Abstract
Rationale:
Less than 1% of breast carcinomas metastasize to the gastrointestinal tract. The diagnosis is frequently not recognized especially when the history of breast carcinoma is remote.
Patient concerns:
A 61-year-old female with a remote history of breast carcinoma presented with a 3-month history of change in bowel habits. Colonoscopy showed a circumferential rectal mass with initial impression of primary rectal cancer. MRI of the rectum showed findings that are atypical for primary rectal cancer.
Diagnoses:
Deep biopsy of the rectal mass confirmed lobular breast carcinoma metastasis to the rectum.
Intervention and outcomes:
The patient was treated with radiotherapy and hormonal therapy. She is symptomatically well 2 years after presentation and remains on hormonal therapy.
Lessons:
Lobular breast cancer which metastasizes to the rectum can mimic primary rectal cancer clinically. The unique MRI features described in our case when present with a concordant history of lobular breast carcinoma should alert the radiologist to the possibility of this diagnosis which has important treatment implications.
Keywords: breast cancer, gastrointestinal tract, metastasis, MRI, rectum
1. Introduction
Breast cancer is the most common malignancy in women. Metastatic breast cancer typically involves the lungs, bones, brain, liver, and lymph nodes.[1] Less than 1% of breast carcinomas metastasize to the gastrointestinal tract (GIT).[2] Hence the diagnosis is frequently not recognized when the GIT is the only site of metastases or when the history of breast carcinoma is remote.
There have been several case reports of barium, computer tomography (CT), and endoscopic features of metastatic breast carcinoma to the GIT, but no reports to our knowledge have elaborated on the magnetic resonance (MR) features. MRI depicts better soft tissue contrast and provides superior delineation of the individual histological layers of the gastrointestinal wall. We present a case of metastatic breast carcinoma to the rectum which was initially mistaken to be primary rectal carcinoma. The unique MRI morphologic features described in our case potentially allow radiologic differentiation from primary rectal adenocarcinoma.
2. Case report
2.1. Ethics review and patient consent
This is a retrospective case report, where the patient's medical history, records, and images were reviewed. Ethics committee approval was not necessary as the case fell within the standard of medical care. Informed consent on diagnostic and therapeutic procedures were given by the patient. Additionally, verbal consent was obtained from the patient for the purposes of this case publication.
2.2. Case
A 61-year-old female presented with a 3-month history of change in bowel habits. She complained of increased bowel frequency, mucous in her stools and reduced stool caliber.
She had a history of stage T1N0M0 right breast lobular carcinoma 11 years ago. She had right breast mastectomy and was given 5 years of Tamoxifen.
Digital per rectal examination revealed circumferential induration and bogginess in the anal canal and rectum. Proctoscopy was not done in view of the patient's discomfort. The plain abdominal and chest radiographs were unremarkable. Colonoscopy revealed a circumferential lesion in the distal rectum causing luminal narrowing (Fig. 1). The overall initial clinical impression was that of primary rectal carcinoma.
Figure 1.
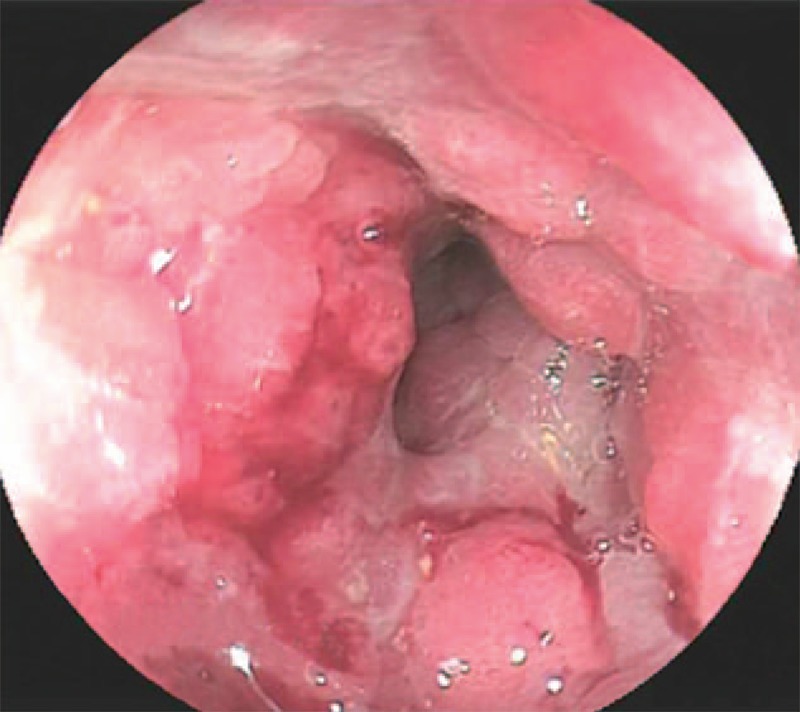
Colonoscopy image shows a concentric, circumferential lesion in the distal rectum with resultant luminal narrowing.
A staging CT of the abdomen and pelvis was performed which revealed a 9 cm segment of diffuse circumferential wall thickening along the mid rectum to anal canal with resultant luminal narrowing (Fig. 2). Marked perirectal fat stranding was observed but there was no significant abdominal-pelvic adenopathy or hepatic metastases.
Figure 2.
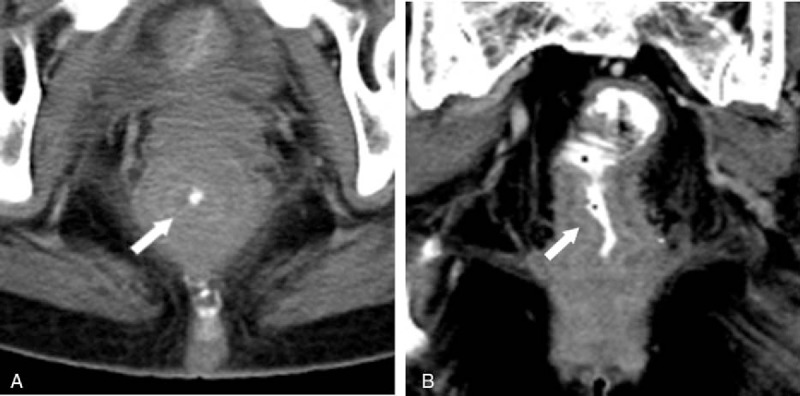
On CT abdomen and pelvis (A and B), a diffuse long segment concentric rectal wall thickening (arrows) is noted with resultant luminal narrowing. There is also marked perirectal stranding but no enlarged mesorectal nodes are seen.
An MRI of the rectum was performed as was routine in our institution for local staging of all primary rectal cancers. This again showed diffuse concentric mural thickening of the rectum and anal canal. MRI was able to demonstrate the wall thickening involved the submucosa and muscularis propria layers and spared the mucosa. On T2-weighted images, the laminated architecture of the histological layers of the gastrointestinal wall was preserved but thickened. In addition, an extramural component of tumor that was markedly hypointense on T2-weighted images also seen. The entire involved segment of the rectum showed intense avid enhancement on the postcontrast images with only mild restricted diffusion. Despite the extensive involvement, there was no enlarged mesorectal or superior rectal node (Fig. 3).
Figure 3.
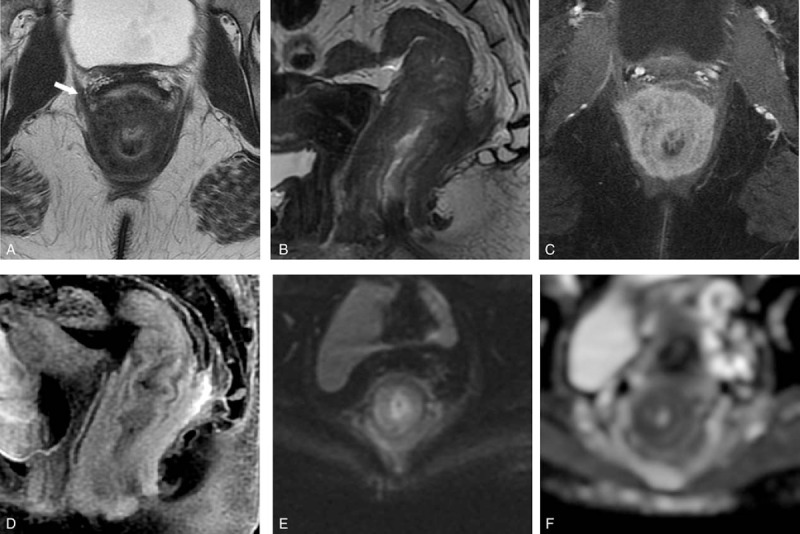
On MRI rectum, noncontrast axial and sagittal T2-weighted images (TR/TE = 2440/82 ms; slice thickness = 4 mm; field of view = 18 cm) (A and B) show a long segment of concentric rectal wall thickening extending to the anal canal, with thickening of the various layers. The mural thickening involves the submucosa and muscularis propria but spares the mucosa. A T2 hypointense, extramural component (arrow) is also seen. Postcontrast axial and sagittal T1-weighted fat saturated image show avid enhancement of the rectal wall (C and D). Axial diffusion-weighted imaging (b = 800 s/mm2) and apparent diffusion coefficient (ADC) maps (E and F) show mild restricted diffusion of the thickened rectal wall (ADC = 1.1 × 10–3 mm2/s).
The initial endoscopic biopsy performed showed colonic mucosa with stromal fibrosis, congestion, and distortion of the crypts but no evidence of active inflammation, granuloma formation, ulceration, dysplasia, or malignancy. In view of high suspicion of malignancy and predominantly submucosal changes on MRI, a deep biopsy was repeated.
Histology showed invasive carcinoma with cord-like infiltrative growth pattern, typical of breast lobular carcinoma. The tumor cells were positive for cytokeratin markers AE1/3, estrogen receptor (ER), and progesterone receptor (PR). Marked desmoplastic stroma typical for lobular carcinoma was also noted (Fig. 4).
Figure 4.
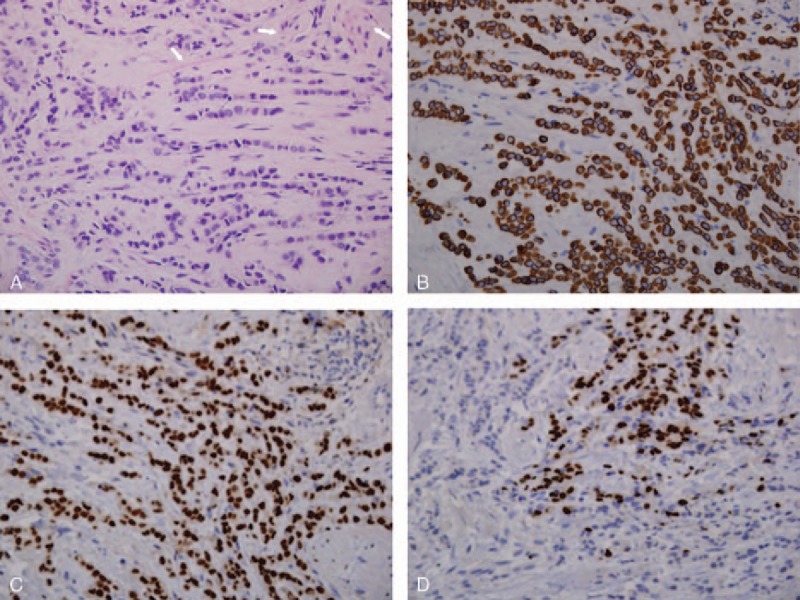
Tumor has cord-like infiltrative growth pattern typical of breast lobular carcinoma. Tumor cells have mild pleomorphic nuclei and high nuclear cytoplasmic ratio. Arrows highlighting muscle fibers (A, hemotoxylin and eosin, ×400). Tumor cells are positive for cytokeratin marker AE1/3 (B, ×400) and ER (C, ×400). Tumor cells also focally express PR (D, ×400).
The patient underwent laparoscopic assisted diverting colostomy and 16 cycles of 40 Gy palliative radiotherapy. She was also started on hormonal therapy, Letrozole 2.5 mg every morning (OM). Serial follow-up MRIs of the pelvis showed decreasing tumor volume. A year after she was started on treatment for metastasis, she had closure of colostomy. She currently remains on Letrozole with stable disease 2 years after presentation. The timeline of events is as Fig. 5.
Figure 5.
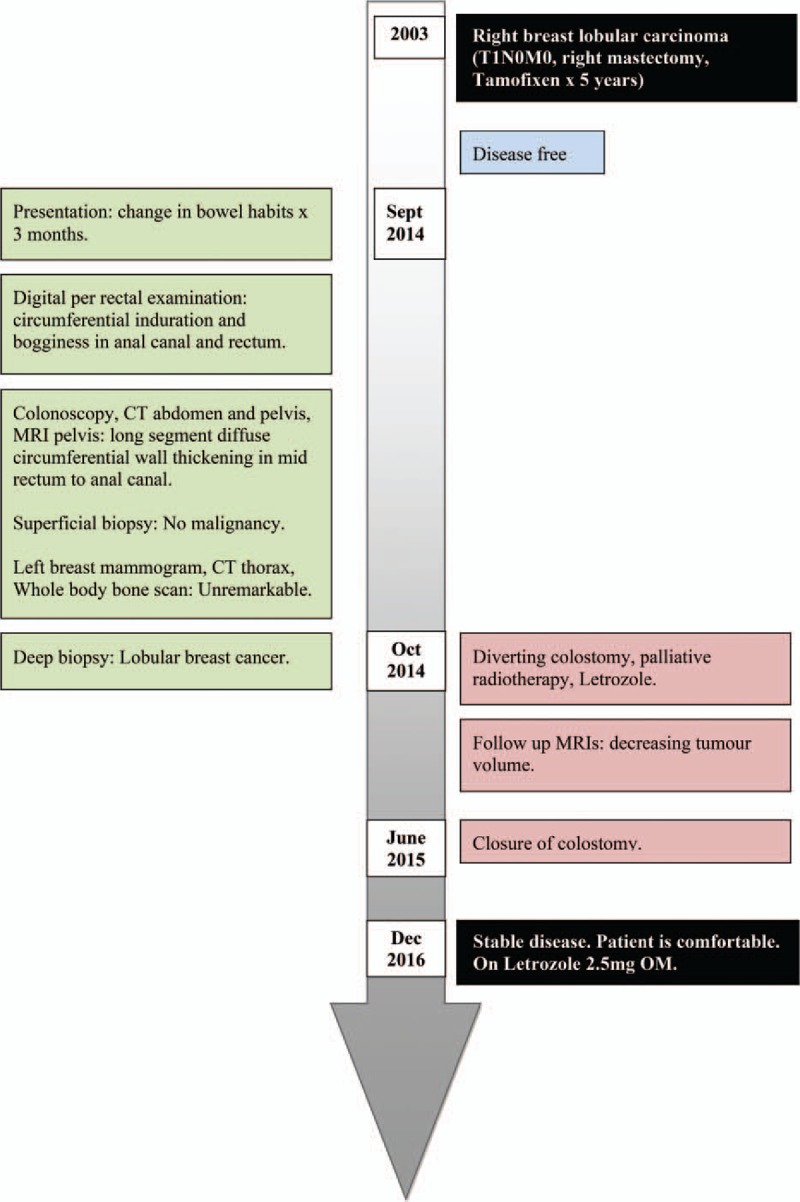
Timeline of events.
3. Discussion
Metastatic presentations of breast cancer are likely to increase as the number of breast cancer survivors continues to rise.[2] The GIT is not a common site of metastatic disease but breast carcinoma is the most common primary tumor to metastasize to the GIT. Interestingly, metastatic disease to the extrahepatic GIT from breast carcinoma usually originates from the lobular carcinoma subtype which accounts for only 8% to 14% of all breast adenocarcinomas, rather than the more common invasive ductal carcinoma.[2] This could be related to a particular tropism of lobular cells to the GIT.
In a large retrospective analysis of patterns of metastatic spread of lobular breast carcinoma, it was shown that the small bowel is the commonest site in the GIT followed by the stomach.[3] Metastases to the colon and rectum were particularly rare. A recent literature review showed that out of 206 patients with reported GIT involvement from breast cancer, only 7% had metastases to the rectum.[4] These patients usually presented with obstruction, abdominal pain, and rectal bleeding.
It has been well documented that recurrence in lobular breast cancer can occur many years after the initial diagnosis of breast cancer, even in early stage tumors. In our case, GIT recurrence occurred 11 years after treatment of T1N0M0 breast cancer. Recurrences of lobular breast cancer have been reported up to 30 years from initial diagnosis.[5] Therefore clinicians and radiologists should be mindful of the relatively high prevalence of late disease recurrence in patients with even a remote history of lobular breast carcinoma.
The CT features of breast metastases to the GIT have previously been described as bowel mural thickening and bowel dilation which are nonspecific findings.[6] Our case is the first report to our knowledge detailing the MRI features of breast cancer metastasizing to rectum. The following MR characteristics seen in our case may be useful for distinction from typical primary rectal carcinoma. First, the diffuse and relatively long segment concentric mural thickening of the rectum which involves the submucosa and musclaris propria layers with sparing of the mucosa is reminiscient of a linitis plastica pattern (circumferentially infiltrating intramural cancer causing significant desmoplastic response with thickened wall and concentric ring pattern on T2-weighted images) not usually seen in primary rectal cancer. In primary rectal carcinoma, there is commonly more eccentric wall thickening and an obvious invasive margin with mucosal disruption, rather than the concentric wall thickening in our case. Second, the more mass like portion of the tumor in our case showed marked T2 hypointensity rather than the intermediate to hyperintense appearance typical of rectal carcinoma. We believe this may be due to the intense desmoplastic stromal reaction with fibrous component found in breast carcinoma. Third, there was also only very mild restricted diffusion in the involved segment of the rectum rather than the markedly restricted diffusion found in most primary rectal cancers. We postulate this could reflect the relative hypocellularity of lobular breast cancer, in which the tumor cells are arranged in loose cords (“Indian file” pattern) among a fibrous stroma rather than the more typical densely packed clusters of tumor cells in rectal carcinoma. Fourth, there were no enlarged mesorectal or pelvic side wall nodes despite the very extensive involvement of the bowel wall, which would be unusual for advanced rectal carcinomas.
Other differentials for a linitis plastica appearance of the rectum would include inflammatory colitis such as ulcerative colitis, ischemic colitis, and infiltrative malignancies such as lymphoma.[7,8] However these conditions do not exhibit the markedly hypointense T2 signal present in this case.
4. Conclusion
Lobular breast cancer which metastasizes to the rectum can mimic primary rectal cancer clinically. We have reported the unique MR imaging features of metastatic lobular breast carcinoma to the rectum which builds on previously published CT reports of this entity. Radiologists should be aware of these features as these cases may be initially misdiagnosed as primary rectal carcinoma. A pertinent history of lobular breast carcinoma in combination with the characteristic MR findings described would enable one to suggest the diagnosis.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, CT = computer tomography, DWI = diffusion-weighted imaging, ER = estrogen receptor, GIT = gastrointestinal tract, Gy = gray, H&E = hemotoxylin and eosin, MR = magnetic resonance, OM = every morning, PR = progesterone receptor, TE = echo time, TNM = TNM Classification of Malignant Tumours, TR = repetition time.
The authors have no conflicts of interest to disclose.
References
- [1].Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol 1998;93:111–4. [DOI] [PubMed] [Google Scholar]
- [2].Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637–41. [PubMed] [Google Scholar]
- [3].Asch MJ, Wiedel PD, Habif DV. Gastrointestinal metastases from carcinoma of the breast. Arch Surg 1968;96:840–3. [DOI] [PubMed] [Google Scholar]
- [4].Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer 2012;2012:439023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benfiguig A, Anciaux ML, Eugène CI, et al. Gastric metastasis of breast cancer occurring after a cancer-free interval of 30 years (article in French). Ann Gastroenterol Hepatol (Paris) 1992;28:175–7. [PubMed] [Google Scholar]
- [6].Winston CB, Hadar O, Teitcher JB, et al. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 2000;175:795–800. [DOI] [PubMed] [Google Scholar]
- [7].Balthazar EJ. CT of the gastrointestinal tract: principles and interpretation. AJR Am J Roentgenol 1991;156:23–32. [DOI] [PubMed] [Google Scholar]
- [8].Ha HK, Jee KR, Yu E, et al. CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. AJR Am J Roentgenol 2000;174:463–6. [DOI] [PubMed] [Google Scholar]


