMc1514509 - ref7-SA1[18] or differences in the research objectives. RIPC has been reported to mitigate neurological dysfunction following haemorrhagic shock, provide protection against ischemia-reperfusion injury in rat brains, and improve spatial learning and memory abilities following focal cerebral ischemia-reperfusion in rats.[9,19–21] Additionally, RIPC may be an effective strategy to improve cerebral perfusion and protect human brain cell membranes from ischemia in patients with intracranial arterial stenosis.[10,22] In the present study, we aimed to evaluate the effects of RIPC on postoperative cognitive function in elderly patients undergoing colon surgery. We used the MoCA, which is a highly sensitive screening instrument,[23] to evaluate cognitive function and found that the MoCA scores of the patients in Group R were markedly higher than the scores of the patients in Group C at 1 day and 3 days postsurgery, as shown in Table 3. RIPC did not improve MoCA scores on the seventh day postsurgery, possibly because the effects of RIPC on cognitive function had diminished. These results imply that RIPC may improve early postoperative cognitive function in patients undergoing colon surgery.
The precise mechanism of action of RIPC is currently unclear, but it has been linked to the modulation of inflammatory responses. Contradictory experimental results regarding the effects of RIPC on inflammation have been described in the literature. Konstantinov et al[24] have shown that RIPC suppresses proinflammatory gene transcription in human leukocytes. Peralta et al[25] have demonstrated that RIPC reduces the systemic release of TNF-α. In addition, Konstantinov and Redington[26] have shown that RIPC inhibits nuclear factor kappa B. In contrast, Albrecht et al[27] have reported that RIPC promotes increases in the levels of low-molecular-weight cytokines, including IL-1β and TNF-α. The factors that potentially influenced the effects of RIPC included the condition and age of the patient and the presence of other concomitant diseases. In our study, we found that RIPC decreased the serum IL-1β and TNF-α concentrations of patients during surgery and on the first day and third day postsurgery, but it did not reduce serum IL-1β and TNF-α concentrations on the seventh day after surgery compared with the control group, as shown in Table 4. These results might be because the effect of RIPC is related to the time and strength of preconditioning.[28,29] Therefore, we should consider the time window and strength of RIPC prior to application, although this finding requires further study.
The pathological mechanisms of POCD remain elusive, but recent studies have demonstrated that this condition is related to surgery-induced neuroinflammation.[30–32] Inflammatory factors that are induced in patients who have undergone surgical procedures penetrate the blood–brain barrier directly via active transport mechanisms or indirectly via vagal nerve stimulation and influence inflammatory processes in the central nervous system.[33,34] In particular, the normal ageing brain is susceptible to neuroinflammation, and activation of the peripheral innate immune system can induce an amplified and prolonged inflammatory response in the brain.[35] Jiang et al[36] have shown that intracisternal administration of an interleukin-6 receptor antagonist alleviates surgery-induced cognitive impairment. In addition, Terrando et al[37] and Cibelli et al[38] have demonstrated that peripheral blockade of proinflammatory factors prevents neuroinflammation and cognitive decline. Neuroinflammation can cause nerve cell dysfunction or death, leading to elevated blood levels of biochemical markers of brain damage.[39] The S100B protein is an acidic calcium-binding protein that is primarily found in astrocytes. The physiological serum concentration of the S100B protein is low, but glial cells are activated and more S100B protein is released into blood circulation after neurocyte damage during the early stages of brain damage. Therefore, the S100B protein is considered a biochemical marker of cognitive dysfunction.[40] In the present clinical trial, the patients who received RIPC had lower S100B protein concentrations at early time points following surgery than the control subjects, which suggests that RIPC has certain nerve protective effects. This could be the reason that RIPC is able to improve cognitive function. Moreover, the trend of change in S100B was similar to those for IL-1β and TNF-α; therefore, we speculated that RIPC decreased the serum concentration of S100B protein through its anti-inflammatory activity. However, more studies are needed to further understand RIPC's effects.
We also closely evaluated the safety of RIPC in elderly patients. Loukogeorgakis et al[41] have reported that RIPC protects against endothelial ischemia-reperfusion injury in young patients. However, whether this technique is safe for elderly patients is unknown. Elderly patients over 65 years of age were enrolled in this study, and none of the elderly patients who received RIPC developed deep venous thrombosis or bruising or exhibited an injury related to the procedure, as determined by vascular ultrasound imaging. These results demonstrate that RIPC is safe for elderly patients. The mechanism underlying the protection of endothelial function by RIPC is unclear, but it might be dependent on COX-2.[42]
Some limitations of this study should be addressed. First, the sample size is not sufficiently large, and multicenter experiments are needed. Second, we only examined elderly patients; thus, it is unclear whether the results of our experiment can be translated to other age groups. Third, due to limited experimental funds, we could only evaluate the patients until the seventh postoperative day. Fourth, the degree of protection rendered against brief ischemia of the arm appeared to be related to ischemia timing and ischemia strength[28,29]; thus, further studies are needed to establish the optimal timing of RIPC for the prevention of postoperative neurological impairment in elderly patients.
In conclusion, these results show that RIPC improves postoperative cognitive function and inhibits the secretion of proinflammatory cytokines in elderly patients following colon surgery. Though this protective effect of RIPC may be short-lived, it represents a nonpharmacological technique that can be used to improve postoperative cognitive function in elderly patients. This procedure has a much higher potential for clinical translation as an interesting alternative to cerebral preconditioning because it is substantially less invasive, and we need more clinical trials to determine the appropriate timing and strength of RIPC.
Acknowledgments
The authors thank the staff of the operating theater and PACU at the Fourth Affiliated Hospital of Harbin Medical University for their friendly assistance with the study. The authors also acknowledge Weiwei Zhao for her assistance with the experimental design and statistical analysis.
Footnotes
Abbreviations: ASA = American Society of Anaesthesiologists, BIS = bispectral index, BMI = body mass index, ELISA = enzyme-linked immunosorbent assay, IL-1β = interleukin-1β, MoCA = Montreal Cognitive Assessment, PACU = postanesthetic care unit, POCD = postoperative cognitive dysfunction, RIPC = remote ischemic preconditioning, S100B = S100B protein, TNF-a = tumor necrosis factor-α.
The experimental funds come from the Natural Science Foundation of China: the Effect of Mild Brain Hypothermia on Mitochondrial Homeostasis of Nerve Cells Following Cerebral Ischemia-Reperfusion Injury (No. 81271456).
The authors have no conflicts of interest to disclose.
References
- [1].Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- [2].Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009;110:548–55. [DOI] [PubMed] [Google Scholar]
- [3].Broadhurst C, Wilson K. Immunology of delirium: new opportunities for treatment and research. Br J Psychiatry 2001;179:288–9. [DOI] [PubMed] [Google Scholar]
- [4].Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One 2013;8:e79624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004;56:163–84. [DOI] [PubMed] [Google Scholar]
- [6].Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation 2011;123:714–21. [DOI] [PubMed] [Google Scholar]
- [7].Walsh SR, Nouraei SA, Tang TY, et al. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovasc Surg 2010;44:434–9. [DOI] [PubMed] [Google Scholar]
- [8].Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke 2004;35:616–21. [DOI] [PubMed] [Google Scholar]
- [9].Hu S, Dong H, Zhang H, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 2012;1459:81–90. [DOI] [PubMed] [Google Scholar]
- [10].Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012;79:1853–61. [DOI] [PubMed] [Google Scholar]
- [11].Cheung MMH, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 2006;47:2277–82. [DOI] [PubMed] [Google Scholar]
- [12].Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575–9. [DOI] [PubMed] [Google Scholar]
- [13].Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci 2005;30:100–7. [PMC free article] [PubMed] [Google Scholar]
- [14].Camara-Lemarroy CR. Remote ischemic preconditioning as prevention of transfusion-related acute lung injury. Med Hypotheses 2014;83:273–5. [DOI] [PubMed] [Google Scholar]
- [15].Sardar P, Chatterjee S, Kundu A, et al. Remote ischemic preconditioning in patients undergoing cardiovascular surgery: evidence from a meta-analysis of randomized controlled trials. Int J Cardiol 2016;221:34–41. [DOI] [PubMed] [Google Scholar]
- [16].Meybohm P, Bein B, Brosteanu O, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 2015;373:1397–407. [DOI] [PubMed] [Google Scholar]
- [17].Hausenloy DJ, Candilio L, Evans R, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015;373:1408–17. [DOI] [PubMed] [Google Scholar]
- [18].Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 2012;56:30–8. [DOI] [PubMed] [Google Scholar]
- [19].Dave KR, Saul I, Prado R, et al. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett 2006;404:170–5. [DOI] [PubMed] [Google Scholar]
- [20].Hu X, Yang Z, Yang M, et al. Remote ischemic preconditioning mitigates myocardial and neurological dysfunction via K (ATP) channel activation in a rat model of hemorrhagic shock. Shock 2014;42:228–33. [DOI] [PubMed] [Google Scholar]
- [21].Hu X, Lu Y, Zhang Y, et al. Remote ischemic preconditioning improves spatial learning and memory ability after focal cerebral ischemia-reperfusion in rats. Perfusion 2013;28:546–51. [DOI] [PubMed] [Google Scholar]
- [22].Gonzalez NR, Hamilton R, Bilgin-Freiert A, et al. Cerebral hemodynamic and metabolic effects of remote ischemic preconditioning in patients with subarachnoid hemorrhage. Acta Neurochir Suppl 2013;115:193–8. [DOI] [PubMed] [Google Scholar]
- [23].Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009;73:1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 2004;19:143–50. [DOI] [PubMed] [Google Scholar]
- [25].Peralta C, Fernández L, Panés J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced p-selectin up-regulation in the rat. Hepatology 2001;33:100–13. [DOI] [PubMed] [Google Scholar]
- [26].Konstantinov IE, Redington AN. Linking gene expression, nuclear factor kappa B, remote ischemic preconditioning, and transplantation: a quest for an elusive Holy Grail or a road to an amazing discovery? J Thorac Cardiovasc Surg 2006;131:507–9. [DOI] [PubMed] [Google Scholar]
- [27].Albrecht M, Zitta K, Bein B, et al. Remote ischemic preconditioning regulates HIF-1α levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol 2013;108:314. [DOI] [PubMed] [Google Scholar]
- [28].Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K (ATP)-channel dependent mechanism. Circulation 2007;116:1386–95. [DOI] [PubMed] [Google Scholar]
- [29].Ren C, Gao X, Steinberg GK, et al. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008;151:1099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hovens IB, Schoemaker RG, van der Zee EA, et al. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun 2014;38:202–10. [DOI] [PubMed] [Google Scholar]
- [31].Zhang X, Dong H, Li N, et al. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation 2016;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang S, Dong H, Zhang X, et al. Cerebral mast cells contribute to postoperative cognitive dysfunction by promoting blood brain barrier disruption. Behav Brain Res 2016;298:158–66. [DOI] [PubMed] [Google Scholar]
- [33].Maier SF, Goehler LE, Fleshner M, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci 1998;840:289–300. [DOI] [PubMed] [Google Scholar]
- [34].Goehler LE, Gaykema RP, Nguyen KT, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci 1999;19:2799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 2005;19:1329–31. [DOI] [PubMed] [Google Scholar]
- [36].Jiang P, Ling Q, Liu H, et al. Intracisternal administration of an interleukin-6 receptor antagonist attenuates surgery-induced cognitive impairment by inhibition of neuroinflammatory responses in aged rats. Exp Ther Med 2015;9:982–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [37].Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010;107:20518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 2010;68:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rasmussen LS, Christiansen M, Hansen PB, et al. Do blood levels of neuron-specific enolase and S-100 protein reflect cognitive dysfunction after coronary artery bypass? Acta Anaesthesiol Scand 1999;43:495–500. [DOI] [PubMed] [Google Scholar]
- [40].Linstedt U, Meyer O, Kropp P, et al. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand 2002;46:384–9. [DOI] [PubMed] [Google Scholar]
- [41].Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, et al. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005;46:450–6. [DOI] [PubMed] [Google Scholar]
- [42].Liu ZB, Yang WX, Fu XH, et al. Remote ischemic precondition prevents radial artery endothelial dysfunction induced by ischemia and reperfusion based on a cyclooxygenase-2-dependent mechanism. Int J Clin Exp Med 2015;8:20946–52. [PMC free article] [PubMed] [Google Scholar]


