Abstract
The association between dietary vitamin K intake and the risk of fractures is controversial. Therefore we perform a meta-analysis of cohort or nested case–control studies to investigate the relationship between dietary vitamin K intake and the risk of fractures. A comprehensive search of PubMed and EMBASE (to July 11, 2016) was performed to identify cohort or nested case–control studies providing quantitative estimates between dietary vitamin K intake and the risk of fractures. Summary relative risk (RRs) with corresponding 95% confidence intervals (CIs) were pooled by using a random-effects model. Four cohort studies and one nested case–control study, with a total of 1114 fractures cases and 80,982 participants, were included in our meta-analysis. Vitamin K intake in all included studies refers exclusively to the intake of phylloquinone (vitamin K1), which is the predominant form of vitamin K in foods. We observed a statistically significant inverse association between dietary vitamin K intake and risk of fractures (highest vs. the lowest intake, RR = 0.78, 95% CI: 0.56–0.99; I2 = 59.2%, P for heterogeneity = .04). Dose–response analysis indicated that the pooled RR of fracture for an increase of 50 μg dietary vitamin K intake per day was 0.97 (95% CI: 0.95–0.99) without heterogeneity among studies (I2 = 25.9%, P for heterogeneity = .25). When stratified by follow-up duration, the RR of fracture for dietary vitamin K intake was 0.76 (95% CI: 0.58–0.93) in studies with more than 10 years of follow-up. Our study suggests that higher dietary vitamin K intake may moderately decrease the risk of fractures.
Keywords: cohort study, fracture, meta-analysis, nested case–control study, vitamin K
1. Introduction
Fractures is one of the major worldwide health problems, which have serious consequences, leading to disability, morbidity, and mortality. Bone mineral density (BMD), body mass index (BMI), low physical activity, cigarette smoking, diabetes mellitus, and stroke have been identified as major risk factors for fractures.[1–6] Lifestyle factors, including nutrition supplements, are believed to play an important role in the prevention of fracture.
Vitamin K, which is best known for function of blood coagulation, may also play a role in bone metabolism and bone-related disease.[7] Lots of epidemiologic studies have reported that lower vitamin K levels may lead to an adverse outcome on BMD and increase fracture risk. [8,9] Booth et al[10] performed a cross-sectional study and found that low plasma vitamin K level was associated with low BMD at the femoral neck in men, and with low BMD at the spine in women without using estrogen replacements. Another case–control study conducted by Torbergsen et al[9] showed that low plasma vitamin K level was associated with risk of hip fracture. The adjusted odds ratio for per ng/mL increase in plasma vitamin K level was 0.07 [95% confidence interval (95% CI): 0.02–0.32]. Huang et al[11] performed a meta-analysis for vitamin K2 supplement, and found that vitamin K2 supplement may improve the vertebral BMD.
However, only few epidemiologic studies focused on the relationship between dietary vitamin K intake and the risk of fracture, and the findings were inconsistent.[12–16] To the best of our knowledge, there has been no comprehensive quantitative assessment of the association between dietary vitamin K intake and the risk of fracture. Therefore, we perform this meta-analysis to estimate the evidence from prospective cohort studies and nested case–control studies on the relationship between dietary vitamin K intake and the risk of fractures. Vitamin K intake in all included studies refers exclusively to the intake of phylloquinone (vitamin K1), which is the predominant form of vitamin K in foods.[12–16]
2. Methods
2.1. Search strategy and data sources
This meta-analysis was according to the PRISMA statement and the MOOSE guidelines.[17,18] Because our study was performed on the basis of previous studies, the ethical approval and informed consent were not required. We performed a systematic literature search of PubMed and EMBASE from January 1966 through July 11, 2016. We used medical subject headings (MeSH) words and free text words (“vitamin K” OR “phylloquinone” OR “menaquinones”) AND “fracture” to identify relevant studies. We also reviewed the reference lists of relevant articles and additional relevant publications. No language restrictions were restricted.
2.2. Selection criteria
Studies were appropriate for inclusion in this study if they met the following inclusion criteria: (1) prospective cohort study or nested case–control study; (2) the exposure of interest was dietary vitamin K intake; (3) the outcome was fracture; and (4) the studies reported relative risks (RRs) and 95% CIs for continuous variable of vitamin K intake or at least 3 quantitative categories of vitamin K intake. The studies were excluded if they did not meet the inclusion criteria. If the same population was investigated in more than 1 prospective cohort study or nested case–control studies, we included the study with the longest follow-up duration.
2.3. Data extraction
Two independent investigators (GH and GZ) extracted related information using a standardized data collection form. For the reliability data extraction, there was a third investigator (XG) checking all the data. The following data were extracted from each study: last name of the first author, publication year of the study, the country of the study, the sex of participants, the age of participants, years of follow-up, total participants, fracture site, the number of fractures, fracture ascertainment, vitamin K assessment, covariates adjusted for in the multivariable analysis, and RR estimates with corresponding 95% CIs with the greatest degree of control for potential confounders. The study quality was assessed by the 9-star Newcastle–Ottawa Scale.
2.4. Statistical analysis
All statistical analyses were performed with Stata version 12.0 (Stata, College Station, TX). For the highest versus lowest mate-analysis, we pooled study-specific RRs and 95% CIs to evaluate the associations between dietary vitamin K intake and the risk of fracture using a random-effects model. The possible heterogeneity among studies was examined by Cochrane Q test and the Cochran I2 statistics.[19] If P value for heterogeneity was <.1, according to Higgins et al,[20]I2 values were considered of <25% as low heterogeneity, 25% to 50% as moderate heterogeneity, and >75% as high heterogeneity.
To assess the dose–response relationship between dietary vitamin K intake and the risk of fractures, we conducted a meta-analysis of dose–response categorical data. Referring to Feskanich et al,[12] who use 50 μg for each quintile, we determined 50 μg/day as a cut-off point. We estimated an RR with 95% CI for an increase of 50 μg dietary vitamin K intake per day for each study. We computed the trend from the correlated log RR estimated across categories of dietary vitamin K intake, according to Greenland and Longnecker [21] and Orsini et al.[22] We used restricted cubic splines with 3 knots by employing generalized least-squares regression to examine the potential nonlinear dose–response associations. A probability value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to 0.[22] Moreover, for each vitamin K category, we assigned the mean intake (or median if mean was not available), along with RR with its 95% lower and upper bounds, the number of cases, and amount of person-years. If the median or mean dietary vitamin K intake for each category was not provided, we assigned the midpoint data of each category as the median intake.[23] If the highest category or the lowest category was open-ended, we assigned that the boundary had the same amplitude as the closest category.[23]
Subgroup analyses according to gender and length of follow-up were performed to assess the potential effect modification of these variables on results. The publication bias was assessed using Begg, Egger tests, and “trim and fill” analysis [24–26]. The “trim and fill” analysis considered the possibility of hypothetical “missing” studies, then imputed their RRs, and got a pooled RR that combined the hypothetical missing studies as if they actually existed.[24,26] All statistical tests were considered P < .05 to be statistically significant.
3. Results
Figure 1 showed the procedure of the study selection. A total of 891 potential studies were identified from the PubMed and Embase databases. After assessment of title and abstract, 17 full texts were retrieved and assessed for more detail. Then, we excluded 12 studies because of lack of relevant outcome of vitamin K intake, fracture, or editorials and comments without original data. Finally, there were 4 cohort studies and 1 nested case–control study, included in our meta-analysis. [12–16]
Figure 1.
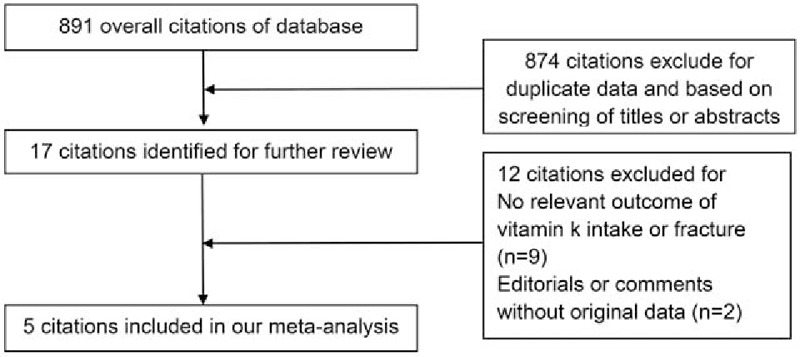
Flowchart for study selection.
3.1.1. The characteristics of included studies
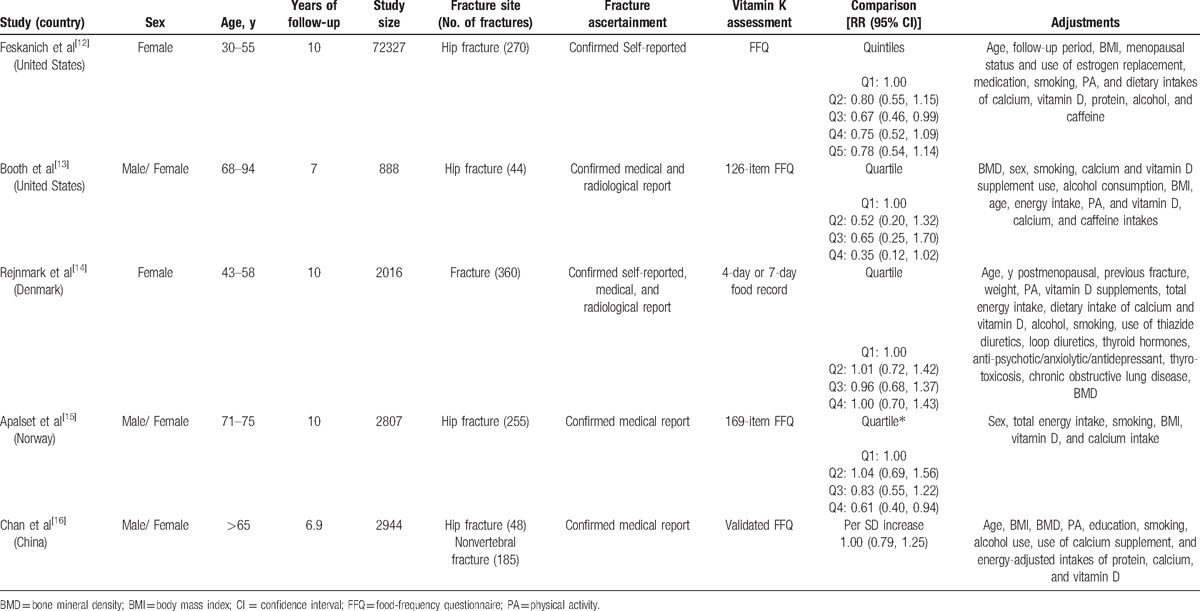
The characteristics of the included studies about dietary vitamin K intake are summarized in Table 1. These studies were published between 1999 and 2012, including 80,982 total subjects and 1114 fracture cases. Three studies included men and women, and 2 studies included only women. Three studies were conducted in the United States, 1 each study was from Denmark, Norway, and China. The fractures were assessed using confirmed self-reported, medical, and radiological report. Dietary vitamin K intake was assessed with a food-frequency questionnaire (FFQ) in 4 studies, only 1 study used 4-day or 7-day food record. Vitamin K intake in all included studies refers exclusively to the intake of vitamin K1. All subjects were more than 30 years old. Duration of follow-up for the included studies ranged from 6.9 to 10 years. Most studies provided RRs that were adjusted for age, BMI, BMD, physical activity, vitamin D and calcium intake, smoking, and alcohol consumption.
Table 1.
Characteristics of the prospective cohort studies included in the meta-analysis.
3.2. Main analysis
Figure 2 showed the pooled RR and 95% CI of fracture for the highest versus lowest dietary of vitamin K intake for each study and all studies combined. The present meta-analysis showed that the pooled RR of dietary vitamin K intake for total fracture is 0.78 (95% CI: 0.56–0.99). Moderate heterogeneity was observed (I2 = 59.2%, P for heterogeneity = .04).
Figure 2.
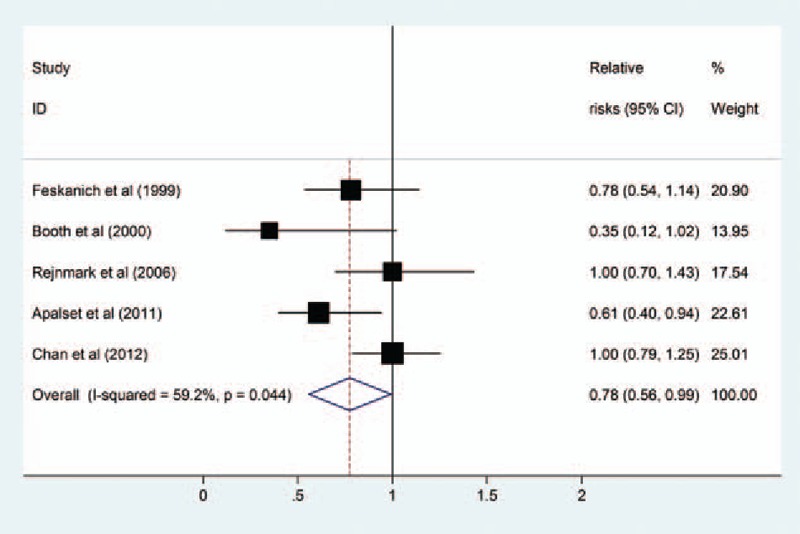
Pooled relative risks (RRs) of dietary vitamin K intake and risk of fractures.
3.3. Subgroup analysis
Subgroup analyses according to gender (Fig. 3) and length of follow-up (Fig. 4) were performed. The results showed that there was a statistically significant inverse association between dietary vitamin K intake and risk of total fractures in studies that contained both men and women (RR = 0.77, 95% CI 0.61–0.94, I2 = 76.6%, P for heterogeneity = .01), but not in studies that contained only women (RR = 0.87, 95% CI 0.64–1.10, I2 = 0%, P for heterogeneity = .36). Moreover, the results stratified by follow-up duration showed that there was a statistically significant inverse association between dietary vitamin K intake and risk of total fractures in studies with more than 10 years of follow-up (RR = 0.76, 95% CI 0.58–0.93, I2 = 30.2%, P for heterogeneity = .24), but not in studies with less than 10 years of follow-up (RR = 0.87, 95% CI 0.66–1.07, I2 = 84.3%, P for heterogeneity = .01).
Figure 3.
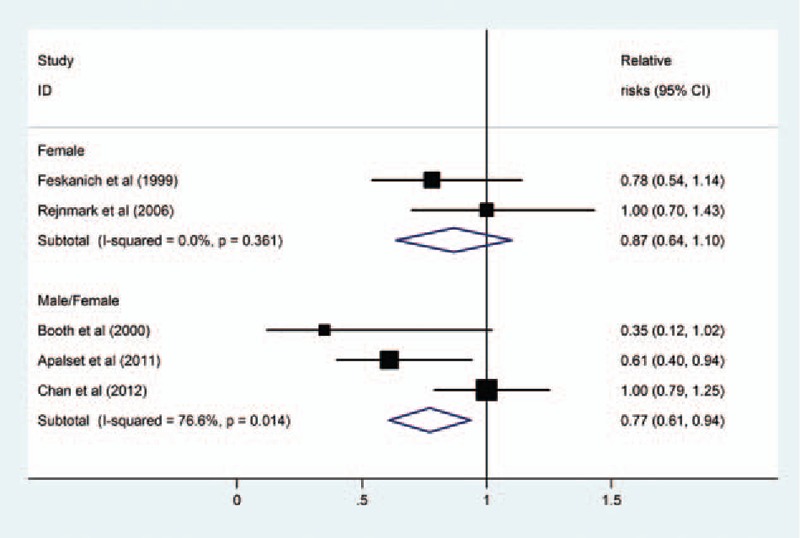
Subgroup analysis of gender for the relationship between dietary vitamin K intake and risk of fractures.
Figure 4.
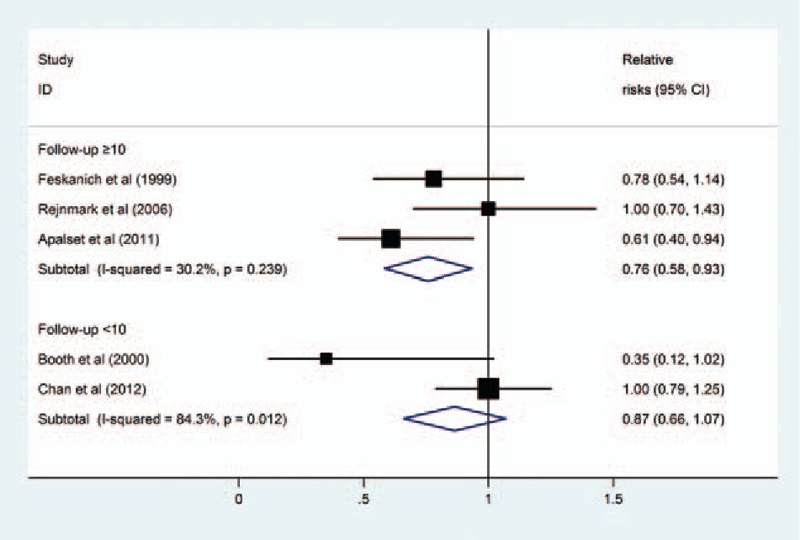
Subgroup analysis of follow-up duration for the relationship between dietary vitamin K intake and risk of fractures.
3.4. Publication bias
The Begg and Egger tests did not show any substantial asymmetry (P = .33 for Begg test and P = .36 for Egger test). Further trim and filled meta-analysis showed that there were no trimming data added.
3.5. Dose–response meta-analysis
Dose–response meta-analysis indicated that the pooled RR of fracture for an increase of 50 μg dietary vitamin K intake per day was 0.97 (95% CI: 0.95–0.99) without heterogeneity among studies (I2 = 25.9%, P for heterogeneity = .25, P for nonlinearity = .13). The Begg and Egger tests did not show any substantial asymmetry (P = .50 for Begg test and P = .32 for Egger tests). Further trim and filled meta-analysis showed that there were no trimming data added.
4. Discussion
To our knowledge, the present study is the first meta-analysis of the association between dietary vitamin K intake and risk of fractures. Our meta-analysis including 80,982 total subjects and 1114 fracture cases showed that there was a significant inverse association between dietary vitamin K intake and risk of fractures. The subjects with the highest intake of dietary vitamin K were found to have a 22% reduction in the risk of fractures (95% CI: 0.56–0.99), when compared with the lowest intake. An increase of 50 μg in intake of dietary vitamin K per day was associated with a 3% decreased risk of total fractures.
Several potential mechanisms have been proposed for the associations between dietary vitamin K and fracture harm. Vitamin K has a classic role in the function of several coagulation factors.[27] Moreover, vitamin K also has an important role within bone formation.[27] Osteocalcin is a vitamin K dependent protein, which is produced by the osteoblasts during bone formation and impact the synthesis and regulation of bone matrix.[28] The apoE genotype, which may play a role in the level of serum cholesterol and triglycerides, has been suggested to influence skeletal health through the transport of vitamin K to bone.[29] The persons who carry the apoE4 allele have a lower BMD and increased risk of fracture, which may be attributed to inadequate vitamin K transport to the bone. [29–32] Multiple signaling pathways related to bone are vitamin K dependent.[33] Experimental study showed that vitamin K can inhibit the differentiation of osteoclast.[34] Moreover, interleukin (IL)-6 is a stimulator for osteoclastic activity in bone remodeling, and if treated with vitamin K can decrease the levels of IL-6 in rats with high levels of IL-6.[35] Moreover, Cadir et al[36] found that administration of vitamin K(1) and fluoride resulted in an additional increase in vertebral bone mass in growing rats. Another study examined the effects of the long-term addition of vitamin K1 or vitamin K2 to a control diet on BMD, bone strength, body composition, and serum parameters in rats. And the results showed that the addition of vitamin K1 to the basic control diet significantly increased the BMD of the femur after 3 months. The addition of vitamin K1 or vitamin K2 significantly decreased the total fat accumulation and serum triglycerides compared with the control.[37]
In addition, several previous meta-analyses investigated the associations between oral vitamin K supplementation and the risk of fractures and BMD. Cockayne et al[38] performed a meta-analysis to assess whether oral vitamin K supplementation can impact bone loss or fracture risks. And the authors found that oral vitamin K supplementation can reduce the fracture risk (odds ratio was 0.40 for vertebral fractures, 0.23 for hip fractures, and 0.19 for nonvertebral fractures). Fang et al[39] performed a meta-analysis focusing on the associations between oral vitamin K supplementation and BMD, and results showed that oral vitamin K supplementation might be efficacious in increasing BMD at the lumbar spine but not at the femoral neck. Another meta-analysis conducted by Huang et al[11] found that oral vitamin K2 supplementation may significantly improve the vertebral BMD for both medium-term and long-term results in postmenopausal women with osteoporosis. These above results may support our conclusion.
Our meta-analysis has several strengths. First, our meta-analysis was based on prospective studies, and the recall and selection bias was minimized. Second, individual epidemiological studies had insufficient statistical power and may lead to inconsistent results. Our meta-analysis including a large number of participants and fracture cases may enhance the statistical power and solve the inconsistency of the studies on the relationship between dietary vitamin K intake and risk of fractures. Moreover, our results showed a significant dose–response relationship between dietary vitamin K intake and risk of fractures, which thereby further strengthened this association.
Our meta-analysis also has several limitations. First, as a meta-analysis, the residual confounding factors are always of concern in the included studies, and the possibility that other risk factors may impact the observed association between dietary vitamin K intake and risk of fractures. However, most of the studies were adjusted for a wide range of potential confounding factors, including age, BMI, BMD, physical activity, vitamin D and calcium intake, smoking, and alcohol consumption. Second, random misclassification of dietary vitamin K intake may impact the results. However, the adjusted RRs were assessed on the basis of the highest categories compared with the lowest categories of dietary vitamin K intake, and the wide ranges of dietary vitamin K intake may reduce this bias. Third, in our meta-analysis, 3 studies included men and women and 2 studies included only women. We could not examine the association between dietary vitamin K intake and risk of fracture in men. Moreover, subgroup analysis showed that no statistically significant association between dietary vitamin K intake and risk of total fractures was observed in studies of women. Because only 2 studies investigated in women, this result should be interpreted with caution. Further well-designed and stratified cohort studies should be conducted to quantitatively assess the relationship between dietary vitamin K intake and risk of total fractures in men and women, respectively.
5. Conclusion
Results from this meta-analysis indicate that intake of dietary vitamin K is significantly associated with reduced risk of fractures. Our meta-analysis offers an additional evidence on the relationship between dietary vitamin K intake and risk of fractures. The benefit of vitamin K should be confirmed in future well-designed prospective cohort studies and clinical trials.
Footnotes
Abbreviations: BMD = Bone mineral density, BMI = body mass index, CI = confidence intervals, IL = interleukin, RR = relative risks.
Authorship: GH, GZ, and XC conceived of and designed the meta-analysis. GH, BZ, and XC reviewed abstracts and full articles. GH, MG, CC, QZ, and GZ extracted the data. GH performed the meta-analysis and wrote the first draft. All authors have read and approved the final draft.
All authors declare that they have no conflict of interest.
References
- [1].Shen GS, Li Y, Zhao G, et al. Cigarette smoking and risk of hip fracture in women: a meta-analysis of prospective cohort studies. Injury 2015;46:1333–40. [DOI] [PubMed] [Google Scholar]
- [2].Janghorbani M, Van Dam RM, Willett WC, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495–505. [DOI] [PubMed] [Google Scholar]
- [3].Yuan ZC, Mo H, Guan J, et al. Risk of hip fracture following stroke, a meta-analysis of 13 cohort studies. Osteoporos Int 2016;27:2673–9. [DOI] [PubMed] [Google Scholar]
- [4].Zhang X, Yu Z, Yu M, et al. Alcohol consumption and hip fracture risk. Osteoporos Int 2015;26:531–42. [DOI] [PubMed] [Google Scholar]
- [5].Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223–33. [DOI] [PubMed] [Google Scholar]
- [6].De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 2005;16:1330–8. [DOI] [PubMed] [Google Scholar]
- [7].Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom 2013;16:409–13. [DOI] [PubMed] [Google Scholar]
- [8].Hodges SJ, Akesson K, Vergnaud P, et al. Circulating levels of vitamins K1 and K2 decreased in elderly women with hip fracture. J Bone Miner Res 1993;8:1241–5. [DOI] [PubMed] [Google Scholar]
- [9].Torbergsen AC, Watne LO, Wyller TB, et al. Vitamin K1 and 25(OH)D are independently and synergistically associated with a risk for hip fracture in an elderly population: a case control study. Clin Nutr 2015;34:101–6. [DOI] [PubMed] [Google Scholar]
- [10].Booth SL, Broe KE, Peterson JW, et al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 2004;89:4904–9. [DOI] [PubMed] [Google Scholar]
- [11].Huang ZB, Wan SL, Lu YJ, et al. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos Int 2015;26:1175–86. [DOI] [PubMed] [Google Scholar]
- [12].Feskanich D, Weber P, Willett WC, et al. Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 1999;69:74–9. [DOI] [PubMed] [Google Scholar]
- [13].Booth SL, Tucker KL, Chen H, et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr 2000;71:1201–8. [DOI] [PubMed] [Google Scholar]
- [14].Rejnmark L, Vestergaard P, Charles P, et al. No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int 2006;17:1122–32. [DOI] [PubMed] [Google Scholar]
- [15].Apalset EM, Gjesdal CG, Eide GE, et al. Intake of vitamin K1 and K2 and risk of hip fractures: the Hordaland Health Study. Bone 2011;49:990–5. [DOI] [PubMed] [Google Scholar]
- [16].Chan R, Leung J, Woo J. No association between dietary vitamin K intake and fracture risk in Chinese community-dwelling older men and women: a prospective study. Calcif Tissue Int 2012;90:396–403. [DOI] [PubMed] [Google Scholar]
- [17].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [22].Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40. [Google Scholar]
- [23].Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012;95:362–6. [DOI] [PubMed] [Google Scholar]
- [24].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Begg CB. A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig, Statistics in Medicine, 2001; 20:641-654. Stat Med. 2002; 21(12):1803; author reply 1804. [DOI] [PubMed] [Google Scholar]
- [26].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shah K, Gleason L, Villareal DT. Vitamin K and bone health in older adults. J Nutr Gerontol Geriatr 2014;33:10–22. [DOI] [PubMed] [Google Scholar]
- [28].Atkins GJ, Welldon KJ, Wijenayaka AR, et al. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol 2009;297:C1358–1367. [DOI] [PubMed] [Google Scholar]
- [29].Kohlmeier M, Salomon A, Saupe J, et al. Transport of vitamin K to bone in humans. J Nutr 1996;126(4 Suppl):1192S–6S. [DOI] [PubMed] [Google Scholar]
- [30].Zajickova K, Zofkova I, Hill M, et al. Apolipoprotein E 4 allele is associated with low bone density in postmenopausal women. J Endocrinol Invest 2003;26:312–5. [DOI] [PubMed] [Google Scholar]
- [31].Johnston JM, Cauley JA, Ganguli M. APOE 4 and hip fracture risk in a community-based study of older adults. J Am Geriatr Soc 1999;47:1342–5. [DOI] [PubMed] [Google Scholar]
- [32].Cauley JA, Zmuda JM, Yaffe K, et al. Apolipoprotein E polymorphism: a new genetic marker of hip fracture risk: the Study of Osteoporotic Fractures. J Bone Miner Res 1999;14:1175–81. [DOI] [PubMed] [Google Scholar]
- [33].Falcone TD, Kim SS, Cortazzo MH. Vitamin K: fracture prevention and beyond. PM R 2011;3(6 Suppl 1):S82–7. [DOI] [PubMed] [Google Scholar]
- [34].Koshihara Y, Hoshi K, Okawara R, et al. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol 2003;176:339–48. [DOI] [PubMed] [Google Scholar]
- [35].Ohsaki Y, Shirakawa H, Hiwatashi K, et al. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem 2006;70:926–32. [DOI] [PubMed] [Google Scholar]
- [36].Cadir B, Kurkcu M, Oz IA, et al. Effects of vitamin K(1) on fluoride-induced bone changes in growing rats: a histomorphometric and radiodensitometric study. Arch Oral Biol 2005;50:889–95. [DOI] [PubMed] [Google Scholar]
- [37].Sogabe N, Maruyama R, Baba O, et al. Effects of long-term vitamin K(1) (phylloquinone) or vitamin K(2) (menaquinone-4) supplementation on body composition and serum parameters in rats. Bone 2011;48:1036–42. [DOI] [PubMed] [Google Scholar]
- [38].Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256–61. [DOI] [PubMed] [Google Scholar]
- [39].Fang Y, Hu C, Tao X, et al. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab 2012;30:60–8. [DOI] [PubMed] [Google Scholar]


