Abstract
Background & aims:
Several studies have shown that proton pump inhibitors (PPIs) use can increase the risk of developing hepatic encephalopathy (HE) in patients with liver dysfunction. However, no definite conclusion is drawn because of study design limitations. Therefore, we conducted a meta-analysis to explore the association between PPIs and HE.
Methods:
We searched PubMed, EMBASE, and the Cochrane Library from inception until November 2016. Data from the identified studies were combined using a random effects model, and odds ratios (ORs) were calculated.
Results:
Three case-control studies were included. Compared with nonusers, hepatic insufficiency patients receiving PPIs therapy had a significantly increased risk of developing HE (OR = 1.76, 95% CI: 1.15–2.69), with notable heterogeneity (I2 = 61.4%, P = .075) and publication bias. No relevance was found between PPIs and HE after using the trim and fill method (OR = 1.360, 95%CI: 0.909–2.035, P = .135).
Conclusions:
PPIs are associated with a higher risk of HE among patients with chronic and acute liver dysfunction. A final conclusion cannot be drawn because of the limited number of studies and a lack of prospective studies.
Keywords: hepatic encephalopathy, meta-analysis, microbiota, proton pump inhibitors
1. Introduction
Hepatic encephalopathy (HE) constitutes a spectrum of neuropsychiatric manifestations associated with both acute and chronic liver dysfunction.[1,2] Previous studies have suggested that an altered gut microbiome may play an essential role in the pathology of HE, possibly by increasing ammonia levels, and interacting with the inflammation and oxidative stress pathways.[3,4] Thus, therapy targeting the regulation of microbiota imbalance may have important implications for management of HE. The quality of life and long-term prognosis for patients who develop HE is discouraging, and a cohort study conducted in a cirrhotic patient population showed a 1-year survival rate of 36% after the onset of HE.[5] Therefore, proper management is needed to lower the incidence of HE, including avoiding abusive use of certain medications that may contribute to HE onset.
Proton pump inhibitors (PPIs) are effective gastric acid suppressants that have been widely prescribed in patients with acute and chronic liver disease, mainly for the prophylaxis and treatment of upper gastrointestinal hemorrhage. Overuse of PPIs is common among cirrhotic patients.[6,7] However, inappropriate use of PPIs can also lead to rare but serious adverse effects including bone fracture, community-acquired pneumonia, Clostridium difficile infection, and acute kidney injury (AKI) or chronic kidney disease (CKD).[8–11] Previous studies have reported some adverse effects of PPIs in patients with acute liver failure and chronic hepatitis or cirrhosis. These studies mainly focused on the relatively high prevalence of spontaneous bacterial peritonitis (SBP) in cirrhotic patients who are prescribed PPIs.[12–15] Recent research from 3 individual centers raised concerns that PPIs may affect the risk of HE in patients with liver dysfunction.[16–18] Therefore, we conducted a meta-analysis to explore the association between PPIs and HE.
2. Methods
2.1. Search strategy
We performed a computerized literature search of 3 electronic databases including PubMed, EMBASE, and The Cochrane Library from inception until November 2016. The search items were (proton pump inhibitors OR rabeprazole OR esomeprazole OR lansoprazole OR omeprazole OR pantoprazole) AND (hepatic encephalopathy). Ethical approval was not necessary because our article is a review.
2.2. Study selection
Two independent reviewers read the abstracts or full-text articles to assess the eligibility of studies in a standardized manner. We also reviewed all references from the included articles and further selected eligible studies. The following criteria were used to select the articles: (i) randomized controlled trial, case-control or cohort studies; (ii) studies conducted in humans; and (iii) the value of the relative risk (RR), hazard ratio (HR), or odds ratio (OR) with corresponding 95% confidence intervals (CIs), or the original data to calculate them were reported. Exclusion criteria were as follows: (i) no control group of patients; (ii) patients with previous brain function impairment were included in the study; and (iii) papers were letters, commentaries, or reviews. Disagreements were resolved by consensus.
2.3. Data extraction
Two investigators independently extracted data from the full text of the included studies. Data collected included study design, study population, years of publication, type of acid-suppressive therapy, comparison of exposure level, dose, and duration of acid-suppressive therapy, and adjusted confounding variables. The estimates of OR/HR, their associated 95% CIs, and the P value were also extracted. We assumed that there was similarity between the OR and HR because hepatic encephalopathy events were relatively rare.[19] Any disagreements or discrepancies were resolved in consensus.
2.4. Statistical analyses
We extracted the OR/HR and 95% CIs from each of the 3 studies. We then calculated the standard error (SE) of the logOR/HR using the following equation: SE = (ln[OR/HR_upper − ln OR/HR_lower])/3.92. We used I2 to evaluate the heterogeneity, and an I2 of 30%–60% was considered to represent moderate heterogeneity.[20] We performed a meta-analysis using a random effect model in a conservative manner.
To evaluate publication bias, we generated a funnel plot and visually examined it for asymmetry. The trim and fill method was used to recalculate the effect if an obvious publication bias was observed. STATA (Version 12.0, StataCorp, College Station, TX) was used to perform all data analysis.
3. Results
3.1. Search results
The computerized search yielded 22 references; no relevant articles were identified from the references. We excluded 19 articles according to our inclusion and exclusion criteria. A total of 3 articles were eventually included, all of which were retrospective studies (Fig. 1).
Figure 1.
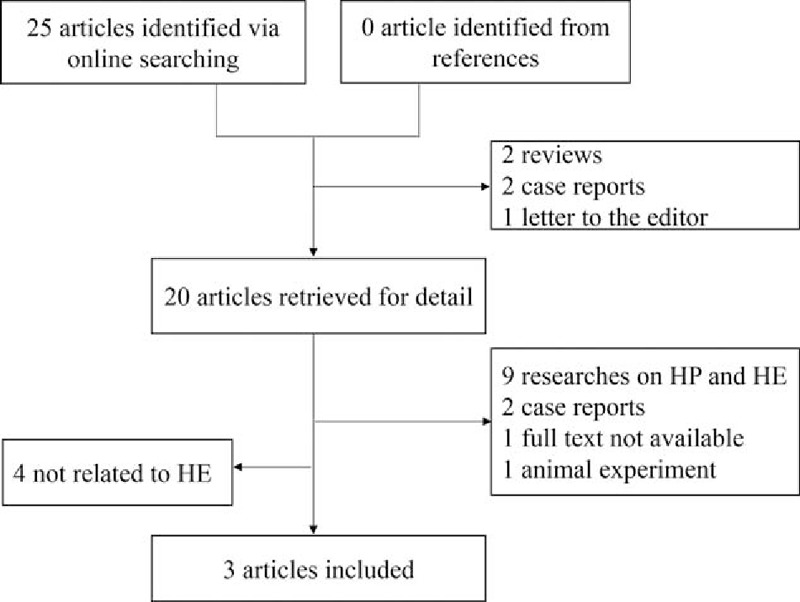
Flowchart of the searching and review of literatures.
3.2. Study characteristics
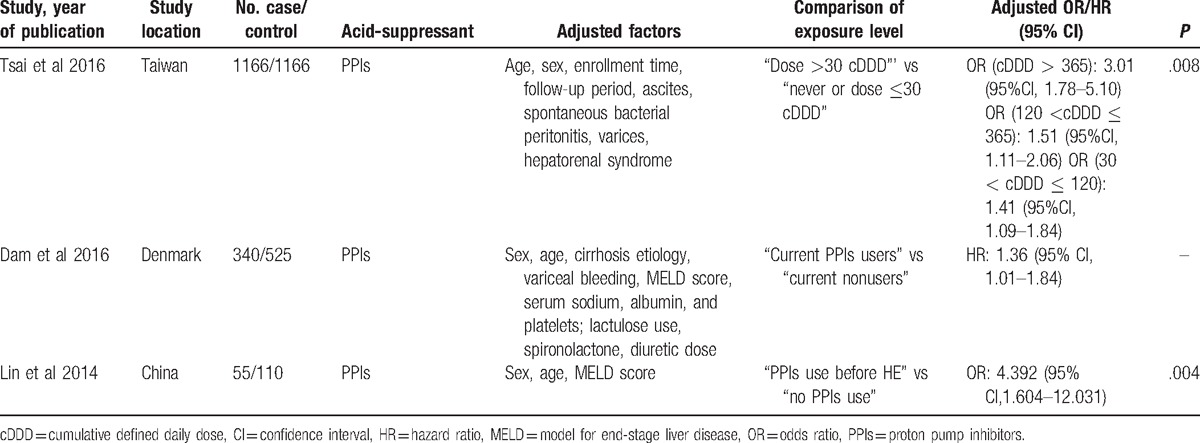
The main study characteristics are listed in Table 1. All 3 studies investigated the association between PPI use and HE, and age and sex were adjusted-for in all these studies. Tsai et al's study included 1166 patients with HE; Dam et al's study included 340 PPI users, of whom 88 subsequently developed HE; and Lin's research comprised a smaller population of 55 HE patients.[16–18] The adjusted ORs of the 3 studies were 1.738, 1.36, and 4.392, respectively.
Table 1.
General characteristics of included studies.
3.3. Pooled results and heterogeneity
The overall OR derived from using a random effects model was 1.76 (95% CI = 1.15, 2.69), indicating a rising risk of onset of HE in PPI users compared to nonusers (Fig. 2). Heterogeneity was significant among the pooled results (I2 = 61.4%, P = .075), when an I2 of 30% to 60% is considered to be a moderate heterogeneity level.[20]
Figure 2.
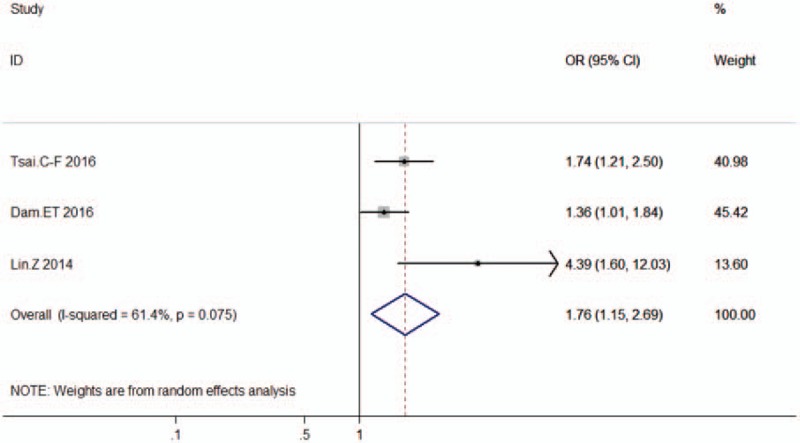
Summary estimates of the association between PPIs use and HE sorted by effect estimate. CI = confidence interval, HE = hepatic encephalopathy, I2 = the percentage of total variation across studies that is due to heterogeneity rather than change, PPIs = proton pump inhibitors.
3.4. Publication bias
A funnel plot was generated from the 3 studies, and it showed visual asymmetry that was mainly caused by the study of Lin et al[17] (Fig. 3). Since the trim and fill method is a well-established method to estimate the number of missing studies and reduce the publication bias,[21] we also performed the trim and fill analysis. Because significant heterogeneity was observed using the fixed effects model (Q = 13.881, P = .008), we used a random effects model. In contrast to previous results, the adjustment for publication bias using the trim and fill procedure resulted in an OR of 1.36 (95% CI: 0.909 to 2.035, P = .131), indicating that there was no relevance between PPIs and HE.
Figure 3.
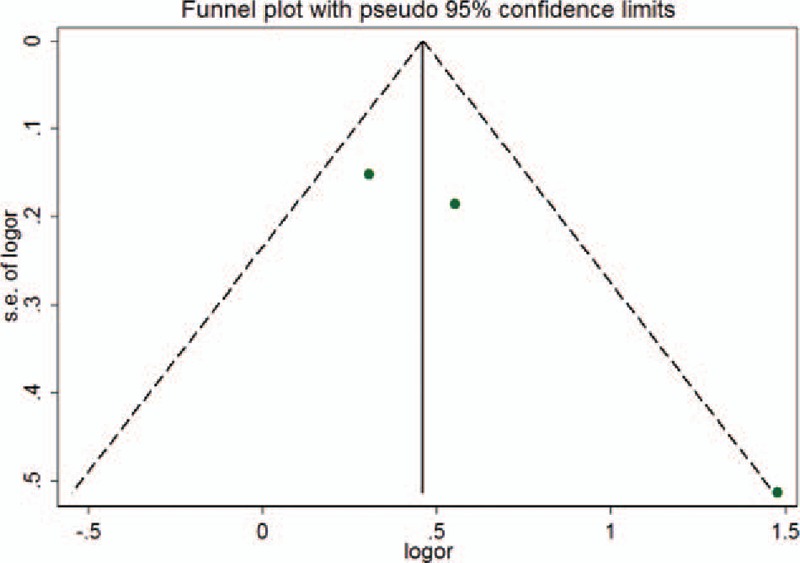
Funnel plot assessing publication bias. Dot lines are 95% pseudo-confidence intervals: OR = odds ratio, SE = standard error.
4. Discussion
To our knowledge, this is the first meta-analysis on the relationship between PPIs and HE. The results from our analysis revealed the association between PPIs and HE, with an average OR of 1.76 (95% CI 1.15, 2.69), which indicates that there is a higher risk of developing HE in PPI users with liver dysfunction. However, when publication bias was taken into consideration, no significant relationship was observed after using the trim and fill procedure, and this needs further investigation. While existing studies based on the hepatic insufficiency population suggest that PPIs may harm brain function, these results should be interpreted with caution because of limited research.
Our study has some limitations. Because there are only 3 articles on association of PPIs and HE, the outcomes of analysis based on this small number of studies can be controversial. Except for Tsai's study, variables such as type, dose, and duration of PPIs, patients’ baseline conditions, and other therapeutic interventions were not well adjusted. In Tsai et al's study,[18] the drug dosage and supply days were extracted, and cumulative defined daily dose (cDDD) was used. They found a dose-dependent risk of HE among PPIs users in cirrhotic patients. However, in the other 2 studies, the dose and duration of PPIs were not mentioned or difficult to obtain due to lack of data. Additionally, all the studies were retrospective and recall bias may be difficult to ignore. Thus, residual confounding factors may influence the results. As described in their methods, hepatic encephalopathy was assessed differently in all the included studies. Clinical features of HE can be confusing, and it is especially difficult to diagnose minimal and Grade 1 HE. Thus, HE morbidity may be underestimated.
Our analysis showed obvious heterogeneity among the 3 included studies, with I2 = 61.4%. There was a high degree of variability in study populations, recruitment, and assessment, as well as differences in the way data was recorded and handled in these retrospective studies. Thus, the included studies showed a high degree of heterogeneity. We assumed that different study populations impacted the heterogeneity; Dam et al.[16] and Tsai et al[18]conducted their studies in cirrhotic patients, whereas Lin et al's study comprised a relatively small population who had acute liver failure. The OR in the study by Lin et al was larger, whereas Tsai et al and Dam et al had reported similar ORs. It can be speculated that acute liver failure was more complicated and resulted in quicker multi-organ damage than chronic liver failure, and thus patients with acute liver failure had more multiorgan damage including brain dysfunction, as reflected by a lower survival rate of 20%. Lin et al defined PPIs use as intravenous, and it is, therefore, possible that patients who subsequently developed HE had a more deteriorated baseline condition than those without HE, and they needed more radical therapy, including intravenous PPIs. Overall, we observed obvious heterogeneity among the 3 studies. However, we did not conduct a sensitivity analysis, subgroup analysis, or regression analysis because of the small number of studies.
Publication bias was observed. Statistically significant results are more likely to be published, and PPIs are considered to be safe for most patients without any contradiction. Thus, it is possible that some studies with negative or null results were not published. Therefore, we conducted a trim and fill analysis to evaluate the stability of the overall results.
The potential underlying mechanism of PPIs’ action in the pathogenesis of HE remains unknown. Recent studies have highlighted the role of gut dysbiosis in the occurrence of HE,[3,4] which suggests that the gastrointestinal microenvironment and associated gut microbiota may play a vital role in the pathogenesis of HE. Researchers believed that accumulation of gut-derived ammonia, inflammation, and oxidative stress cause the underlying symptoms of HE. In addition to ammonia, which is recognized to be crucial in HE pathogenesis, recent studies proposed that synergy between systemic inflammation and infection may be involved.[22] Accumulating evidence indicates that alterations in gut micro-biota could lead to impaired gut motility, small intestinal bacterial overgrowth (SIBO), and increased gut permeability, with subsequent endotoxemia and systemic inflammation that impose toxic effect on central nervous system, eventually impair cognition and psychological status.[22,23] This would partly explain why rifaximin, a poorly absorbable synthetic antibiotic, can lower the risk of HE in cirrhotic patients through modulating the gut microbiota.[24,25]
PPIs have been reported to contribute to alterations in the gut microbiota, mainly causing bacterial overgrowth.[3] However, other studies found no such significant association.[26] Accumulating evidence suggests that there is a relationship between PPIs and the microbiota. PPIs are powerful gastric acid-suppressing drugs, and they can directly target the proton pumps of the bacteria, or affect the microenvironment of the flora by changing the pH within the alimentary tract; both of these can result in gastrointestinal microbiota dysbiosis. Some studies have shown negative results,[27,28] but convincing evidence shows that PPIs alter the gut microbiota and possibly increase the occurrence of SBP.[13,29–31] Recent retrospective studies also showed that PPIs are implicated during the onset of HE both in acute and chronic liver dysfunction, and Dam et al[16] found that PPIs were an independent risk factor for SBP, which could be an infection that is caused by PPIs. Thus, we postulate that PPIs act as a risk factor for HE by promoting gut microbiota translocation and subsequent bacterial infection. Oxidative stress and systemic inflammation implicated with dysbiomia may partially account for HE pathophysiology.
Our results may be restricted because of the study design and the inclusion of a relatively small number of studies. However, considering the wide use of PPIs in hospitalized patients and their potential risk for developing of HE, it is important to evaluate the positives and negatives of PPI administration, to provide guidance for healthcare practitioners. We found that PPIs did not increase the risk of HE after trim and fill analysis, which is in contrast to the conclusions drawn by the included studies. Therefore, additional prospective studies are needed to address these controversies.
Footnotes
Abbreviations: HE = hepatic encephalopathy, PPIs = proton pump inhibitors, SBP = spontaneous bacterial peritonitis, SIBO = small intestinal bacterial overgrowth.
Financial Support: This work was supported by International Science and Technology Cooperation Projects (2015DFA30650 and 2010DFB33720), Capital Special Research Project for Health Development (2014–2–4012), Capital research project for the characteristics clinical application (Z151100004015170), and Program for New Century Excellent Talents in University (NCET-11–0288).
Authorship: JB and AW designed and wrote the manuscript; JL performed data analysis; LW and SW read and selected the literature; HH and XY extracted data; YX, XL, and HZ revised the data and gave final approval to the manuscript.
XL, YX, and HZ share senior co-authorship.
JB, AW, and JL contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768–73. [DOI] [PubMed] [Google Scholar]
- [2].Wijdicks EF. Hepatic encephalopathy. N Engl J Med 2016;375:1660–70. [DOI] [PubMed] [Google Scholar]
- [3].Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol 2013;58:1020–7. [DOI] [PubMed] [Google Scholar]
- [4].Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol 2015;5suppl 1:S29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- [6].Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015;62:1056–60. [DOI] [PubMed] [Google Scholar]
- [7].Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moayyedi P, Leontiadis GI. The risks of PPI therapy. Nature reviews. Gastroenterol Hepatol 2012;9:132–9. [DOI] [PubMed] [Google Scholar]
- [9].Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Int Med 2016;176:172–4. [DOI] [PubMed] [Google Scholar]
- [10].Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci 2011;56:931–50. [DOI] [PubMed] [Google Scholar]
- [11].Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947–53. [DOI] [PubMed] [Google Scholar]
- [12].O’Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:753–9.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014;40:695–704. [DOI] [PubMed] [Google Scholar]
- [14].Bajaj JS, Ratliff SM, Heuman DM, et al. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther 2012;36:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang KW, Kuan YC, Luo JC, et al. Impact of long-term gastric acid suppression on spontaneous bacterial peritonitis in patients with advanced decompensated liver cirrhosis. Eur J Int Med 2016;32:91–5. [DOI] [PubMed] [Google Scholar]
- [16].Dam G, Vilstrup H, Watson H, et al. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64:1265–72. [DOI] [PubMed] [Google Scholar]
- [17].Lin ZN, Zuo YQ, Hu P. Association of proton pump inhibitor therapy with hepatic encephalopathy in hepatitis B virus-related acute-on-chronic liver failure. Hepat Mon 2014;14:e16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsai CF, Chen MH, Wang YP, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with Cirrhosis in population study. Gastroenterology 2016;152:134–41. [DOI] [PubMed] [Google Scholar]
- [19].Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ (Clinical Research ed) 1998;316:989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deeks JJ, Higgins JP, Altman DG. Higgins JP, Green S, et al. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, UK: John Wiley & Sons, Ltd; 2011;doi: 10.1002/9780470712184.ch92008. [Google Scholar]
- [21].Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- [22].Shawcross DL, Shabbir SS, Taylor NJ, et al. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2010;51:1062–9. [DOI] [PubMed] [Google Scholar]
- [23].Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 2013;14:851–8. [DOI] [PubMed] [Google Scholar]
- [24].Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther 2016;43suppl 1:11–26. [DOI] [PubMed] [Google Scholar]
- [25].Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071–81. [DOI] [PubMed] [Google Scholar]
- [26].Vesper BJ, Jawdi A, Altman KW, et al. The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab 2009;10:84–9. [DOI] [PubMed] [Google Scholar]
- [27].Ratuapli SK, Ellington TG, O’Neill MT, et al. Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth. Am J Gastroenterol 2012;107:730–5. [DOI] [PubMed] [Google Scholar]
- [28].Khan MA, Kamal S, Khan S, et al. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2015;27:1327–36. [DOI] [PubMed] [Google Scholar]
- [29].Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology 2015;149:883–5.e889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


