Abstract
Barcelona clinic liver cancer-stage C (BCLC-C) encompasses a broad spectrum of tumor burdens, liver function statuses, patient prognoses, and treatment strategies. Currently, sorafenib is the only recommended treatment for patients with BCLC-C and outcomes remain suboptimal. The aims of this study were to assess the heterogeneity of BCLC-C hepatocellular carcinoma (HCC) cases, propose a novel subclassification for these cases, and suggest optimal treatment strategies other than sorafenib.
We retrospectively analyzed 196 consecutive BCLC-C HCC patients who were diagnosed and treated between January 2008 and December 2015.
All 196 patients were classified according to the modified Union for International Cancer Control (Stage I, 0.0%; Stage II, 8.2%; Stage III, 64.3%; Stage IVA, 21.9%; and Stage IVB, 5.6%) and American Joint Committee on Cancer TNM staging systems (Stage I, 0.0%; Stage II, 16.3%; Stage IIIA, 27.6%; Stage IIIB, 49.5%; Stage IIIC, 1.5%; Stage IVA, 1.0%; and Stage IVB, 4.1%). First-line treatment modalities included surgical resection (8.7%), transarterial chemoembolization (49.5%), hepatic arterial infusion therapy (5.6%), sorafenib therapy (9.2%), radiotherapy (9.2%), and best supportive care (10.7%). In univariate analysis, Child-Pugh score, tumor size, distant metastasis, multinodular or infiltrative/diffuse type of HCC, main portal vein invasion, hepatic vein invasion, and bile duct invasion were significantly associated with survival (P < .001). Tumor size, distant metastasis, HCC type, and bile duct invasion remained significantly associated with 1-, 3-, and 5-year survival rates in multivariate Cox regression analyses. Using these 4 characteristics, a novel subclassification of BCLC-C was developed and applied to the patient cohort. The subclassification included 5 substages (stages C0–C4), as defined based on the number of characteristics that were present in each HCC case (0–4). The subclassification showed significant associations with survival, with median survival times of 3026 days, 605 days, 224 days, 126 days, and 82 days for patients with Stage C0, C1, C2, C3, and C4 disease, respectively (P < .001). Additionally, diverse survival rates were observed when different treatment modalities were selected for cases within each substage.
The proposed BCLC-C subclassification of HCC patients is effective in providing better prognostic subclassifications and more appropriate treatment strategies.
Keywords: barcelona clinic liver cancer-stage C, hepatocellular carcinoma, survival
1. Introduction
Hepatocellular carcinoma (HCC) is a common cancer worldwide, and is associated with increasing medical expenses and health problems. HCC is also one of the most frequent types of cancer in Korea.[1] Although there are numerous classification systems for HCC, the Barcelona clinic liver cancer (BCLC) staging system has been used most widely because of its simplicity.[2] The BCLC staging system stratifies patients according to clinical condition and also provides treatment strategies.[3] However, it does not provide an accurate reflection of the various factors that are present in the clinical setting. In particular, BCLC-stage C (BCLC-C) HCC encompasses a broad spectrum of tumors (including uninodular, multinodular, or infiltrative HCC; portal vein invasion [PVI]; and other characteristics) and is associated with varying degrees of liver function and overall performance status (PS). Owing to the heterogeneity of the BCLC-C group, it is associated with a wide range of prognoses and survival outcomes.
At the time of HCC diagnosis, portal vein tumor thrombosis (PVTT) is detected in approximately 10.0% to 40.0% of patients,[4,5] and HCC with PVTT is classified as BCLC-C according to BCLC staging system. PVTT is associated with an extremely poor prognosis; the median survival time is 2 to 4 months for patients who have unresectable HCC with PVTT, but 10 to 24 months for patients who have unresectable HCC without PVTT.[4,6] The extremely poor prognosis of patients with PVTT is the result of hematogenous metastasis, portal hypertension, and hepatic failure.[7] PVTT also restricts the treatment strategies that can be employed, with the majority of HCC guidelines considering PVTT as a contraindication for curative treatment modalities (e.g., liver transplantation, surgical resection, and transarterial chemoembolization [TACE]).[2,8] Currently, sorafenib is the only recommended treatment option for BCLC-C HCC patients with or without PVTT in the European Association for the Study of the Liver guidelines.[3] However, the increases in overall survival (OS) rates associated with sorafenib are disappointing, and better treatment options or combination therapies are urgently required.[9]
The BCLC staging system does not provide any subgroup stratification of BCLC-C HCC patients, which poses a challenge to the development of more specific and effective treatment strategies. There have been diverse efforts to improve the management of patients with BCLC-C HCC by providing alternatives to sorafenib (the current standard treatment). However, these potential alternatives require further validation.[10,11] Therefore, the aims of this study were to assess the heterogeneity of BCLC-C HCC patients and propose a novel subclassification and treatment strategy.
2. Materials and methods
2.1. Patient selection
This study was conducted at Chonnam National University Hospital, a 1000-bed medical center in Gwangju (South Korea), and Hwasun Chonnam National University Hospital, a 500-bed medical center in Hwasun (South Korea) that specializes in cancer. Data were collected and retrospectively reviewed from the medical histories of HCC patients who were diagnosed and treated between January 2008 and December 2015. Patients with missing data or unavailable follow-up information were excluded from our prognostic analysis. In total, 196 consecutive BCLC-C HCC patients were selected and analyzed. The following factors were investigated: age, sex, viral markers (hepatitis B and C), presence of liver cirrhosis, Child-Pugh score (CPS), alpha-fetoprotein (AFP) levels, tumor characteristics (number of lesions, diameter of the largest lesion, nodularity, vessel invasion, bile duct invasion [BDI], PVTT, and distant metastasis), first-line treatment modalities, and survival status. Survival was defined as the time interval between the date of HCC diagnosis and the date of death or last follow-up. According to the Korean Association for the Study of the Liver and National Cancer Center guidelines,[12–14] the enrolled patients with BCLC-C HCC were treated with 5 different modalities as first-line therapies for HCC, including surgical resection, TACE, hepatic arterial infusion (HAI) therapy, sorafenib therapy, and radiotherapy (RT). A proportion of BCLC-C HCC patients were treated with combination therapy and a small number of patients received best supportive care only.
2.2. HCC diagnoses and treatment
HCC diagnosis was based on the American Association for the Study of Liver Disease criteria.[2] Diagnosis and treatment were performed according to the Korean Association for the Study of the Liver, National Cancer Center guidelines, and the BCLC staging system.[12–14] Tumor characteristics were assessed by abdominal computed tomography or magnetic resonance imaging, and the PS was rated according to the Eastern Cooperative Oncology Group scale.[15] Images were retrospectively reviewed by an expert senior liver imaging radiologist (S.S.S.).
2.3. Surgical resection
Patients were selected for surgical resection if they had a PS of 0 with a Child-Pugh classification of A or B7, on the basis of their hepatic functional reserves, on the basis of their predicted remnant liver volumes, or according to their tumor stage. A macroscopic PVTT classification has been proposed by the Liver Cancer Study Group of Japan.[16] Vp1 is defined as the presence of a tumor thrombus in the third-order (or higher) branches of the portal vein. Vp2 is defined as the presence of a tumor thrombus in the second-order branches of the portal vein. Vp3 is defined as the presence of a tumor thrombus in the first-order branches of the portal vein. Vp4 is defined as the presence of a tumor thrombus in the main portal vein or a branch of the portal vein contralateral to the primary involved lobe (or both).
2.4. TACE
TACE was performed with a selective injection of a mixture of epirubicin (50 mg) and lipiodol (10 mL), followed by embolization with Gelfoam fragments. TACE was repeated 6 to 8 weeks later unless clear progression or serious adverse events had occurred. Additional TACE procedures were planned “on demand,” according to the results of radiological and serum AFP measurements conducted every 8 to 12 weeks. The European Association for the Study of the Liver criteria, based on bidimensional measurements of a tumor's enhanced viable component, were used to evaluate tumor responses.[17]
2.5. Hepatic arterial infusion therapy
A drug delivery system was positioned and patients received scheduled arterial infusions of chemotherapy via the injection port. Chemotherapy comprised daily administration of cisplatin (7 mg/m2), followed by 5-flurouracil (170 mg/m2) on days 1 to 5. Days 6 and 7 were rest days.[18]
2.6. Sorafenib therapy
The initial dose of sorafenib was determined according to several factors (e.g., PS and residual liver function). Patients with a Child-Pugh classification of A received 400 mg twice daily. A reduction in the dose of sorafenib or a temporary stoppage was permitted depending on the type and severity of any adverse events (i.e., Grade 2 or higher in the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0). Sorafenib therapy was continued unless intolerable toxicity occurred or clinical disease progression was observed. Computed tomography and/or magnetic resonance imaging were used to evaluate tumor responses every 3 months.
2.7. RT
Radiation doses for palliation varied from 8 Gy delivered in 1 fraction for patients with a poor PS or widespread disease to 50 Gy delivered in 20 fractions for patients with isolated metastasis and a good PS.[19] We only included those patients who received RT for reducing PVTT, and excluded patients who received RT for other purposes (e.g., extrahepatic/lymph node metastasis or debulking). Patients who were treated with other first-line treatment modalities (e.g., TACE, HAI therapy, and sorafenib therapy), as well as RT, were also included in this group.
2.8. Best supportive care
The reasons for providing best supportive care were diverse and related to comorbidities (e.g., old age, advanced tumor stage, insufficient residual liver function, and refusal of treatment by the patient or their relatives), meaning it was difficult to implement any therapeutic management approaches.
2.9. Ethical considerations
All participants provided written informed consent. The study protocol was approved by the institutional review board committee (CNUH-2016–157) of Chonnam National University Hospital (Gwangju, South Korea). Research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
2.10. Statistical analyses
Categorical variables were presented as absolute numbers and percentages and compared using Fisher exact tests. Continuous variables were presented as median values and ranges and compared using Mann-Whitney U tests. Cumulative OS was calculated using the Kaplan-Meier method and compared by the log-rank test. Variables with P ≤ .05 in the univariate analysis were entered into the multivariate Cox regression analysis. The null hypotheses of no differences were rejected for P ≤ .05 or, equivalently, if the 95% confidence interval (CI) of the hazard ratio (HR) estimates excluded one. All statistical analyses were conducted using the Statistical Package for the Social Sciences for Windows, software version 20.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Baseline characteristics of the enrolled patients
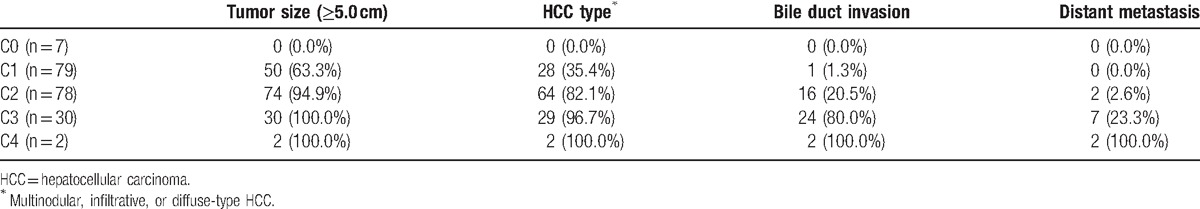
A summary of the baseline characteristics of the enrolled patients is provided in Table 1. The cohort comprised 160 men (81.6%) and 36 women (18.4%). The mean age of all 196 patients was 60.0 (range, 25–97) years. The median follow-up duration was 221 (range, 3–2996) days. One hundred five patients (53.6%) were infected with the hepatitis B virus and 26 patients (13.3%) were infected with the hepatitis C virus. One hundred sixty-four (83.7%) patients had liver cirrhosis at the time of HCC diagnosis. The mean CPS was 5.7 (range, 5–9). One hundred fifty-eight patients (80.6%) had a Child-Pugh classification of A and 38 patients (19.4%) had a Child-Pugh classification of B. The mean tumor size was 9.0 (range, 1.0–20.0) cm. Seventy-five patients (38.3%) had uninodular HCC, 93 patients (47.4%) had multinodular HCC, and 28 patients (14.3%) had infiltrative or diffuse type HCC. One patient with distant metastases (0.5%) did not have PVI. Of the remaining 195 patients, 83 patients (42.3%) had subsegmental PVI, 58 patients (29.6%) had left or right PVI, 15 patients (7.7%) had both (left and right) PVI, and 39 patients (19.9%) had main PVI. Eighty-three patients (42.3%) did not have hepatic vein invasion (HVI). Of the remaining 113 patients, 76 patients (38.8%) had solitary HVI, 27 patients (13.8%) had dual (HVI), and 10 patients (5.1%) had triple HVI. BDI was present in 43 patients (21.9%) and was absent in 153 patients (78.1%; Table 1).
Table 1.
Baseline characteristics of all 196 patients.
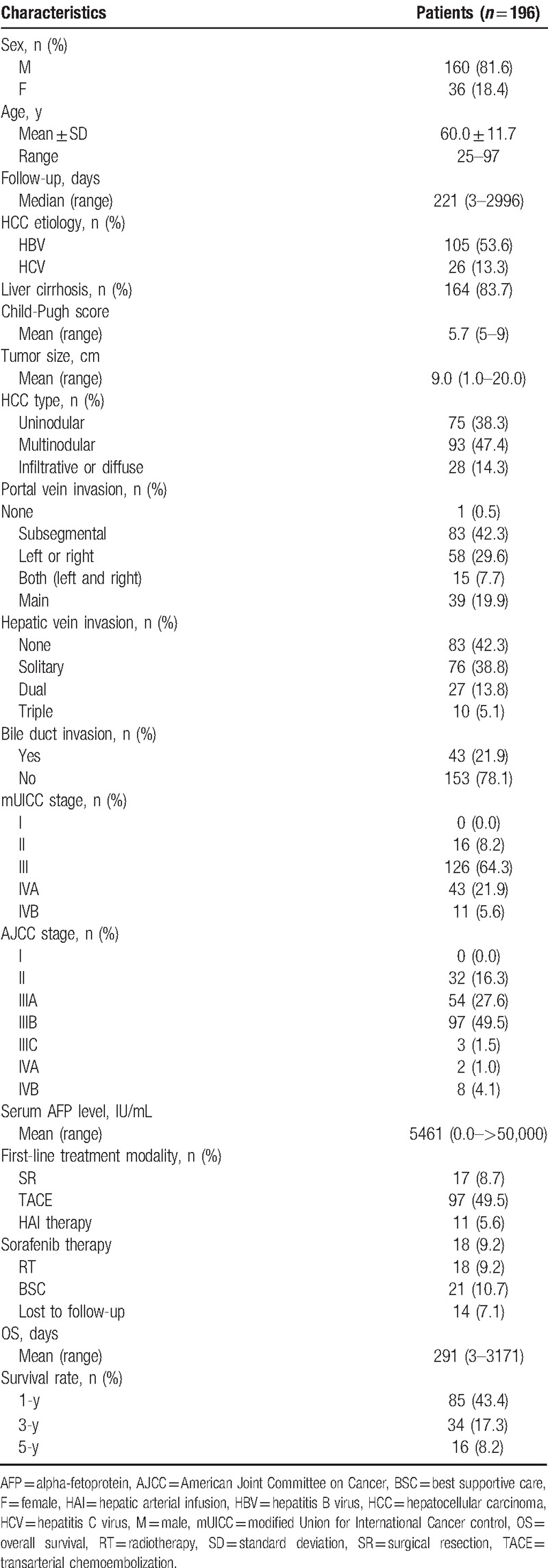
All 196 BCLC-C HCC patients were classified according to the modified Union for International Cancer Control (Stage I, 0.0% [n = 0]; Stage II, 8.2% [n = 16]; Stage III, 64.3% [n = 126]; Stage IVA, 21.9% [n = 43]; and Stage IVB, 5.6% [n = 11]) and American Joint Committee on Cancer TNM staging systems (Stage I, 0.0% [n = 0]; Stage II, 16.3% [n = 32]; Stage IIIA, 27.6% [n = 54]; Stage IIIB, 49.5% [n = 97]; Stage IIIC, 1.5% [n = 3]; Stage IVA, 1.0% [n = 2]; and Stage IVB 4.1% [n = 8]). The mean serum AFP level was 5461 (range, 0.0–>50,000) IU/mL. First-line treatment modalities included surgical resection in 17 patients (8.7%), TACE in 97 patients (49.5%), HAI therapy in 11 patients (5.6%), sorafenib therapy in 18 patients (9.2%), RT in 18 patients (9.2%), and best supportive care in 21 patients (10.7%). Liver transplantation was not performed in BCLC-C HCC patients at our centers, owing to the high cost involved and shortage of donors. The median OS time was 291 (range, 3–3,171) days. The 1-, 3-, and 5-year OS rates were 43.4%, 17.3%, and 8.2%, respectively (Table 1).
3.2. Analysis of survival according to different first-line treatment modalities
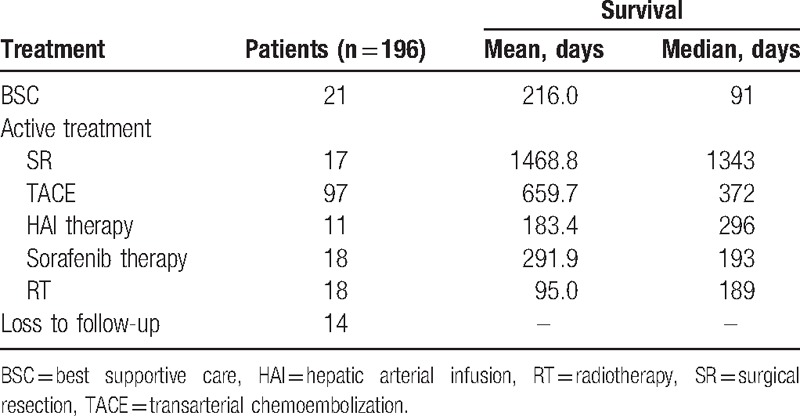
The survival durations of patients with different first-line treatment modalities are displayed in Table 2. The 21 patients who received best supportive care only survived for a median of 91 days. The 17 patients treated with surgical resection, 97 patients treated with TACE, 11 patients treated with HAI therapy, 18 patients treated with sorafenib therapy, and 18 patients treated with RT survived for a median of 1343 days, 372 days, 296 days, 193 days, and 189 days, respectively (P < .001).
Table 2.
Survival durations of patients with best supportive care or active treatment.
3.3. Analysis of prognostic factors for 1-, 3-, and 5-year survival rates
Clinical factors that may be related to survival were analyzed. Univariate analysis of 5-year survival rates among all 196 patients identified a CPS of ≥7 (P < .001), a tumor size of ≥5.0 cm (P < .001), distant metastasis (P < .001), multinodular or infiltrative/diffuse type HCC (P < .001), main PVI (P < .001), HVI (P < .001), and BDI (P < .001) as significant poor prognostic factors for BCLC-C HCC. Four independent prognostic factors associated with 1-, 3-, and 5-year survival rates in the multivariate Cox regression analysis are listed in Table 3. The 5-year survival HRs and 95% CIs for a tumor size of ≥5.0 cm, distant metastasis, multinodular or infiltrative/diffuse type HCC, and BDI were 3.1 (2.0–4.7), 2.4 (1.3–4.4), 2.4 (1.7–3.4), and 2.3 (1.5–3.3), respectively. The associated Kaplan-Meier curves are displayed in Figure 1A–D. As illustrated, the 5-year survival rates of each prognostic factor decreased with statistical significance (P < .001).
Table 3.
Multivariate Cox regression analysis of factors associated with 1-, 3-, and 5-year survival rates.
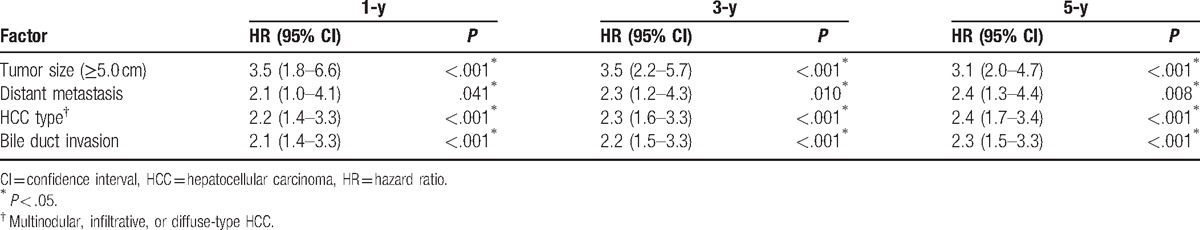
Figure 1.
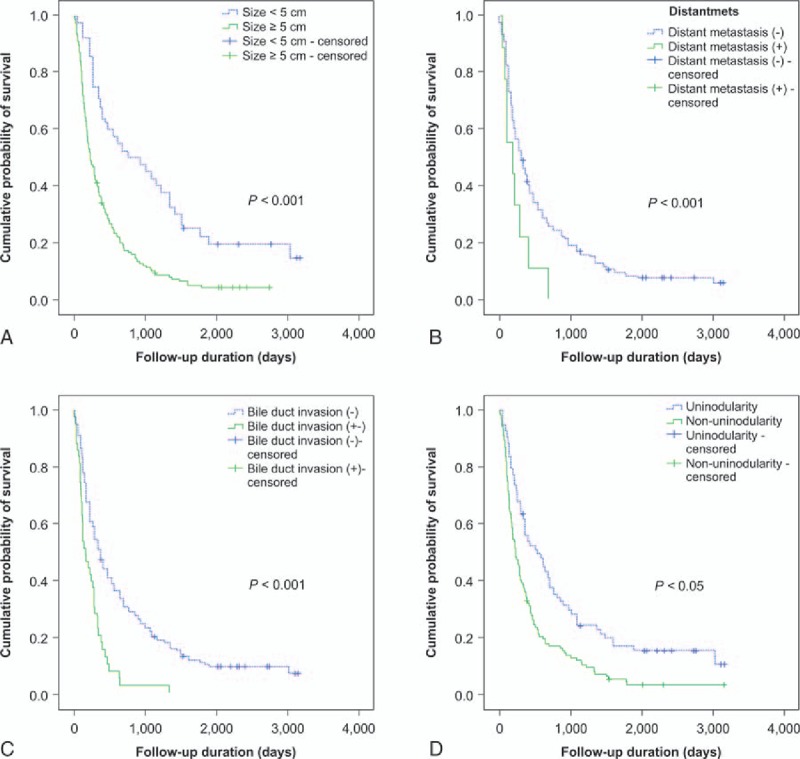
Kaplan-Meier curves of 5-year survival rates after diagnosis stratified according to (A) tumor size (log-rank test; P < .001), (B) distant metastasis (log-rank test; P < .001), (C) bile duct invasion (log-rank test; P < .001), and (D) nodularity (log-rank test; P < .05).
3.4. Subclassification and survival of BCLC-C HCC patients
Based on the results of the multivariate Cox regression analysis, patients were stratified according to a tumor size of ≥5.0 cm, distant metastasis, multinodular or infiltrative/diffuse type HCC, and BDI. Patients were categorized into 5 substages according to a new BCLC-C subclassification system: Stage C0, patients with no prognostic factors; Stage C1, patients with 1 prognostic factor; Stage C2, patients with 2 prognostic factors; Stage C3, patients with 3 prognostic factors; and Stage C4, patients with all 4 prognostic factors. The new BCLC-C subclassification system and survival curves were analyzed. Figure 2A shows the survival curve for the entire patient cohort, whereas Figure 2B shows the survival curve for each subclassification. Analysis of the Kaplan-Meier curves demonstrated that there was a statistically significant difference in survival among the substages (log-rank test; P < .001) (Fig. 2B). Seven patients (3.6%) were classified as Stage C0, 79 patients (40.3%) as Stage C1, 78 patients (39.8%) as Stage C2, 30 patients (15.3%) as Stage C3, and 2 patients (1.0%) as Stage C4, respectively. The median survival times progressively declined in patients with a higher substage (Stage C0, 3026 days; Stage C1, 605 days; Stage C2, 224 days; Stage C3, 126 days; and Stage C4, 82 days) (P < .001; Fig. 3). The 5-year OS rates of each of the substages analyzed by multivariate Cox regression analysis are displayed in Figure 3. Patients with a higher substage had a statistically significant shorter 5-year OS rate (P < .001). Therefore, BCLC-C subclassification correlated with survival.
Figure 2.
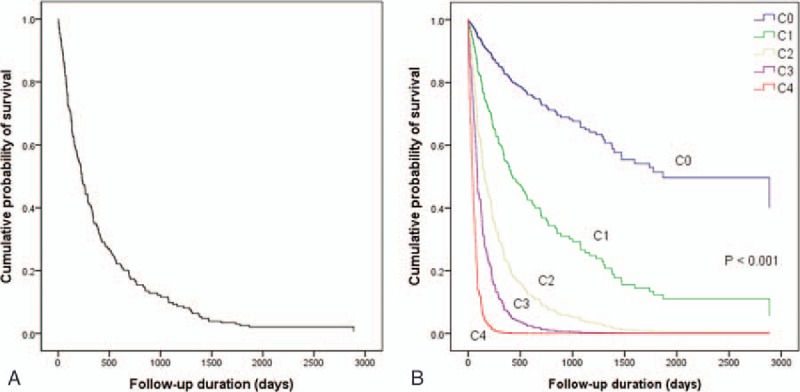
(A) Kaplan-Meier curve of 5-year survival rates of 196 enrolled patients. (B) Kaplan-Meier curve of 5-year survival rates after diagnosis stratified according to the Barcelona Clinic Liver Cancer-Stage C subclassification (Cox regression analysis; P < .001).
Figure 3.
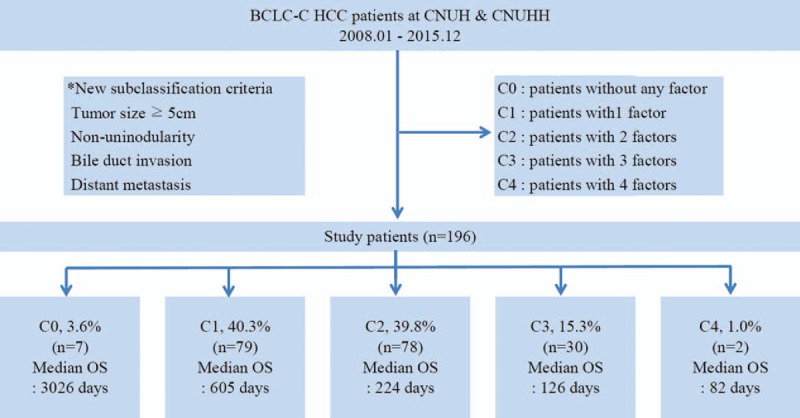
Patient numbers and median overall survival (OS) stratified according to the BCLC-C classification. BCLC-C = Barcelona Clinic Liver Cancer-Stage C, CNUH = Chonnam National University Hospital, CNUHH = Chonnam National University Hospital Hwasun, HCC = hepatocellular carcinoma, OS = overall survival.
Table 4 summarizes the distribution of prognostic factors in each subclass. Tumor size (≥5.0 cm) was the most commonly observed prognostic factor in Stages C1, C2, and C3. HCC type and BDI were the second- and third-most commonly observed factors in Stages C1, C2, and C3.
Table 4.
Occurrence of the prognostic factors in each subclass.
3.5. Treatment modalities according to BCLC-C subclassification
Of the enrolled patients who received active treatment (n = 161), 17 patients (10.6%) underwent surgical resection, 97 patients (60.2%) were treated with TACE, 11 patients (6.8%) were treated with HAI therapy, 18 patients (11.2%) were treated with sorafenib therapy, and 18 patients (11.2%) were treated with RT as first-line therapy (Table 2).
The distribution of treatment approaches is presented in Table 5, as stratified by BCLC-C subclassification. Four Stage C0 patients (57.1%), 9 Stage C1 patients (11.4%), and 4 Stage C2 patients (5.1%) underwent surgical resection. Two Stage C0 patients (28.6%), 54 Stage C1 patients (68.4%), 31 Stage C2 patients (39.7%), and 10 Stage C3 patients (33.3%) were treated with TACE. One Stage C1 patient (0.5%), 10 Stage C2 patients (12.8%), and 1 Stage C3 patient (3.3%) were treated with HAI therapy. Three Stage C1 patients (3.8%), 8 Stage C2 patients (10.3%), 6 Stage C3 patients (20.0%), and 1 Stage C4 patient (50.0%) were treated with sorafenib therapy. Fourteen Stage C2 patients (17.9%) and 3 Stage C3 patients (10.0%) were treated with RT (Table 5).
Table 5.
Comparison of treatment approaches according to the proposed BCLC-C classification.
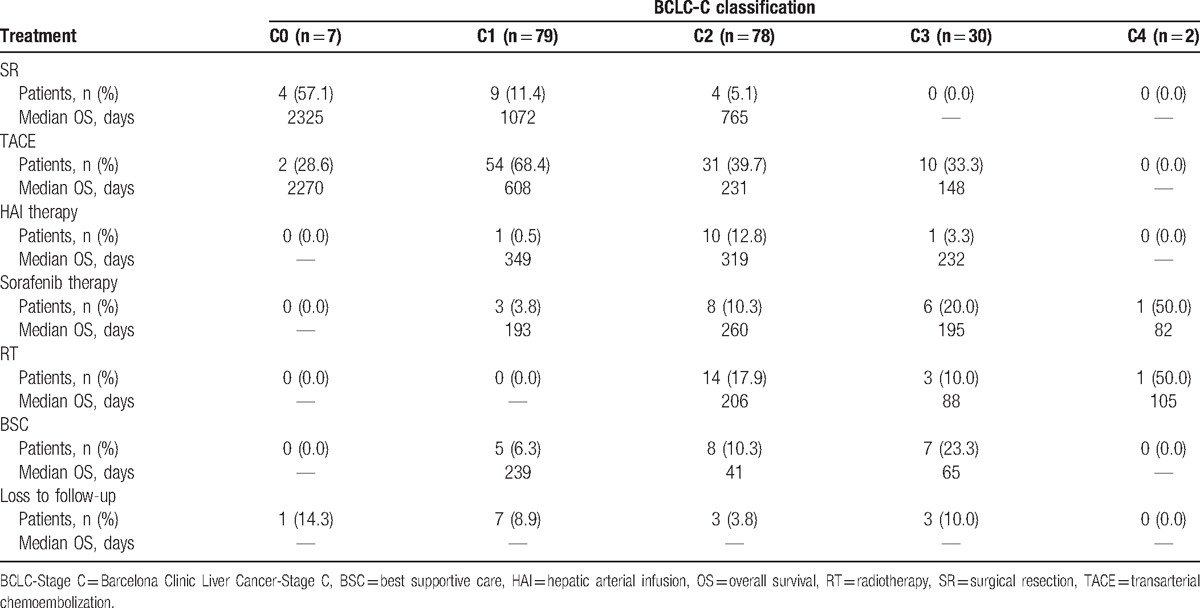
Surgical resection was performed as the first-line treatment in 4 Stage C0 patients (57.1%). TACE was the most commonly employed first-line therapeutic approach, and was performed in 54 Stage C1 patients (68.4%), 31 Stage C2 patients (39.7%), and 10 Stage C3 patients (33.3%), with median OS times of 608 days, 231 days, and 148 days, respectively. Additionally, HAI therapy was performed as the first-line treatment in 10 Stage C2 patients (12.8%) and 1 Stage C3 patient (3.3%), with median OS times of 319 days and 232 days, respectively (Table 5).
4. Discussion
There are many persistent, unmet clinical needs in the approach to managing BCLC-C HCC patients with or without PVTT. [20,21] The need for better treatment strategies has not been satisfied. Recently, there have been numerous attempts to develop alternatives to the current standard treatment (sorafenib) and to develop combined treatment modalities.[10,11] Since the present BCLC-C staging system includes heterogeneous patients with HCC, and treatment is limited to sorafenib only, sub-classification of BCLC-C HCC should be considered an important first step toward developing more specific and effective approaches to the management of these patients. Therefore, we investigated treatment outcomes and prognostic factors for BCLC-C HCC patients and proposed a new sub-classification system for BCLC-C. Our study demonstrates that a new subclassification system for BCLC-C can aid clinicians in further refining the stratification of patients with HCC and improving their treatment. The proposed subclassification system of BCLC-C is not aimed at simply substituting the reference BCLC-C staging system or its treatment algorithm. Instead, it is intended to serve as an additional prognostic tool that complements this classification. In addition, treatments other than the standard therapy that is currently recommended by the BCLC guidelines (sorafenib) were analyzed and their clinical outcomes were compared with each other. Our analysis specifically included surgical resection, TACE, HAI therapy, and RT.
Previous studies[22–25] have reported that tumor size, serum AFP levels, CPS, extrahepatic involvement, multinodular HCC, vascular invasion, and BDI are poor prognostic factors for survival in patients with HCC. In agreement with these studies, CPS, tumor size, distant metastasis, HCC nodularity, PVI, HVI, and BDI were associated with survival rates in the present study. Based on a multivariate analysis, tumor size, distant metastasis, HCC nodularity, and BDI were confirmed as independent prognostic factors for 5-year survival in our study. Although serum AFP levels and CPS exhibited significant associations with survival in our univariate analysis, the associations did not remain statistically significant in our multivariate Cox regression analysis. These findings may be explained by the fact that the majority of patients with BCLC-C HCC had elevated serum AFP levels (>400 IU/mL) and the majority of the enrolled BCLC-C HCC patients had a low CPS. Therefore, we stratified BCLC-C HCC patients into 5 substages, using the 4 prognostic factors that were significantly associated with survival in our multivariate analysis (tumor size ≥5.0 cm, distant metastasis, multinodular or infiltrative/diffuse type HCC, and BDI). Our proposal for a new BCLC-C subclassification system for HCC patients is as follows: Stage C0, patients with no prognostic factors; Stage C1, patients with 1 prognostic factor; Stage C2, patients with 2 prognostic factors; Stage C3, patients with 3 prognostic factors; and Stage C4, patients with all 4 prognostic factors. The median survival times progressively declined in patients with a higher substage (Stage C0, 3026 days; Stage C1, 605 days; Stage C2, 224 days; Stage C3, 126 days; and Stage C4, 82 days; P < .001). These findings suggest that BCLC-C substages correlate with survival. Although the small numbers of patients with Stage C0 (n = 7) and Stage C4 (n = 2) disease is a limiting factor for survival stratification, our proposed subclassification could be validated in larger patient cohorts. Therefore, large prospective studies are warranted.
Sinn et al[26] suggested su-classifying BCLC-C based on the extent of PVI and type of extrahepatic spread, noting that this subclassification may minimize within-stage tumor heterogeneity and help to provide better predictions of survival. Although CPS, tumor size, PVI, distant metastasis, and AFP were significantly associated with survival rates in their study, Sinn et al subclassified BCLC-C based on PVI and distant metastasis, which are tumor-related factors that define BCLC-C. In contrast, our study included a multivariate analysis of all of the factors that were significantly associated with survival in univariate analyses. We then defined our subclassification based on the 4 factors that continued to show significant associations with survival in the multivariate analysis. Sinn et al[26] showed that other treatment modalities may have better outcomes than sorafenib, but their study did not include any comparison of the survival rates within each substage for treatment modalities other than sorafenib. We assessed treatment outcomes, as evaluated according to both substages and treatment modalities, and suggest the application of different treatment methods, as individualized based on tumor status.
Regarding first-line treatment modalities, 17 patients (8.7%) underwent surgical resection, 97 patients (49.5%) were treated with TACE, 11 patients (5.6%) were treated with HAI therapy, 18 patients (9.2%) were treated with sorafenib therapy, and 18 patients (9.2%) were treated with RT. TACE was the most commonly used first-line treatment modality, with a median survival time of 372 days. Surgical resection exhibited the longest median survival time of 1343 days. Liver transplantation was not performed in BCLC-C HCC patients at our centers, owing to the high costs involved and shortage of donors.
Generally, PVTT is regarded as a contraindication for TACE because of the potential for extensive hepatic necrosis in patients whose blood supply is already compromised.[7,27] However, PVTT was not considered an absolute contraindication for TACE or surgical resection at our centers, as suggested by other studies and guidelines.[28,29] Our study suggests that surgical resection or TACE may be appropriate for a proportion of BCLC-C HCC patients, especially Stage C0–1 BCLC-C HCC patients, which is consistent with other reports.[30–32] Recent studies[33,34] have revealed that TACE is effective in prolonging the survival of HCC patients with PVTT in comparison with more conservative treatments. Furthermore, a recent study[35] comparing TACE and sorafenib in patients with BCLC-C HCC reported that the median OS times of patients treated with TACE (9.2 [95% CI: 6.1–12.3] months) were comparable to those of patients treated with sorafenib (7.4 [95% CI: 5.6–9.2] months; P = .38).
In our study, TACE was associated with a longer median OS time than sorafenib in Stage C0 and Stage C1 patients, but a shorter median OS time than sorafenib in Stage C2 patients. Of the 12 patients with a median OS time of <180 days in the Stage C2 group, 4 patients (33.3%) were treated with TACE as first-line therapy because of HCC rupture. HCC rupture is a life-threatening complication, and the higher proportion of HCC rupture patients in the Stage C2 group treated with TACE, as compared with the StageC2 group treated with other modalities, may have contributed to the shorter median OS times of Stage C2 patients treated with TACE as first-line therapy.
Ando et al[36] reported that HAI therapy with cisplatin plus 5-fluorouracil was effective in HCC patients (n = 48) with PVTT(5-year survival rate, 11.0% and median survival time,10.2 months). In our study, HAI therapy was also an effective first-line treatment for HCC patients with PVTT (median OS time, 9.7 months).
In our study, surgical resection was associated with the longest median OS time of 1343 days. However, small sample sizes and the bias that treating physicians have in favor of operating on candidates may represent limiting factors for the confirmation of survival differences among other treatment groups. The majority of HCC patients with Vp4 are considered technically inappropriate for curative resection.[7] Peng et al[37] reported that surgical resection was associated with increased survival durations for resectable HCC patients with PVTT (1-, 3-, and 5-year OS rates: 42.0%, 14.1%, and 11.1%, respectively) compared to those treated with TACE (1-, 3-, and 5-year OS rates: 37.8%, 7.3%, and 0.5%, respectively; P < .001). Some authors[30] have also proposed extending the indications for surgery to include more advanced-stage HCC patients.
A recent study[19] demonstrated that proton-beam RT, 3-dimensional conformal RT, and stereotactic body RT provide increased superselective delivery of radiation doses to the tumor, while minimizing radiation doses to normal tissue. Nakazawa et al[38] reported survival differences between sorafenib and RT in inoperable HCC patients with PVTT (Vp3/Vp4). The median OS times of the sorafenib-treated group and the RT-treated group were initially comparable (4.3 vs.5.9 months; P = .12). However, after matching on propensity scores, the RT-treated group had a higher median OS time than the sorafenib-treated group (n = 28 per group; 10.9 vs.4.8 months; P < .05). In our study, a statistically significant difference in median OS times was not observed between the sorafenib-treated group and the RT-treated group (6.2 vs. 6.4 months; P = .940).
Considering these findings together, a small proportion of patients may benefit from sorafenib treatment. Although sorafenib is currently the only approved treatment for BCLC-C HCC, its therapeutic effect appears marginal. Therefore, different treatment strategies or combination modalities should be considered for improving survival outcomes in these patients. We recommend surgery-based combination therapies for resectable HCC patients with PVTT and TACE-based multimodal therapies for unresectable HCC patients with PVTT. This suggestion may help physicians select optimal treatment strategies for HCC patients with PVTT. In our study, HAI therapy or RT proved to be more effective than conventional sorafenib treatment. However, small sample sizes limit our evaluations of their efficacies, warranting further investigation.
Because our study did not have prospective design, there was no standardized indication for selecting treatment modalities. Instead, the treatment modality was decided based on the patient's tumor state, physical activity, and economic status, as well as the physician's personal preferences. Hence, because of preexisting differences in patient characteristics, selection biases may have affected the survival differences that were observed between treatment modalities in our study. Additionally, patients who have higher substages are more likely to have shorter survival times because of their tumors showing more unfavorable characteristics.
There were some limitations to this study. First, our study had a dual-center retrospective design that is susceptible to potential biases, which may preclude definitive conclusions. Therefore, future prospective multicenter clinical trials are needed to confirm these findings. Second, the lack of patients treated with combination therapies may have affected the results. Lastly, the small sample sizes may have also been a limiting factor.
5. Conclusions
In this study, we proposed a new subclassification of BCLC-C HCC that offers improved prognostic ability and better guidance for treatment selection. Multidisciplinary approaches combining surgical resection, TACE, HAI therapy, and RT in selected patients may provide better clinical outcomes than current first-line sorafenib treatment alone. However, future prospective randomized controlled studies are needed to validate these findings.
Acknowledgments
None.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, AJCC = American Joint Committee on Cancer, BCLC = Barcelona Clinic Liver Cancer, BDI = bile duct invasion, BSC = best supportive care, CI = confidence interval, CPS = Child-Pugh score, F = female, HAI = hepatic arterial infusion, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, HVI = hepatic vein invasion, M = male, mUICC = modified Union for International Cancer control, OS = overall survival, PS = performance status, PVI = portal vein invasion, PVTT = portal vein tumoral thrombus, RT = radiotherapy, SD = standard deviation, SR = surgical resection, TACE = transarterial chemoembolization.
C.H.J. and J.H.Y. contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. [DOI] [PubMed] [Google Scholar]
- [2].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Sem Liver Dis 1999;19:329–38. [DOI] [PubMed] [Google Scholar]
- [4].Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World JGastroenterol 2006;12:7561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62–7. [DOI] [PubMed] [Google Scholar]
- [6].Schoniger-Hekele M, Muller C, Kutilek M, et al. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut 2001;48:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lau WY, Sangro B, Chen PJ, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium–90. Oncology 2013;84:311–8. [DOI] [PubMed] [Google Scholar]
- [8].Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology Int 2010;4:439–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [10].Han K, Kim JH, Ko GY, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol 2016;22:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu SJ, Kim YJ. Effective treatment strategies other than sorafenib for the patients with advanced hepatocellular carcinoma invading portal vein. World J Hepatol 2015;7:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61–74. [DOI] [PubMed] [Google Scholar]
- [13].Practice guidelines for management of hepatocellular carcinoma 2009. [Article in Korean] Korean J Hepatol 2009;15:391–423. [DOI] [PubMed] [Google Scholar]
- [14].Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis 2014;32:764–77. [DOI] [PubMed] [Google Scholar]
- [15].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [16].Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today 2014;44:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ando E, Yamashita F, Tanaka M, et al. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer 1997;79:1890–6. [DOI] [PubMed] [Google Scholar]
- [19].Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer 2006;106:1653–63. [DOI] [PubMed] [Google Scholar]
- [20].Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 2012;48:1452–65. [DOI] [PubMed] [Google Scholar]
- [21].Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009;29:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang YH, Chen CH, Chang TT, et al. Evaluation of predictive value of CLIP, Okuda, TNM and JIS staging systems for hepatocellular carcinoma patients undergoing surgery. J Gastroentero Hepatol 2005;20:765–71. [DOI] [PubMed] [Google Scholar]
- [24].Navadgi S, Chang CC, Bartlett A, et al. Systematic review and meta-analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB 2016;18:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760–9. [DOI] [PubMed] [Google Scholar]
- [26].Sinn DH, Cho JY, Gwak GY, et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One 2015;10:e0124434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v59–64. [DOI] [PubMed] [Google Scholar]
- [28].Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146: 1691-1700:e1693. [DOI] [PubMed] [Google Scholar]
- [29].Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg 2001;233:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929–37. [DOI] [PubMed] [Google Scholar]
- [31].Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82–8. [DOI] [PubMed] [Google Scholar]
- [32].Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413–20. [DOI] [PubMed] [Google Scholar]
- [34].Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258:627–34. [DOI] [PubMed] [Google Scholar]
- [35].Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012;263:590–9. [DOI] [PubMed] [Google Scholar]
- [36].Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95:588–95. [DOI] [PubMed] [Google Scholar]
- [37].Peng ZW, Guo RP, Zhang YJ, et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2012;118:4725–36. [DOI] [PubMed] [Google Scholar]
- [38].Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]


