Abstract
Adrenocortical carcinoma (ACC) is a rare and malignant tumor. The main treatment is primary surgical resection with or without mitotane therapy. The role of radiation therapy is still controversial. We aim to investigate the survival efficacy of radiotherapy in a large population-based cohort.
We queried the Surveillance, Epidemiology, and End Results (SEER) database (1973–2013) to identify cases with ACC. Traditional multivariate Cox regression and propensity score analysis were used to evaluate the effect of radiotherapy on cancer survival. The survival outcomes included overall survival and cancer-specific survival. The treatment effect was evaluated using a hazard ratio (HR) and its 95% confidence interval (95% CI).
Five hundred thirty patients diagnosed with ACC were identified. Among them, 74 patients received radiotherapy. In the multivariate Cox regression, radiotherapy did not increase the overall survival (HR 0.794, 95% CI 0.550–1.146, P = .218) or cancer-specific survival (HR 0.842, 95% CI 0.574–1.236, P = .388). In the propensity score analysis, the results consistently showed no survival benefit of radiotherapy regardless of the different propensity score analysis methods.
Radiotherapy did not improve overall or cancer-specific survival in ACC patients. Further confirmation is needed from multi-institutional prospective studies in the future.
Keywords: adrenocortical carcinoma, radiation, radiotherapy, survival
1. Introduction
Adrenocortical carcinoma (ACC) is a highly malignant and rare tumor with a median of 35.2 months overall survival time.[1] Surgical resection is still the mainstay treatment for resectable ACCs and even some metastatic cases.[2,3] More than half the patients with ACC experienced tumor recurrence after surgery.[4,5] Adjuvant treatment is needed to control and delay tumor progression. Mitotane, as an adrenotoxic agent that blocks cortisol synthesis, was suggested to be efficient for reducing recurrence risk,[6] but validation trial is still needed. Moreover, whether adjuvant mitotane after surgery would improve overall survival is not consistent.[6,7] In addition, the mitotane plasma levels should be continuously monitored.[4] Radiotherapy is an alternative choice for ACC patients. It is mainly applied in advanced ACC patients for palliative care.[3,8] ACC is deemed to be resistant to radiation therapy based on previous case series. Recently, radiotherapy has demonstrated benefits through reducing recurrence risk. However, the effect of radiation therapy is still controversial.[7,9–12] The bone of contention is whether radiotherapy improves oncological outcomes. We intended to investigate the effect of radiotherapy using a national population-based database with a large sample size.
2. Methods
2.1. Data acquisition and selection
We queried the Surveillance, Epidemiology, and End Results (SEER) database (1973–2013) for cases diagnosed with ACC. Cases were identified from the SEER∗stat client using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). The initial condition was set as Site Recode B ICD-O-3/WHO 2008: Adrenal Gland. In all, 5321 cases with an adrenal malignant tumor were identified. After that, inclusion and exclusion criteria were applied. The screening conditions were ACC, being an adult, first tumor, and unilateral ACC. Patients without survival data or deterministic treatment modalities were excluded. Ultimately, 530 cases without missing data were identified for analysis. Ethical approval was not needed because the study was exempt from Institutional Review Board review. Variables included age, race, gender, marital status, tumor laterality, surgery, regional lymph node dissection (RLND), tumor size, and tumor stage. For tumor stage, we used the European Network for the Study of Adrenal Tumors (ENSAT) staging system[13] to evaluate the ACC stage according to the codes of the Collaborative Stage (http://cancerstaging.org/cstage/about/Pages/default.aspx). Stage I and stage II were combined and classified as the reference. The end outcomes were overall survival and cancer-specific survival.
2.2. Statistical analysis
Continuous variables were described with medians and interquartile range (IQR), and Mann–Whitney U tests were used to compare the differences between the groups. For categorical variables, we used Fisher exact test to evaluate the differences between the 2 groups. The primary outcome was overall survival. For cancer-specific survival, we adopted the cause of death listed in the SEER dataset. The follow-up cutoff date was December 31, 2013. A hazard ratio (HR) and its 95% confidence interval (95% CI) were used to estimate the risk of mortality from multivariate Cox regression analysis. Compared with traditional multivariate analysis incorporating numerous independent variables, we also performed propensity score analysis, including propensity score adjustment (PSA), inverse probability treatment weighting (IPTW), standardized mortality ratio weighting (SMRW), and propensity score matching (PSM). PSM was performed using the 1:1 nearest-neighbor method. For the propensity score matching cohort, survival differences were visualized and tested by the Kaplan–Meier method. All statistical analyses were performed using Stata 14.2 (Stata Corp, College Station, TX). A 2-tailed P value less than .05 was considered statistically significant.
3. Results
Five hundred thirty cases were identified after inclusion and exclusion criteria. All cases were diagnosed from 2004 to 2013 (relatively modern samples due to consideration of radiation technique development). Among them, 74 patients received radiation therapy. Three hundred eighteen patients (318/530) died during follow-up. The distribution of tumor stage was significantly different between the radiotherapy and no radiotherapy groups (P = .002). No differences between the 2 groups were observed on age, gender, race, marital status, tumor laterality, surgery, tumor size, or RLND status (Table 1).
Table 1.
Characteristics of the included 530 patients.
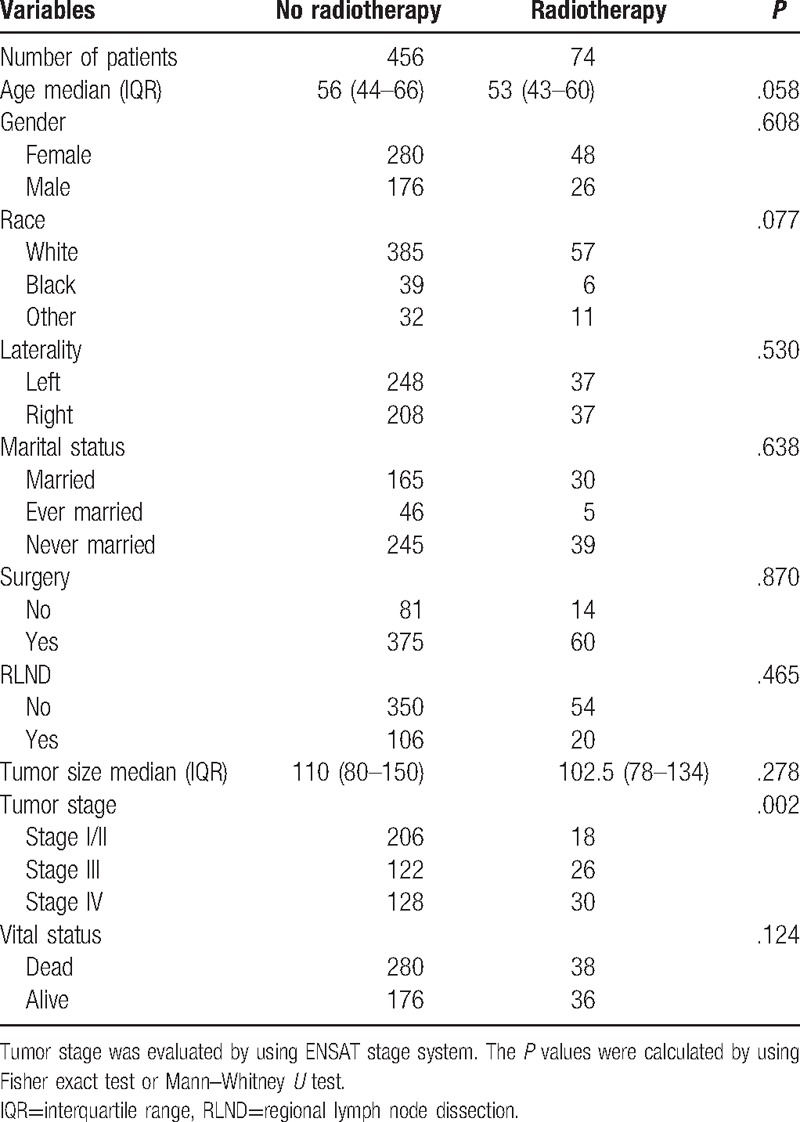
Table 2 presents the traditional multivariate survival analysis of overall and cancer-specific survival. Radiotherapy did not increase the overall survival (HR 0.794, 95% CI 0.550–1.146, P = .218) or cancer-specific survival (HR 0.842, 95% CI 0.574–1.236, P = .388). Prognostic factors were age, surgery, and tumor stage. Table 3 summarizes the results of the various propensity score analysis. The results consistently presented no survival benefits regardless of the use of PSA, IPTW, SMRW, and PSM. After propensity score matching, the survival curves of the 2 groups were similar (Fig. 1). The P values of the log-rank tests were .7223 and .8051 for overall survival and cancer-specific survival, respectively. Considering the survival difference, we performed a subgroup survival analysis stratified by tumor stage. We stratified the stage into 2 subgroups. Group 1 was stage I/II and stage III, group 2 was stage IV. Overall mortality risk for radiation was similar to nonradiation patient in group 1 (HR 1.026, 95% CI 0.599–1.757, P = .926) and group 2 (HR 0.807, 95% CI 0.484–1.344, P = .410), respectively. All results above indicated the stability of the survival analysis results.
Table 2.
Multivariate Cox regression for radiotherapy on overall survival and cancer-specific survival.
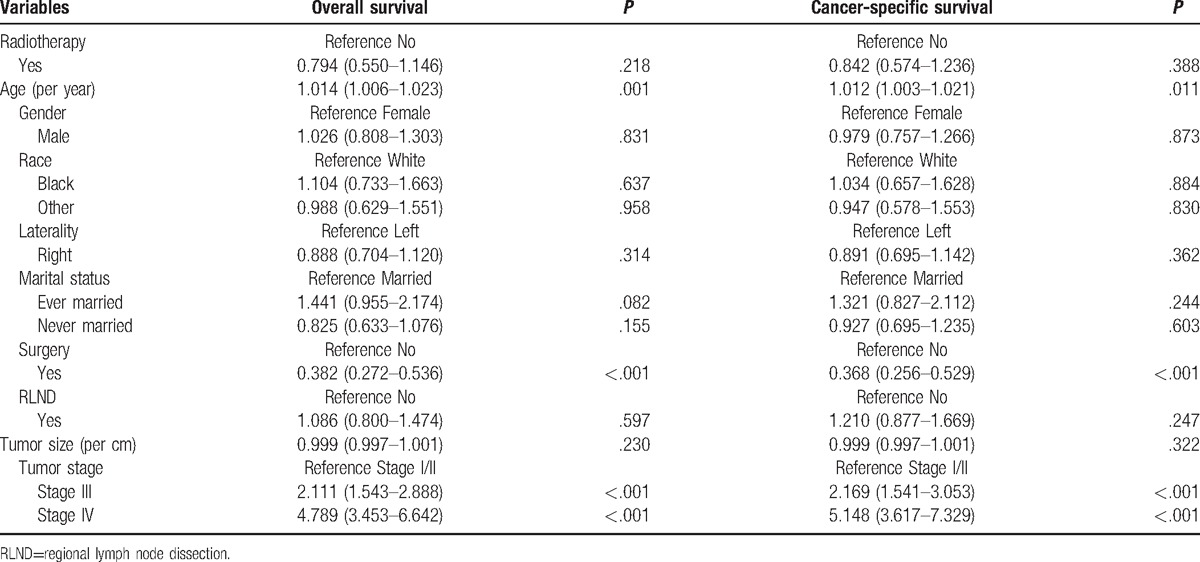
Table 3.
Propensity score analysis for the efficacy of radiotherapy on overall survival and cancer-specific survival.
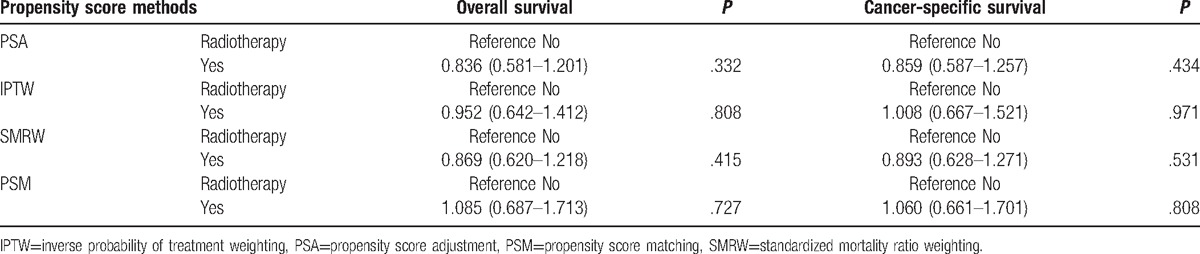
Figure 1.
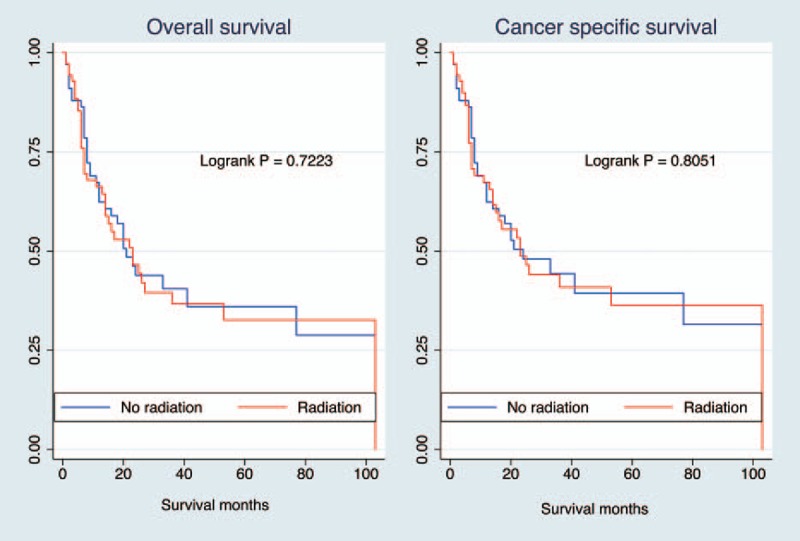
Kaplan–Meier survival curves according to radiotherapy after propensity score matching. Both results showed no significant survival difference in overall survival (log-rank P = .722) or cancer-specific survival (log-rank P = .805) between radiotherapy subgroups.
4. Discussion
In this study, we used a relatively large cohort to demonstrate the efficacy of radiotherapy on ACC. All results consistently showed that no oncologic survival improvement from radiotherapy was observed for ACC patients. The results were stable on the basis of traditional multivariate analysis and propensity score analysis. An incisive criticism of the aforementioned studies is that all previous outcomes were based on a limited sample size that may have had low statistical power to demonstrate significance. We included 530 complete ACC cases with 318 events during follow-up. The outcomes were similar to previous studies.[1,11] Because the ACC was highly malignant, the majority of causes of death were ACC-related causes. Thus, cancer-specific mortality risk was similar to overall mortality.
ACC was deemed to be radiation-resistant, and only a very small portion of patients received radiotherapy, mainly as palliative treatment. It was reported that radiotherapy could reduce the risk of local recurrence but would not change the overall survival.[10,14] The benefit of recurrence control seemed to conflict with null results. These null results may be due to the intrinsically high malignancy of ACC. Mild benefits could not reverse the ultimate end. Moreover, radiotherapy did not reduce the risk of metastasis.[10] In the study by Else et al,[1] it was mitotane, not radiation, that improved recurrence-free survival in the multivariate analysis. The results of this study also indicated the synergism of mitotane combined with radiation.[1] Despite no overall survival improvement, radiotherapy has some potential benefits. However, the side effects of radiation are assignable. Radiation is given to the adrenal bed including multiple organs and tissues. Thus, radiotherapy for ACC could damage adjacent organs and tissues such as the kidney, vascular vessels, spinal cord, diaphragm, and stomach. A precise and modest radiation dose is critical. However, the literature reported that adverse effects of radiotherapy in ACC were mild to moderate.[15] Newly developed radiation techniques such as stereotactic body radiation therapy (SBRT) have been reported to be effective for controlling metastatic lesions.[16] In summary, radiotherapy could not improve overall survival but may ameliorate tumor-related symptoms and achieve local control of ACCs.
Our study has some limitations. This study is based on the SEER database and may have sizeable selection bias. Massive missing data are the flaw for the SEER dataset. Some important roles were not included in the analysis. For instance, the doses and courses were not explicitly recorded. These factors played a key role in terms of the efficacy of the radiotherapy.[15] As the development of radiation technique, different radiation modalities used by different medical teams could lead to variations in outcomes. In addition, the number of patients who received radiotherapy was relatively small (74 patients) and accounted for almost 14% of the total included population. The modality of radiation was not included. It could affect the effect of radiation therapy. The mitotane administration situation was not evaluated, although the contention of efficacy existed.[6,7] The resection margin status was not assessed due to no related records. Resection margin played a critical role in cancer survival. For future clinical studies, multiple center collaborative cooperation is critically urgent.
5. Conclusion
Radiotherapy did not improve overall or cancer-specific survival in ACC patients. Further confirmation is needed from multi-institutional prospective studies in the future.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ACC = adrenocortical carcinoma, ENSAT = European Network for the Study of Adrenal Tumors, Epidemiology, and End Results database, HR = hazard ratio, IPTW = inverse probability treatment weighting, IQR = interquartile range, PSA = propensity score adjustment, PSM = propensity score matching, RLND = regional lymph node dissection, SEER = the Surveillance, Epidemiology, and End Results, SMRW = standardized mortality ratio weighting.
The authors declare that there are no conflicts of interest.
References
- [1].Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2014;99:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Livhits M, Li N, Yeh MW, et al. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery 2014;156:1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stigliano A, Chiodini I, Giordano R, et al. Management of adrenocortical carcinoma: a consensus statement of the Italian Society of Endocrinology (SIE). J Endocrinol Invest 2016;39:103–21. [DOI] [PubMed] [Google Scholar]
- [4].Terzolo M, Baudin AE, Ardito A, et al. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol 2013;169:263–70. [DOI] [PubMed] [Google Scholar]
- [5].Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol 2014;21:3509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 2007;356:2372–80. [DOI] [PubMed] [Google Scholar]
- [7].Postlewait LM, Ethun CG, Tran TB, et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: a 13-institution study by the US Adrenocortical Carcinoma Group. J Am Coll Surg 2016;222:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23Suppl 7:vii:131–8. [DOI] [PubMed] [Google Scholar]
- [9].Hermsen IG, Groenen YE, Dercksen MW, et al. Response to radiation therapy in adrenocortical carcinoma. J Endocrinol Invest 2010;33:712–4. [DOI] [PubMed] [Google Scholar]
- [10].Sabolch A, Feng M, Griffith K, et al. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys 2011;80:1477–84. [DOI] [PubMed] [Google Scholar]
- [11].Habra MA, Ejaz S, Feng L, et al. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sabolch A, Else T, Griffith KA, et al. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys 2015;92:252–9. [DOI] [PubMed] [Google Scholar]
- [13].Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer 2010;46:713–9. [DOI] [PubMed] [Google Scholar]
- [14].Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab 2006;91:4501–4. [DOI] [PubMed] [Google Scholar]
- [15].Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer 2009;115:2816–23. [DOI] [PubMed] [Google Scholar]
- [16].Milgrom SA, Goodman KA. The role of radiation therapy in the management of adrenal carcinoma and adrenal metastases. J Surg Oncol 2012;106:647–50. [DOI] [PubMed] [Google Scholar]


