Supplemental Digital Content is available in the text
Keywords: coronary artery disease, glycosylated hemoglobin, mortality, myocardial infarction, prediabetes
Abstract
A number of studies assessed the prognostic value of HbA1c level in nondiabetic patients with coronary artery disease (CAD). The purpose of this meta-analysis was to assess the association between the HbA1c level and clinical outcomes.
We searched PubMed, EMBASE, MEDLINE, and the Cochrane Library from their inception to 10 April 2016. Studies evaluated the outcomes according to HbA1c levels in CAD patients without diabetes mellitus were eligible.
Twenty studies involving 22,428 patients were included. In nondiabetic patients with CAD, a high HbA1c level was associated with a higher rate of long-term death (odds ratio 1.76, 95% confidence interval 1.44–2.16, P < .001), and myocardial infarction (MI, odds ratio 1.69, 95% confidence interval 1.07–2.67, P = .026), but not a higher rate of early deaths (odds ratio 1.08, 95% confidence interval 0.92–1.27, P = .359). These findings for death remained the same after sensitivity analyses and the trim and fill method, but the risk difference for MI became nonsignificant after adjustment for potential publication bias.
Elevated HbA1c level increased the risks of long-term mortality and MI, but not the risk for early deaths in nondiabetic patients with CAD. High-quality large-scale studies with less bias are needed to confirm these findings.
1. Introduction
Acute glycemic disorder, indicated by a high plasma glucose level, is a powerful predictor of prognosis in patients with acute myocardial infarction (AMI) but without diabetes mellitus (DM).[1,2] Increased glucose level may suggest previously undiagnosed DM[3]; however, the glycemic test is rarely used to diagnose diabetes in patients encountering AMI because the glucose level could increase at the acute phase of cardiac events in all patients.[4,5] Glycated hemoglobin (HbA1c), reflecting the average blood glucose level of the past 2 to 3 months, is a well-known biomarker of long-term glycometabolic control[6] and is also recommended in the diagnosis of DM since 2010.[7] Previous studies demonstrated that the HbA1c level was significantly associated with all-cause mortality in nondiabetic patients with coronary artery disease (CAD).[8–13] Subgroup analysis of a meta-analysis also got similar conclusion.[14] However, a body of recent studies provided inconsistent findings.[15–28] Therefore, we performed a meta-analysis to determine the predictive effect of HbA1c level on clinical outcomes in nondiabetic patients with CAD.
2. Methods
The present study was conducted according to MOOSE (Meta-analysis Of Observational Studies in Epidemiology) recommendations,[29] following a registered protocol on the PROSPERO database (CRD42016037303).
2.1. Data sources and search strategy
We comprehensively searched PubMed, EMBASE, MEDLINE, and the Cochrane Library from database inception to 10 April 2016 without restrictions of language and publication status. The following search terms were used: (“coronary artery disease” or “coronary heart disease” or “myocardial infarction” or “acute coronary syndrome” or “percutaneous coronary intervention”) and (“glycated hemoglobin” or “hemoglobin A1c” or “HbA1c”). We also manually reviewed references of the identified articles and relevant reviews.
2.2. Study selection
Two reviewers (GJ and YZ) independently assessed available studies. Any discrepancies were solved by discussion with a third author (BX). Studies with any study design were eligible if they compared the outcomes between high and low HbA1c levels in CAD patients without a history of DM. A threshold of 6.5% was preferred as the HbA1c cutoff because it is recommended as the diagnostic value for diabetes in recent guideline.[7] Also, all studies had to be followed up for at least 12 months and reported the outcomes for mortality or MI. We excluded abstracts and unpublished studies.
2.3. Data extraction
The primary endpoint was long-term mortality, and the second outcomes were early deaths and MI. Two reviewers (GJ and YZ) independently extracted studies characteristics and clinical and demographic information of enrolled patients in each study, and a third one (BX) verified. We extracted 30-day mortality as early deaths; for studies not reporting 30-day mortality, we used in-hospital mortality instead. We contacted the authors for any missing or unclear data.
2.4. Quality assessment
We assessed the quality of included studies using the Newcastle–Ottawa scale criteria, which includes 9 terms in 3 domains (selection, comparability, and outcome).[29] Studies with 8 or more terms were deemed to be of low risk of bias. Two reviewers (GJ and YZ) independently evaluated the quality, and a third one (BX) solved discrepancies.
2.5. Statistical analysis
Stata 12.0 was employed to analyze the pooled effects with odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity was assessed using the χ2-base Q test the I2 test. A P value less than .10 or an I2 more than 50% suggests significant heterogeneity.[30] We used the fixed effect model (Mantel–Haenszel method) for pooled analysis preferentially[31]; if high heterogeneity was indentified, we used the random effect model (DerSimonian and Larid method) instead.[32] Given considerable heterogeneity, we also performed sensitivity analyses by excluding 1 study at 1 time to evaluate the contribution of including studies for heterogeneity. Publication bias was estimated using Egger's test and funnel plot with the trim and fill method,[33,34] which was also utilized to adjust for publication bias from potential unpublished studies. For primary outcome, we also did subgroup analyses according to clinical status of enrolling patient, timing of HbA1c measurement, performing regions, the HbA1c cutoffs and percent of patients with newly diagnosed diabetes and undergoing percutaneous coronary intervention. Statistical significance was considered when a 2-tailed P value less than .05 was observed.
3. Results
Twenty studies comprising 22,428 nondiabetic patients with CAD from were included (Fig. 1).[8–13,15–28] All studies were published in English, except one in Spanish.[28] The mean age of enrolled patients across studies ranged from 51 to 70 years, and 71% of participants were men. All studies were of either retrospective or prospective cohort design, except 1 study, which was a prespecified substudy of a randomized controlled trial.[12] Sixteen studies were conducted in patients with MI and 15 studies used the HbA1c level at admission to categorize the high and low HbA1c group in nondiabetic patients. The HbA1c cutoffs ranged from 5.1% to 6.0%. Patients with newly diagnosed DM were excluded in 14 studies. Detailed characteristics of eligible studies and nondiabetic patients with CAD are presented in Table 1 of Supplement 1. Quality assessment of eligible studies according to the Newcastle–Ottawa scale criteria is available in Supplement 2 (Table 1). Fourteen studies were considered to have low risk of bias.
Figure 1.
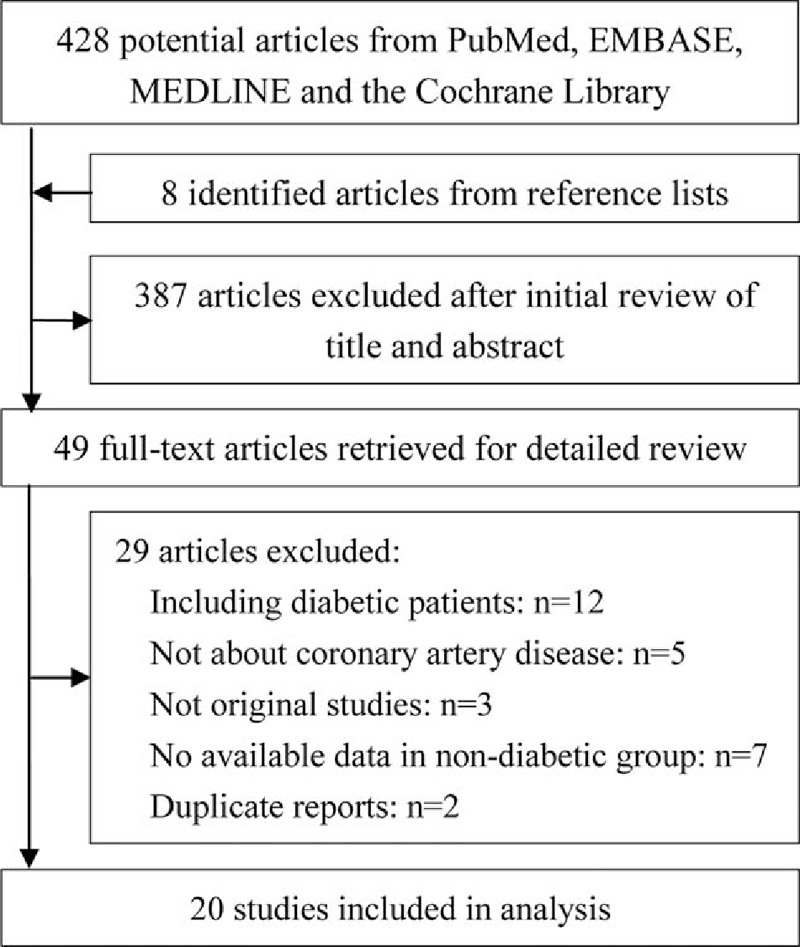
Flow diagram of the meta-analysis.
Table 1.
Subgroup analyses of long-term mortality using a random effect model.
3.1. Long-term mortality
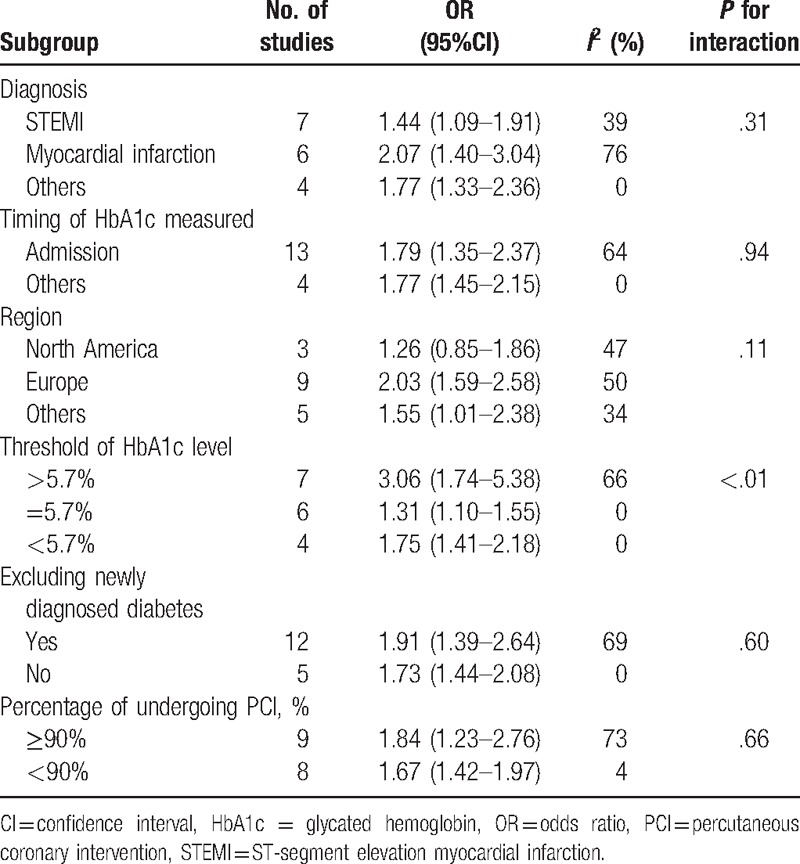
A total of 14 studies provided long-term mortality data with a median follow-up of 2.5 years (range 1–7 years).[8–13,15,18,19,21–28] Three studies did not report mortality data based on HbA1c levels in the articles; but we obtained data of 2 studies from a prior meta-analysis,[9,11] and data of the other study by contacting the primary authors.[23] In total, 18,041 participants were included for the pooled analysis, which showed a significantly increased risk of long-term mortality in patients with a high HbA1c level than those with a low HbA1c level (OR 1.76, 95%CI 1.44–2.16, P < .001, Fig. 2). There were significant heterogeneity among these studies (I2 = 56.6%, P = .002). Subgroup analyses showed that these findings were independent from the clinical status of enrolling patients, the timing of HbA1c measurement, the HbA1c cutoffs, and the percent of patients having percutaneous coronary intervention. Also, whether newly diagnosed diabetes was included or not in the study did not affect the results (Table 1). However, it should be noted that the increased ratio was more remarkable when the HbA1c cutoff was higher (P for interaction < .01).
Figure 2.
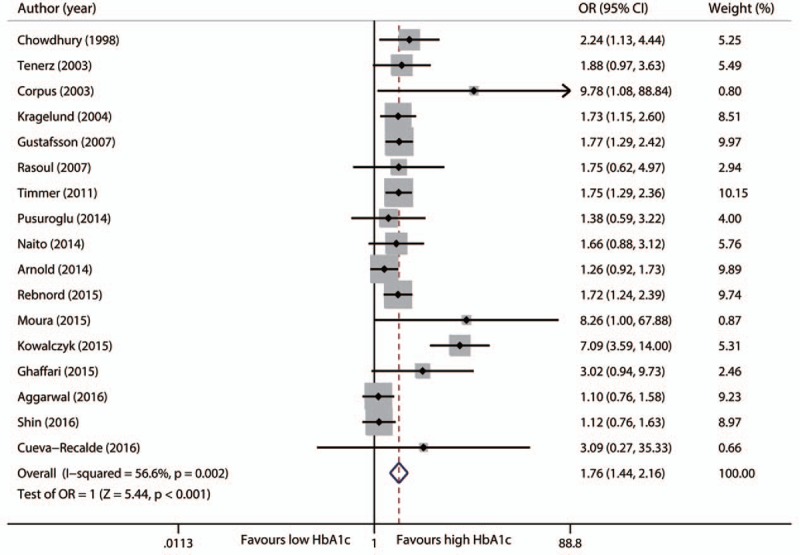
Forest plot for long-term mortality.
Sensitivity analyses showed no alteration of the main outcome after elimination of each study. Specially, study heterogeneity was much less when the study by Kowalczyk was omitted (I2 = 17.7%, P = .251), and the pooled estimate of OR was 1.56 (95%CI 1.36–1.80, P < .001).[24]
Egger's test was statistical significant (P = .037) and visual inspection of the funnel plot seemed to be asymmetric. The trim and fill method suggested there might be 6 unpublished studies (Fig. 1 of Supplement 2). Using the trim and fill method, our finding of long-term mortality remained significant after adjustment for 6 unpublished studies (OR 1.51, 95%CI 1.20–1.91, P = .001).
Four studies also provided data adjusted for other confounding factors.[10,24,26,27] Pool analysis of these 4 study confirmed the conclusion that the elevated HbA1c level was associated with higher long-term mortality (OR 2.46, 95%CI 2.19–2.73, P < .001). A significant heterogeneity was noted (I2 = 98.2%, P < .001).
3.2. Early deaths
Nine studies with 13609 patients contributed to the analysis of early death.[13,15–18,20,25–27] Meta-analysis did not find significant difference in rate of early deaths between patients with high HbA1c levels and those with low HbA1c levels (OR 1.08, 95%CI 0.92–1.27, P = .359), with no evidence of heterogeneity across the studies (I2 = 0%, P = .494, Fig. 3). We found no change of pooled estimate effect and heterogeneity after sensitivity analyses. Egger's test revealed no statistical significant (P = .169); however, the funnel plot seemed to be asymmetric, indicating potential publication bias. We found that there might be 2 unpublished studies with the trim and fill approach (Fig. 2 of Supplement 2). The difference still remained nonsignificant after adding these 2 potential unpublished studies (OR 1.06, 95CI% 0.86–1.32, P = .567).
Figure 3.
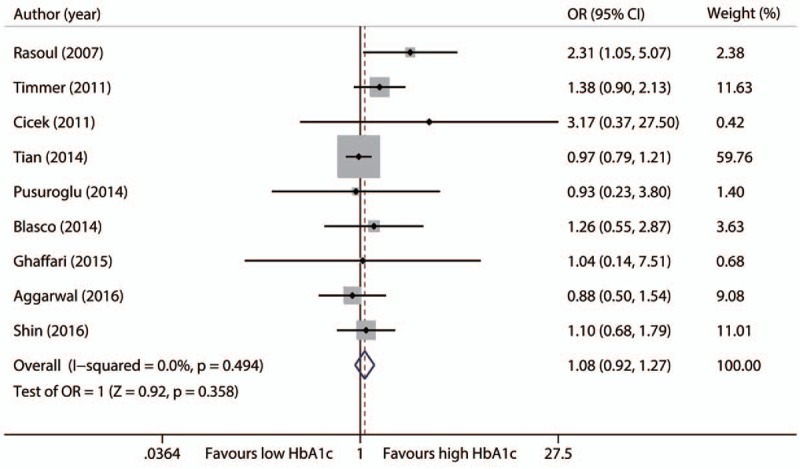
Forest plot for early deaths.
3.3. Myocardial infarction
Five studies with 3664 nondiabetic patients reported data on MI.[18,19,23,27,28] High HbA1c levels were associated with a significantly higher risk of MI (OR 1.69, 95%CI 1.07–2.67, P = .026). No significant heterogeneity was found (I2 = 0%, P = .407, Fig. 4). The association disappeared after adjustment for potential publication bias by the trim and fill method (OR 1.57, 95%CI 0.99–2.48, P = .053) (Fig. 3 of Supplement 2). This risk difference also became nonsignificant after either the study of Pusuroglu and colleagues (OR 1.51, 95%CI 0.88–2.57, P = .133) or the study of Moura and colleagues (OR 1.52, 95%CI 0.92–2.53, P = .104) was excluded.[18,23]
Figure 4.
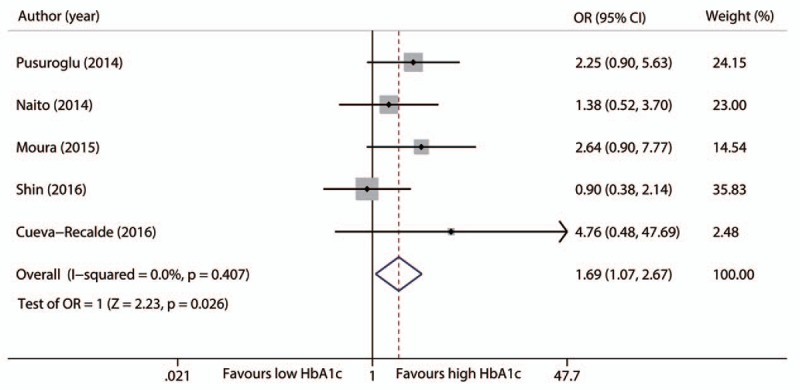
Forest plot for reinfarction during follow-up.
4. Discussion
This meta-analysis showed that the elevated HbA1c level was associated with long-term mortality, but not with early deaths in nondiabetic patients with CAD. These findings were further confirmed by sensitivity analyses and the trim and fill method. Moreover, patients with a high HbA1c level had a higher incidence of MI.
Our results were similar to a previous meta-analysis by Liu,[14] which also showed an increased mortality in nondiabetic patients with elevated HbA1c levels. However, this study differed in the following aspects. First, Liu included 7 studies comprising 5944 nondiabetic patients for the subgroup analysis of mortality,[14] while we added recent 14 studies including more than 20 thousands subjects. Second, we provided short- and long-term mortality data in nondiabetic population and also provided the data of MI by pooled analysis from 3664 nondiabetic patients. Third, the cut-off HbA1c levels to distinguish the high and low HbA1c levels included 7% and 7.5% in previous meta-analysis, suggesting that there were also diabetic individuals included in the subgroup analysis of nondiabetic population.
The association of increased blood glucose with short-term mortality has been well established.[1,2] The high blood glucose level is often observed in CAD patients with an acute event owing to stress response.[35] It is reported that 25% of AMI patients had newly diagnosed DM.[3] AMI patients with newly diagnosed DM had a nearly 2-fold risk of long-term mortality compared with nondiabetic ones.[26] However, using the glucose test, we may fail to identify the undiagnosed DM due to high prevalence of stress hyperglycemia in this population. HbA1c reflects long-term glycometabolic control,[6] and its level as higher than 6.5% is now considered as an alternative category of DM.[7] Our data provided the relationship between the HbA1c level and clinical outcomes, indicating that HbA1c might have a better prognostic value in nondabetic patients.
HbA1c had no predictive value for short-term outcomes based on our results. One possible explanation is that high HbA1c levels result from long-term insulin resistance, leading to dyslipidemia, hypercoagulability inflammation, and subsequent cardiovascular events.[36,37] Besides, nondiabetic individuals with high HbA1c levels have an increased risk of developing DM, which may need a long-term follow-up. CAD patients with DM, even the ones with newly diagnosed DM, have excess risk for developing adverse outcomes.[26] However, in a short-term follow-up, the ability to detect the difference in deaths may be limited by small numbers of incident DM. Moura et al[23] reported a high incidence of newly diagnosed DM in patients with high HbA1c levels, but found no association between newly diagnosed DM and outcomes. However, these results might be hampered by the small sample size. More studies are needed to evaluate the association of newly diagnosed DM with long-term mortality in larger sample size and longer follow-up.
According to a recent guideline for DM diagnosis, HbA1c from 5.7% to 6.5% is considered as prediabetes.[7] In the present meta-analysis, the threshold of HbA1c was 5.7% in 8 studies, and subgroup analysis by HbA1c levels showed that prediabetes was significantly associated with long-term mortality (OR 1.31, 95%CI 1.10–1.55). The prevalence of prediabetes and CAD are 15.5% and 1.76% in China respectively,[38,39] suggesting that there are approximately 4 million nondiabetic patients with CAD in China and will be much more across the world. More interventions, such as lifestyle change or pharmacological therapy, need to be assessed in this population to decrease the risk of adverse outcomes. Lifestyle intervention or metformin may reduce the incidence of DM in nondiabetic individuals[40] and may be beneficial for improving clinical outcomes in theory. Results from large-scale studies with high quality are critically needed to evaluate the effect of therapeutic options in nondiabetic patients with CAD.
There are several limitations presented in this meta-analysis. First, the pooled data were derived from observational studies and baseline characteristics of included patients differed in some confounders. We conducted the meta-analysis of adjusted mortality from 4 studies and got consistent result. Besides, sensitivity analysis and trim and fill method did not alter the results of our primary outcome, lessening the adverse effect from this limitation. However, for the secondary outcomes, no relevant data of adjusted results were obtained. Second, our results of long-term mortality had moderate heterogeneity. We found no significant heterogeneity after exclusion of Kowalczyk's study because Kowalczyk only enrolled patients with impaired glucose tolerance in the nondiabetic group.[24] Diverse regions of the patients in these studies may also explain part of the moderate heterogeneity according to our results of sub-group analyses. Third, there was significant publication bias for long-term mortality given the results of Egger's test and the funnel plot. For early deaths and MI, statistical analyses reached no significance, but potential publication bias still existed based on visual inspection of the funnel plot. We improved the credibility of results in long-term mortality and early deaths using sensitivity analysis and the trim and fill method. The result of MI, however, was still less conclusive. Finally, in-hospital and follow-up managements, such as intervention procedures and medications, may influence the clinical outcomes. We conducted subgroup analysis according to the percentage of percutaneous coronary intervention and found no alternation of the primary endpoint. Besides, we speculate that incident of DM may be partially contribute to the higher long-term mortality in nondiabetic patients with CAD. However, scant comparative data of medication and newly diagnosed diabetes are available for further analysis.
5. Conclusions
In conclusion, we found that the elevated HbA1c level is associated with long-term mortality and MI, but not with early deaths in nondiabetic patients with CAD. These findings may be partially attributed to newly diagnosed DM during follow-up. Future studies are required to confirm this assumption and identify whether lifestyle and pharmacological intervention can improve the long-term outcomes in this population.
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Andrei C. Sposito for providing their unpublished data included in this meta-analysis.
Footnotes
Abbreviations: AMI = acute myocardial infarction, CAD = coronary artery disease, CI = confidence interval, DM = diabetes mellitus, HbA1c = glycated hemoglobin, OR = odds ratio.
JG and YZ contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773–8. [DOI] [PubMed] [Google Scholar]
- [2].Zhao CJ, Hao ZX, Liu R, et al. Admission glucose and risk of early death in non-diabetic patients with ST-segment elevation myocardial infarction: a meta-analysis. Med Sci Monit 2015;21:1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 2002;359:2140–4. [DOI] [PubMed] [Google Scholar]
- [4].Iwakura K. Stress hyperglycemia and microvascular obstruction after acute myocardial infarction. J Cardiol 2015;65:270–1. [DOI] [PubMed] [Google Scholar]
- [5].Knudsen EC, Seljeflot I, Abdelnoor M, et al. Abnormal glucose regulation in patients with acute ST-elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol 2009;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007;50:2239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33suppl 1:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chowdhury TA, Lasker SS. Elevated glycated haemoglobin in non-diabetic patients is associated with an increased mortality in myocardial infarction. Postgrad Med J 1998;74:480–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tenerz A, Nilsson G, Forberg R, et al. Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med 2003;254:494–503. [DOI] [PubMed] [Google Scholar]
- [10].Corpus RA, O’Neill WW, Dixon SR, et al. Relation of hemoglobin A1c to rate of major adverse cardiac events in nondiabetic patients undergoing percutaneous coronary revascularization. Am J Cardiol 2003;92:1282–6. [DOI] [PubMed] [Google Scholar]
- [11].Kragelund C, Snorgaard O, Kober L, et al. Hyperinsulinaemia is associated with increased long-term mortality following acute myocardial infarction in non-diabetic patients. Eur Heart J 2004;25:1891–7. [DOI] [PubMed] [Google Scholar]
- [12].Gustafsson I, Kistorp CN, James MK, et al. Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J 2007;154:470–6. [DOI] [PubMed] [Google Scholar]
- [13].Rasoul S, Ottervanger JP, Bilo HJ, et al. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med 2007;65:95–100. [PubMed] [Google Scholar]
- [14].Liu Y, Yang YM, Zhu J, et al. Prognostic significance of hemoglobin A1c level in patients hospitalized with coronary artery disease. A systematic review and meta-analysis. Cardiovasc Diabetol 2011;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Timmer JR, Hoekstra M, Nijsten MW, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation 2011;124:704–11. [DOI] [PubMed] [Google Scholar]
- [16].Cicek G, Uyarel H, Ergelen M, et al. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:131–7. [DOI] [PubMed] [Google Scholar]
- [17].Tian L, Zhu J, Liu L, et al. Prediabetes and short-term outcomes in nondiabetic patients after acute ST-elevation myocardial infarction. Cardiology 2014;127:55–61. [DOI] [PubMed] [Google Scholar]
- [18].Pusuroglu H, Akgul O, Cakmak HA, et al. Long-term prognostic value of admission haemoglobin A1c (HbA1c) levels in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Postepy Kardiol Interwencyjnej 2014;10:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Naito R, Miyauchi K, Ogita M, et al. Impact of admission glycemia and glycosylated hemoglobin A1c on long-term clinical outcomes of non-diabetic patients with acute coronary syndrome. J Cardiol 2014;63:106–11. [DOI] [PubMed] [Google Scholar]
- [20].Blasco ML, Sanjuan R, Palacios L, et al. Prognostic value of admission glycated haemoglobin in unknown diabetic patients with acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2014;3:347–53. [DOI] [PubMed] [Google Scholar]
- [21].Arnold SV, Lipska KJ, Li Y, et al. Prevalence of glucose abnormalities among patients presenting with an acute myocardial infarction. Am Heart J 2014;168: 466–70 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rebnord EW, Pedersen ER, Strand E, et al. Glycated hemoglobin and long-term prognosis in patients with suspected stable angina pectoris without diabetes mellitus: a prospective cohort study. Atherosclerosis 2015;240:115–20. [DOI] [PubMed] [Google Scholar]
- [23].Moura FA, Figueiredo VN, Teles BS, et al. Glycosylated hemoglobin is associated with decreased endothelial function, high inflammatory response, and adverse clinical outcome in non-diabetic STEMI patients. Atherosclerosis 2015;243:124–30. [DOI] [PubMed] [Google Scholar]
- [24].Kowalczyk J, Mazurek M, Zielinska T, et al. Prognostic significance of HbA1c in patients with AMI treated invasively and newly detected glucose abnormalities. Eur J Prev Cardiol 2015;22:798–806. [DOI] [PubMed] [Google Scholar]
- [25].Ghaffari S, Niafar F, Separham A, et al. Association between HbA1c levels with severity of coronary artery disease and short-term outcomes of acute ST-elevation myocardial infarction in nondiabetic patients. Ther Adv Cardiovasc Dis 2015;9:305–13. [DOI] [PubMed] [Google Scholar]
- [26].Aggarwal B, Shah GK, Randhawa M, et al. Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2016;117:749–53. [DOI] [PubMed] [Google Scholar]
- [27].Shin D, Ahn J, Cha KS, et al. Impact of initial glycosylated hemoglobin level on cardiovascular outcomes in prediabetic patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2016;27:40–6. [DOI] [PubMed] [Google Scholar]
- [28].Cueva-Recalde JF, Ruiz-Arroyo JR, Roncales Garcia-Blanco F. Prediabetes and coronary artery disease: outcome after revascularization procedures. Endocrinol Nutr 2016;63:106–12. [DOI] [PubMed] [Google Scholar]
- [29].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [30].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [31].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [32].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [33].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [35].Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–7. [DOI] [PubMed] [Google Scholar]
- [36].Bansilal S, Farkouh ME, Fuster V. Role of insulin resistance and hyperglycemia in the development of atherosclerosis. Am J Cardiol 2007;99:6B–14B. [DOI] [PubMed] [Google Scholar]
- [37].Geng J, Lu W, Hu T, et al. Subclinical hyperthyroidism increases risk of coronary heart disease events in type 2 diabetes mellitus. Endocrine 2015;49:557–9. [DOI] [PubMed] [Google Scholar]
- [38].Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [39].Geng J, Ye X, Liu C, et al. Outcomes of off- and on-hours admission in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: a retrospective observational cohort study. Medicine (Baltimore) 2016;95:e4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


