Abstract
Background:
Nasopharyngeal carcinoma (NPC) is a special subtype of head and neck cancer (HNC). At present, there are no highly specific prognostic markers to aid in tumor grading and guide patient treatment modalities for NPC. The prognostic value of pretreatment 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-PET-CT) in NPC patients is controversial and no consensus exists as to its predictive capability.
Methods:
To analyze the predictive efficacy of 18F-PET-CT imaging in NPC patients, data from MEDLINE, EMBASE, the Cochrane library, CBM, CNKI, and VIP (inception to July 2016) were accessed. Results from prospective and retrospective observational studies that used 18F-FDG PET to predict disease prognosis in NPC patients were used for analysis. Two authors independently assessed study quality and extracted data. Event-free survival (EFS) was considered the primary endpoint and overall survival rate (OS) was considered the secondary endpoint.
Results:
Data from 14 studies and 1134 patients were included in our analysis. The hazard ratios (HRs) of maximum standardized uptake value of primary tumor (SUVmax-T), metabolic tumor volume of primary tumor (MTV-T), and total lesional glycolysis of primary tumor (TLG-T) for EFS were 1.31 (95% confidence interval [CI], 1.11–1.55, P = .001), 2.38 (95% CI 1.53–3.70, P < .001), and 1.65 (95% CI 0.76–3.59, P = .21), respectively. Among studies including TLG-T, those with a fixed SUV of 2.5 had an HR of 3.55 (95% CI, 1.42–8.84, P = .007). The HRs of SUVmax-T and MTV-T for OS were 2.19 (95% CI, 1.47–3.27, P < .001) and 2.69 (95% CI, 1.01–7.17, P = .05), respectively. Among studies including MTV-T, those with a fixed SUV of 2.5 had an HR of 4.07 (95% CI, 2.22–7.46, P < .001). Tests used for assessing predictive value of pretreatment SUVmax, MTV, and TLG of lymph nodes for EFS and OS showed that these parameters did not have significant predictive value (P>.05).
Conclusion:
Our results suggested that SUVmax, MTV, and TLG (with a fixed SUV of 2.5) of primary tumors before treatment initiation may be independent prognostic factors for NPC patients; however, SUVmax, MTV, and TLG of metastatic lymph nodes are not.
Keywords: 18F-flurorodeoxyglucose positron emission tomography, metabolic tumor volume, nasopharyngeal carcinoma, total lesion glycolysis
1. Introduction
Nasopharyngeal carcinoma (NPC) is a special subtype of head and neck cancer (HNC), characterized by unique epidemiology, histopathology, methods of treatment, and patient response patterns.[1] The 5-year overall survival (OS) for NPC patients is approximately 80%. Important prognostic factors for patients include pretreatment status of several parameters like T/N and clinical stage, tumor volume, and hemoglobin level.[2,3] However, these factors are not predictive of the entire patient population with diverse genetic backgrounds.[4,5] Studies show that some NPC patients characterized as early T/N and clinical stage shows rapid progression of disease in a short duration after radical chemoradiotherapy. It is difficult to reconcile this phenomenon with the prognosis as predicted by the status of the above-mentioned parameters. There is an urgent need for more accurate and specific indices of disease prognosis.
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is an important molecular imaging technology that is being widely used for precision tumor staging and evaluation of treatment outcome in recent years.[6] Maximum standardized uptake value (SUVmax), a semiquantitative parameter, shows the highest intensity of 18F-FDG uptake within the region of interest or volume of interest (VOI), and is the predictive parameter of choice in clinical practice. It is generally accepted that a SUVmax value greater than or equal to 2.5 indicates a malignant tumor. It is believed that FDG PET or PET/ computed tomography (CT) can be used for risk stratification to gauge progression and predict survival. It is believed that patients with positive PET images may benefit from more aggressive treatment.[7] SUVmax of primary tumors has been regarded as a useful biomarker for the prediction of OS and event-free survival (EFS) of NPC patients.[4,8] However, complete consensus does not exist in this regard, and conflicting reports have been published.[9–11] Chang et al[9] showed that SUVmax could not predict EFS or OS. The limitation of the parameter SUVmax is that while it accurately represents uptake within the region of interest, it cannot represent uptake for the entire tumor mass. To overcome this limitation, other metabolic parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are attracting increasing interest. MTV is measured by contouring margins defined by thresholds, while TLG is calculated by multiplying MTV by mean SUV. It has been proposed that MTV and TLG can be used to gauge disease burden and tumor aggressiveness by quantitating the metabolic volumetric burden or activity of tumors, and this can be easily achieved with the use of commercially available tools.[6] Recently, a systematic review and meta-analysis of prognostic value of MTV and TLG in HNC showed that patients with a high MTV or TLG have a higher risk of adverse events or death.[12] However, there is variability even within data from the same patient group pertaining to MTV and TLG, resulting from lack of clear criteria for determining cut-off values and thresholds.
Although many studies have demonstrated that a high MTV or TLG indicates a higher risk of adverse events or death,[13–15] conflicting data does exist. For example, Shi et al[16] demonstrated that MTV or TLG could not predict EFS or OS. There is still no agreement about the prognostic value of pretreatment 18F-PET-CT SUVmax, MTV, and TLG parameters in NPC patients. Thus, we conducted this meta-analysis to assess whether these parameters are useful in understanding tumor burden and patient response to treatment in NPC.
2. Methods
2.1. Search strategy and selection criteria
We searched the MEDLINE, EMBASE, Cochrane library, CBM, CNKI, and VIP (inception to July 2016) databases for studies meeting the following criteria: 18F-FDG PET scans performed before chemotherapy or radiotherapy treatment, human studies with NPC patients, and survival data reported. Study criteria for exclusion were focus on the performance of PET or PET/CT in diagnosis, staging, or monitoring recurrence or metastasis; reviews, abstracts, and studies with significant overlap in patient population; insufficient information provided by investigators. Searches and screening of studies were performed by 2 authors independently and any disagreement was reconciled by discussion. The main search terms used were “nasopharyngeal carcinoma (NPC), PET, and prognostic value.”
2.2. Data extraction and study quality assessment
Database searches were performed to retrieve results and abstracts. This information was reviewed by 2 independent investigators (YH and MF). Any differences in interpretation of results were resolved through discussion. For each publication, details such as first author, year of publication, study design (prospective/retrospective), number of patients, staging, and endpoints were recorded. The 2 reviewers performed quality assessment of the chosen studies in accordance with the scoring method previously described by Berghmans et al.[12,17,18]
The quality scale was derived based on 4 factors: generalizability, scientific design, analysis of results, and PET reports. Each factor had 5 associated parameters, each scored on a scale of 0 to 2 points. The quality scale of a study was defined as the study score expressed as a percentage of the maximum achievable score of 40 points. All of the chosen studies were published in peer-reviewed journals and explicitly stated institutional ethics committee approval and patient consent.
2.3. Statistical analysis
EFS is an important parameter, defined as the period from the date of initiation of therapy to the date of disease recurrence or metastasis. According to 2 similar meta-analysis studies performed to understand the prognostic value of PET/CT in head and neck cancer and cervical cancers, EFS has been considered the primary outcome.[12,19] OS was taken as the secondary outcome. The effect of SUVmax, MTV, and TLG on survival was quantitatively weighed by hazard ratio (HR) and its 95% confidence intervals (CIs). We extracted HR and CIs directly from articles where available. In cases where this data was not available, we estimated HR and CIs indirectly by analyzing associated statistical data (number of events, number at risk, P values of the log-rank test, and 95% CI).[20] Engauge Digitizer (version 3.0; http://digitizer.sourceforge.net) was used to read survival rates on the Kaplan–Meier curves to reconstruct the HR estimate and its variance. An HR >1 represented worse survival (EFS or OS) for patients in high SUVmax, MTV, or TLG survival distributions group, whereas an HR <1 implied a better survival prognosis. Statistically significant difference in survival was concluded for data when P < .05 (2 sides). Data was analyzed using Review Manager (RevMan, version 5.2; The Nordic Cochrane Centre, The Cochrane Collaboration). Assessment of test heterogeneity between studies was done using the χ2 test and I2 statistics, while funnel plots were employed to detect publication bias. Fixed model was chosen for meta-analysis where I2 was less than 35% and heterogeneity between studies could be ignored; otherwise, random model analysis was performed.
3. Results
3.1. Study selection and characteristics of studies
A total of 266 articles were identified from an initial database search. After carefully reviewing the abstracts, 174 studies were excluded, based on the exclusion criteria defined earlier. Several studies did not employ PET scans on patients before treatment (N = 54) and were excluded. Other studies were excluded because they did not involve human subjects (N = 21), prognostic tests were not performed (N = 43), duplicated records were found (N = 23), or were publications of case reports (N = 14) and reviews (N = 19). Subsequent to the initial round of exclusions, another set of 78 studies were removed after reviewing the full-text of articles. Several studies did not have all the required data (N = 42), some did not involve pretreatment PET scans (N = 12), others were purely diagnostic studies (N = 13) or did not perform any prognostic tests (N = 11). After thorough vetting of all the records retrieved, 14 studies satisfied all inclusion criteria, and these involved data from 1134 patients. These 14 studies were included in the final analysis, out of which 5 were prospectively designed while the other 9 studies were retrospectively designed. The study selection procedure and reasons for exclusion are presented in Fig. 1. Pertinent details of the studies selected for analysis are summarized in Table 1.
Figure 1.
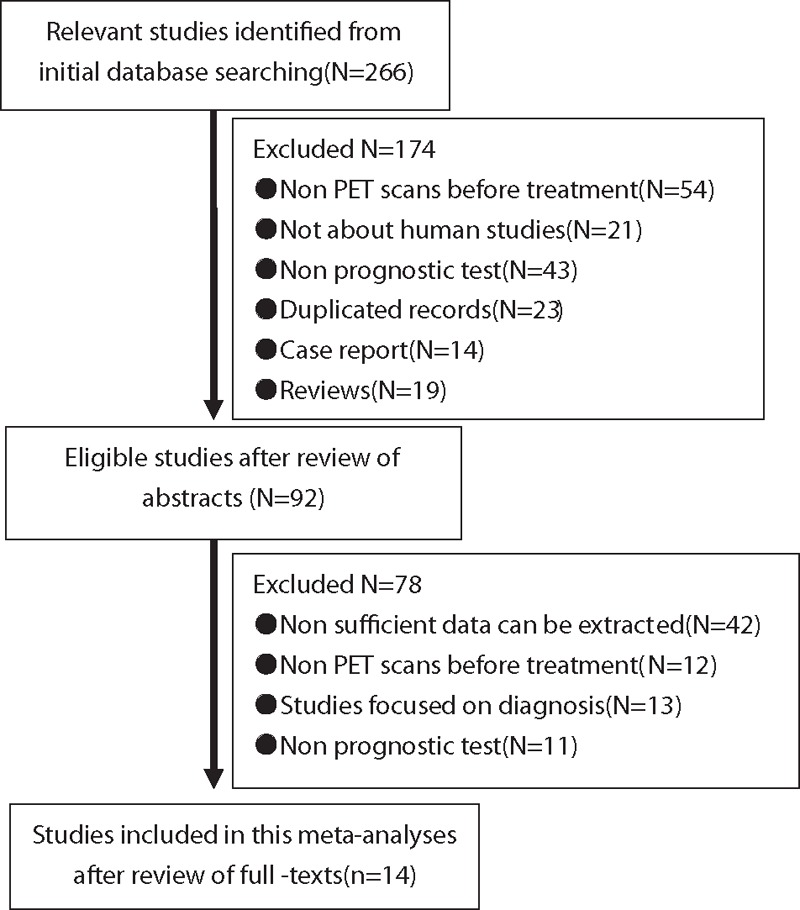
Study selection process for this study.
Table 1.
Characteristics of studies included in meta-analysis.
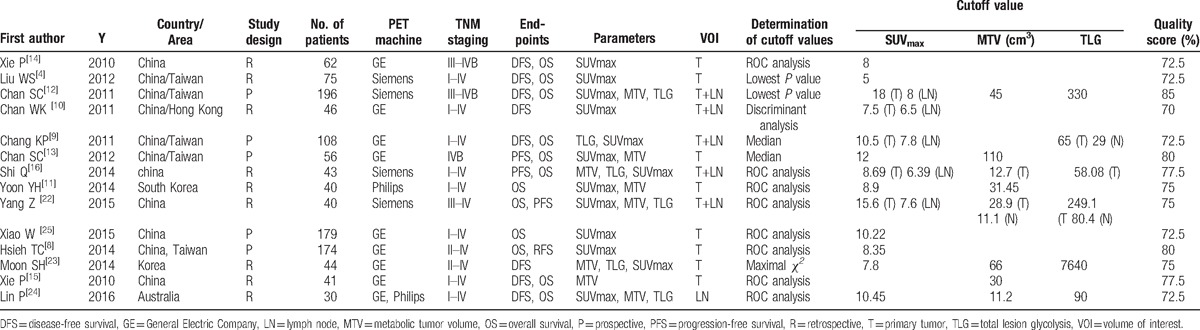
There was variability in the number of parameters of interest that were monitored in studies selected for further analysis. Five studies measured all the desired parameters (SUVmax, MTV, and TLG).[16,21–24] While there was 1 study that measured SUVmax and TLG[9], 2 others reported SUVmax and MTV.[11,13] There were 5 studies that measured only SUVmax,[4,8,10,14,25] and 1 that reported only MTV.[15] There were differences in definitions and terminology used among studies. While 8 studies defined the measurements on tumors alone as VOI,[4,8,11,13–15,23,25] 5 studies included both tumor and lymph node (LN) measurements to define VOI.[9,10,16,21,22] One study used LN measurements alone to report VOI.[21] Determination of cutoff values was based on ROC analysis in 8 studies,[8,11,14–16,21,22,25] median in 2 studies,[10,13] lowest P value in 2 studies,[4,21] and other methods in 2 studies.[10,23] The cutoff values for SUVmax-T and SUVmax-LN ranged from 5 to 18 and from 6.35 to 8, respectively, while those for MTV-T and TLG-T ranged from 12.7 to 110 and from 58.08 to 7640, respectively. The average quality score was 75.5% (72.5%–85%). A Funnel plot of the data suggested no evidence of publication bias. Details of all the studies included in the analysis are summarized in Table 1.
3.2. Predictive value of pretreatment SUVmax, MTV, and TLG for EFS
The HR of SUVmax of primary tumors (SUVmax-T) and that of LNs (SUVmax-LN) for EFS was 1.31 (95% CI, 1.11–1.55, P = .001) and 1.35 (95% CI, 0.80–2.06, P = .29), respectively. The combined HR as calculated using the fixed model (χ2 = 25.39, P = .02, I2 = 49%) was 1.31 (95% CI, 1.12–1.53, P < .001). The HR of predictor parameter MTV of primary tumors (MTV-T) and that of LNs (MTV-LN) for EFS was 2.38 (95% CI 1.53–3.70, P < .001) and 1.96 (95% CI 0.50–7.61, P = .33), respectively, while the combined HR was 2.34 (95% CI, 1.53–3.56, P < .001) in fixed model (χ2 = 0.5.02, P = .54, I2 = 0%). The HR associated with TLG of primary tumors (TLG-T) and with LNs (TLG-LN) for EFS was 1.65 (95% CI 0.76–3.59, P = .21) and 1.53 (95% CI 0.36–6.44, P = .56), respectively, while the combined HR was 1.60 (95% CI, 0.84–3.05, P = .16) as calculated random model analysis (χ2 = 23.25, P = .002, I2 = 70%). Forest plots of SUVmax, MTV, and TLG are shown in Fig. 2.
Figure 2.
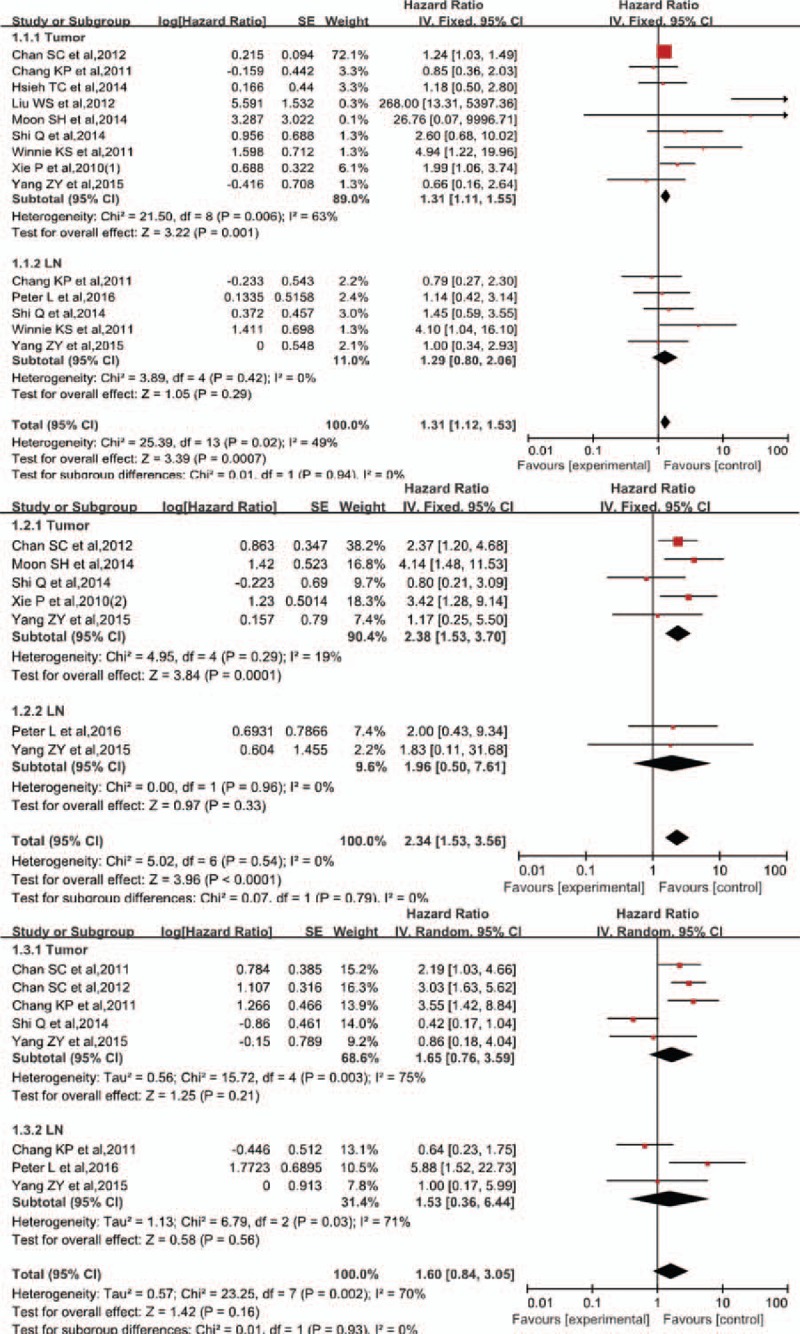
Forest plots of HR for events with SUVmax, MTV, and TLG. 1.1. Forest plots of HR for events with SUVmax. 1.2. Forest plots of HR for events with MTV. 1.3. Forest plots of HR for events with TLG. HR = hazard ratio, MTV = metabolic tumor volume, SUVmax = maximum standardized uptake value, TLG = total lesional glycolysis.
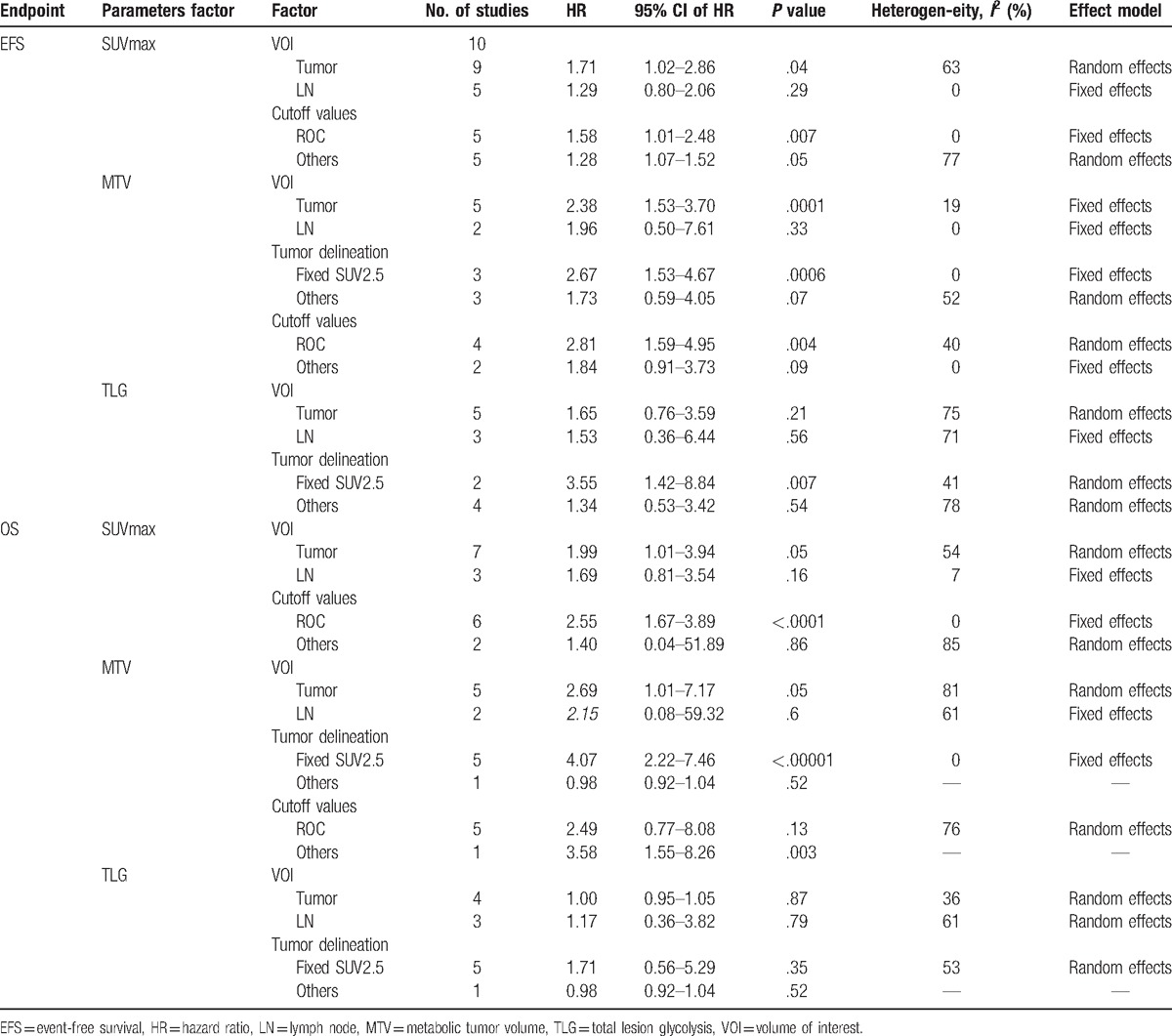
Subgroup analysis was carried out after defining cutoff values and tumor delineation (Table 2). Among studies including MTV and TLG, those with a fixed SUV of 2.5 had an HR of 2.67 (95% CI, 1.53–4.67, P < .001) and 3.55 (95% CI, 1.42–8.84, P = .007), respectively, while those with thresholds other than 2.5 showed no significant results (P = .07, P = .54 respectively). Among studies including SUVmax and MTV, those with cutoff values determined using ROC had an HR of 1.58 (95% CI, 1.01–2.48, P = .007) and 2.81 (95% CI, 1.59–4.95, P = .004). Studies that adopted other methods showed no statistically significant correlations (P = .05, P = .09 respectively).
Table 2.
Subgroup analyses.
3.3. Predictive value of pretreatment SUVmax, MTV, and TLG for OS
Data from 7 studies was used to analyze pretreatment SUVmax-T and data from 2 studies was employed for SUVmax-LN assessment as predictors of OS. The HRs of SUVmax-T and SUVmax-LN for OS were 2.19 (95% CI, 1.47–3.27, P < .001) and 1.69 (95% CI, 0.81–3.54, P = .16), respectively. The combined HR was 2.07 (95% CI, 1.45–2.94, P < .001) in fixed model (χ2 = 15.66, P = .54, I2 = 0%). Subgroup analysis showed that among studies including SUV, those with cutoff values determined employing ROC had an HR of 2.55 (95% CI, 1.67–3.89, P < .001), while those that adopted other methods yielded results with no statistical significance (P = .86). Predictive value of pretreatment MTV-T for OS was analyzed using data from 5 studies, and the HR of MTV-T for OS was 2.69 (95% CI, 1.01–7.17, P = .05).
Subgroup analysis showed that among studies that reported MTV, those with a fixed SUV of 2.5 had an HR of 4.07 (95% CI, 2.22–7.46, P < .001), while those with cutoff thresholds different from 2.5 did not yield statistically significant correlations (P = .52). Test for predictive value of pretreatment TLG-T and TLG-LN for OS showed no significant results (P = .64, P = .68 respectively). Our analyses conclusively showed that tumors with a higher SUVmax-T and MTV-T, especially those with a fixed SUV cutoff of 2.5, are associated with worse OS. Forest plots of SUVmax, MTV, and TLG for OS are shown in Fig. 3, and subgroup analysis is shown in Table 2.
Figure 3.
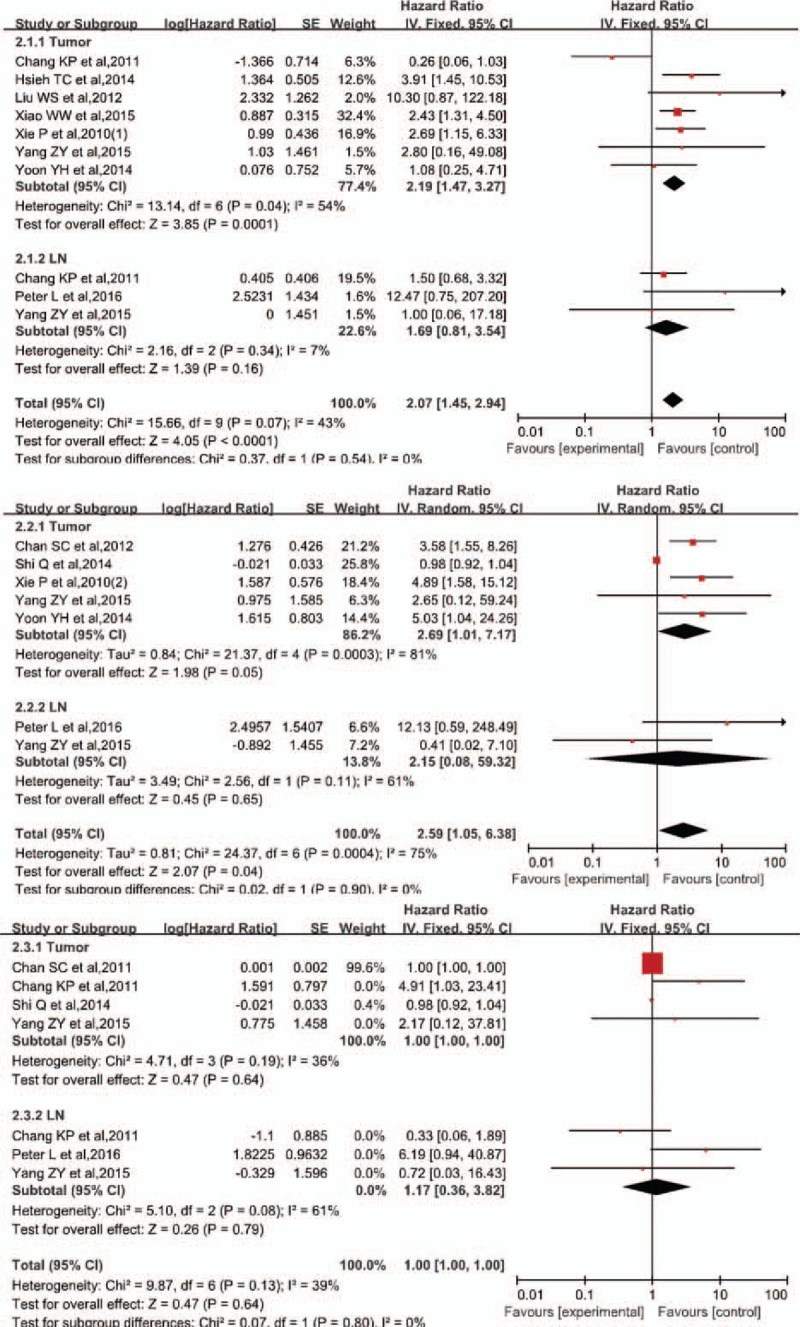
Forest plots of HR for death with SUVmax, MTV, and TLG. 2.1. Forest plots of HR for death with SUVmax. 2.2. Forest plots of HR for death with MTV. 2.3. Forest plots of HR for death with TLG. HR = hazard ratio, MTV = metabolic tumor volume, SUVmax = maximum standardized uptake value, TLG = total lesional glycolysis.
4. Discussion
As a noninvasive imaging tool, 18F-FDG PET plays an important role in tumor staging and assessment of treatment efficacy in cancer patients.[26,27] It is reported that this technique is highly sensitive (96%), has great specificity (94%), and produces highly accurate data (95%) when applied to tumor staging.[28] A recent randomized pilot trial has demonstrated that PET/CT-guided dose escalation radiotherapy was well tolerated in locally advanced NPC patients.[29] Furthermore, 18F-FDG PET-CT has been widely applied to predict patient survival because it is also able to give reliable data about tumor burden and aggressiveness. Patients with primary tumors characterized by a high SUV value before treatment have been demonstrated to have poor survival rates in cases of head and neck squamous cell carcinoma, lung cancer, esophageal cancer, and cervical cancer.[17–19,30] Another study involving meta-analysis of HNC patient data concluded that primary tumors with a high MTV or TLG before treatment are associated with worse patient survival rates.[12] The use of SUV, MTV, and TLG parameters for predicting disease prognosis is worthy of systematic evaluation in both HNC and head and neck squamous cell carcinoma (HNSCC), based on studies cited above. Variables such as tumor delineation, cutoff value, and definition of VOI may affect MTV or TLG. Subgroup analysis was performed to evaluate the effects of these variables on the HRs of EFS and OS for SUV, MTV, and TLG. Compared with patients with a low SUVmax-T before treatment, patients with a high SUVmax-T had a 1.71-fold-higher risk of adverse events and 2.19-fold-higher risk of death. Patients with a high MTV-T (with a fixed SUV more than 2.5) before treatment had a 2.67-fold-higher risk of adverse events and a 4.07-fold-higher risk of death than patients with a low MTV-T. Patients with a high TLG-T (with a fixed SUV cutoff of more than 2.5) before treatment had a 3.55-fold-higher risk of adverse events than patients with a low TLG-T. The results of our meta-analysis suggest that increased SUVmax and MTV of primary tumors are indicators of poor prognosis for survival in patients with NPC.
LN metastasis has been demonstrated to be an independent prognostic factor for NPC patients.[30–32] A recent meta-analysis survey has suggested that PET-CT is a highly reliable diagnostic tool for detecting LN metastasis.[33] Prognostic efficacy of SUV, MTV, TLG parameters of LNs before treatment has been studied by many investigators, but there have been conflicting conclusions.[9,10,17,18] Chang et al have reported that neither SUVmax nor TLG of metastatic LNs is a reliable predictor of OS in NPC patients, while Lin et al demonstrated that patients with a high SUVmax, TLG, and MTV of LNs have a worse OS prognosis.[12,24] In this context, we comprehensively evaluated the prognostic value of these PET-CT parameters in NPC patients. The results of our meta-analysis suggest that there is no significant correlation between pretreatment SUVmax, MTV, TLG status of metastatic LNs and disease prognosis. Further, we also conclude that LN metastasis is not a reliable predictor of OS or DFS. It is possible that LN metastasis correlates better with distant metastasis-free survival (DMFS).[30,31] Since the data included in our analysis did not contain DMFS values, this hypothesis has to be verified with further studies. Hung et al[34] suggested that the SUVmax of the LNs in the neck region is an independent prognostic factor for DMFS in NPC patients treated with intensity-modulated radiation therapy (IMRT). This adds credence to the idea that further clinical trials are needed to evaluate the prognostic values of MTV-LN and TLG-LN in NPC.
Difference in methods applied to define VOI may affect the value of TLG or MTV.[35] Six out of 15 studies included in this meta-analysis used an SUV cutoff value of 2.5 to define VOI of TLG or MTV. The underlying presumption was that lesions with SUVmax value greater than or equal to 2.5 are malignant.[10,18] However, other studies included in our analysis used a fixed cutoff value (3.0) or expressed SUVmax as a percentage value (75%) to define VOI.[11,24] It is well known that the method used to determine the MTV or TLG of a tumor has an impact on the absolute value of the parameter, for the same tumor. In this study, subgroup analysis showed that studies defining MTV and TLG with a fixed SUV cutoff of 2.5 reported a 2.67 and 3.55-fold-higher risk of adverse events, respectively. Interestingly, these same studies reported a 4.07-fold-higher risk of death as well. As such, an SUV cutoff of 2.5 might be a good standard value for threshold of VOI delineation not only in differentiating between benign and malignant lesions, but also in predicting survival. Despite this, studies included in our meta-analysis employed statistical parameters such as ROC, minimum P value, and median value to establish parameter cutoff values. ROC, a commonly used method in this regard, was used in 8 of 14 studies. The cutoff values of MTV and TLG for primary tumors in this meta-analysis ranged from 12.87 to 110 cm3 and from 58.08 to 7640, respectively. Alternative methods of analysis are also prevalent in the literature, for example, Moon et al[23] evaluated prognostic efficacy of MTV and TLG with continuous variables (1 cm3 increases for MTV and 10 unit increase for TLG).
Our meta-analysis adds significantly to the understanding of the applications and limitations of the 18F-FDG PET parameters SUVmax, MTV, and TLG and their relationship with survival prognosis in NPC patients. However, there are several shortcomings that remain to be addressed. The main limitation is that most of the studies (9/14) included in this meta-analysis are retrospective studies. Hence, the evidence extracted from these studies is not very strong. In addition, we were not able to obtain all the pertinent details about the VOI and threshold methods from these studies, increasing the potential risk of bias in this study.
Footnotes
Abbreviations: 18F-FDG PET = 18F-fluorodeoxyglucose positron emission tomography, CI = confidence interval, CT = computed tomography, DFS = disease-free survival, DMFS = distant metastasis-free survival, EFS = event-free survival, HNC = head and neck cancer, HNSCC = head and neck squamous cell carcinoma, HR = hazard ratio, LN = lymph node, MTV = metabolic tumor volume, MTV-LN = metabolic-tumor-volume of lymph node, MTV-T = metabolic tumor volume of primary tumor, NPC = nasopharyngeal carcinoma, OS = overall survival, ROC = receiver operating characteristic curve, SUVmax = maximum standardized uptake value, SUVmax-LN = maximum standardized uptake value of LN, SUVmax-T = maximum standardized uptake value of primary tumor, TLG = total lesional glycolysis, TLG-LN = total lesional glycolysis of lymph node, TLG-T = total lesional glycolysis of primary tumor, VOI = volume of interest.
YH, MF, and QH contributed equally to this work.
Language-editing service (Wolters Kluwer) was used in the final revision.
This study was funded by Cadre Health Care Funds of Sichuan Province (NO. 2015-801) and Science and Technology Program Project Funds of Sichuan Province (NO. 2015SZ0053).
The authors have no conflicts of interest to disclose.
References
- [1].Bruce JP, Yip K, Bratman SV, et al. Nasopharyngeal cancer: molecular landscape. J Clin Oncol 2015;33:3346–55. [DOI] [PubMed] [Google Scholar]
- [2].Feng M, Wang W, Fan Z, et al. Tumor volume is an independent prognostic indicator of local control in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Radiat Oncol 2013;8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aribas BK, Cetindag F, Ozdogan Z, et al. Nasopharyngeal carcinomas: prognostic factors and treatment features. J Egypt Natl Canc Inst 2008;20:230–6. [PubMed] [Google Scholar]
- [4].Liu WS, Wu MF, Tseng HC, et al. The role of pretreatment FDG-PET in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:561–6. [DOI] [PubMed] [Google Scholar]
- [5].Chan SC, Chang JT, Wang HM, et al. Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral Oncol 2009;45:52–8. [DOI] [PubMed] [Google Scholar]
- [6].Sh M, Sh H, Jy C. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol 2013;14:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Paidpally V, Chirindel A, Lam S, et al. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med 2012;4:633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hsieh TC, Hsieh CY, Yang TY, et al. [18F]-fluorodeoxyglucose positron emission tomography standardized uptake value as a predictor of adjuvant chemotherapy benefits in patients with nasopharyngeal carcinoma. Oncologist 2015;20:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang KP, Tsang NM, Liao CT, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med 2012;53:21–8. [DOI] [PubMed] [Google Scholar]
- [10].Chan WK, Kwong DL, Yeung DW, et al. Prognostic impact of standardized uptake value of F-18 FDG PET/CT in nasopharyngeal carcinoma. Clin Nucl Med 2011;36:1007–11. [DOI] [PubMed] [Google Scholar]
- [11].Yoon YH, Lee SH, Hong SL, et al. Prognostic value of metabolic tumor volume as measured by fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in nasopharyngeal carcinoma. Int Forum Allergy Rhinol 2014;4:845–50. [DOI] [PubMed] [Google Scholar]
- [12].Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014;55:884–90. [DOI] [PubMed] [Google Scholar]
- [13].Chan SC, Hsu CL, Yen TC, et al. The role of 18F-FDG PET/CT metabolic tumour volume in predicting survival in patients with metastatic nasopharyngeal carcinoma. Oral Oncol 2013;49:71–8. [DOI] [PubMed] [Google Scholar]
- [14].Xie P, Yue JB, Fu Z, et al. Prognostic value of 18F-FDG PET/CT before and after radiotherapy for locally advanced nasopharyngeal carcinoma. Ann Oncol 2010;21:1078–82. [DOI] [PubMed] [Google Scholar]
- [15].Xie P, Yue JB, Zhao HX, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2010;136:883–9. [DOI] [PubMed] [Google Scholar]
- [16].Shi Q, Yang Z, Zhang Y, et al. Adding maximum standard uptake value of primary lesion and lymph nodes in 18F-fluorodeoxyglucose PET helps predict distant metastasis in patients with nasopharyngeal carcinoma. PLoS One 2014;9:e103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Berghmans T, Dusart M, Paesmans M, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12. [DOI] [PubMed] [Google Scholar]
- [18].Pan L, Gu P, Huang G, et al. Prognostic significance of SUVon PET/CT in patients with esophageal cancer: a systematic review, meta-analysis. Eur J Gastroenterol Hepatol 2009;21:1008–15. [DOI] [PubMed] [Google Scholar]
- [19].Zhao Q, Feng Y, Mao X, et al. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography or PET-computed tomography in cervical cancer: a meta-analysis. Int J Gynecol Cancer 2013;23:1184–90. [DOI] [PubMed] [Google Scholar]
- [20].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [21].Chan SC, Chang JT, Lin CY, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun 2011;32:989–96. [DOI] [PubMed] [Google Scholar]
- [22].Yang Z, Shi Q, Zhang Y, et al. Pretreatment (18)F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma—a retrospective study. Radiat Oncol 2015;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moon SH, Choi JY, Lee HJ, et al. Prognostic value of volume-based positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma treated with concurrent chemoradiotherapy. Clin Exp Otorhinolaryngol 2015;8:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin P, Min M, Lee M, et al. Prognostic utility of (18)F-FDG PET-CT performed prior to and during primary radiotherapy for nasopharyngeal carcinoma: index node is a useful prognostic imaging biomarker site. Radiother Oncol 2016;120:87–91. [DOI] [PubMed] [Google Scholar]
- [25].Xiao W, Xu A, Han F, et al. Positron emission tomography-computed tomography before treatment is highly prognostic of distant metastasis in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy treatment: a prospective study with long-term follow-up. Oral Oncol 2015;51:363–9. [DOI] [PubMed] [Google Scholar]
- [26].Wei J, Pei S, Zhu X. Comparison of (18)F-FDG PET/CT, MRI and SPECT in the diagnosis of local residual/recurrent nasopharyngeal carcinoma: a meta-analysis. Oral Oncol 2016;52:11–7. [DOI] [PubMed] [Google Scholar]
- [27].Mohandas A, Marcus C, Kang H, et al. FDG PET/CT in the management of nasopharyngeal carcinoma. AJR Am J Roentgenol 2014;203:W146–57. [DOI] [PubMed] [Google Scholar]
- [28].Chen YK, Su CT, Ding HJ, et al. Clinical usefulness of fused PET/CT compared with PET alone or CT alone in nasopharyngeal carcinoma patients. Anticancer Res 2006;26:1471–7. [PubMed] [Google Scholar]
- [29].Wang J, Zheng J, Tang T, et al. A randomized pilot trial comparing position emission tomography (PET)-guided dose escalation radiotherapy to conventional radiotherapy in chemoradiotherapy treatment of locally advanced nasopharyngeal carcinoma. PLoS One 2015;10:e124018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tang LL, Guo R, Zhou G, et al. Prognostic value and staging classification of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. PLoS One 2014;9:e108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guo Q, Pan J, Zong J, et al. Suggestions for lymph node classification of UICC/AJCC staging system: a retrospective study based on 1197 nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Medicine (Baltimore) 2015;94:e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yin L, Bian XH, Wang X, et al. Long-term results of concurrent chemoradiotherapy for advanced N2-3 stage nasopharyngeal carcinoma. PLoS One 2015;10:e137383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shen G, Zhang W, Jia Z, et al. Meta-analysis of diagnostic value of 18F-FDG PET or PET/CT for detecting lymph node and distant metastases in patients with nasopharyngeal carcinoma. Br J Radiol 2014;87:20140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hung TM, Wang HM, Kang CJ, et al. Pretreatment (18)F-FDG PET standardized uptake value of primary tumor and neck lymph nodes as a predictor of distant metastasis for patients with nasopharyngeal carcinoma. Oral Oncol 2013;49:169–74. [DOI] [PubMed] [Google Scholar]
- [35].Lin JC, Wang WY, Liang WM, et al. Long-term prognostic effects of plasma Epstein-Barr virus DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1342–8. [DOI] [PubMed] [Google Scholar]


