Abstract
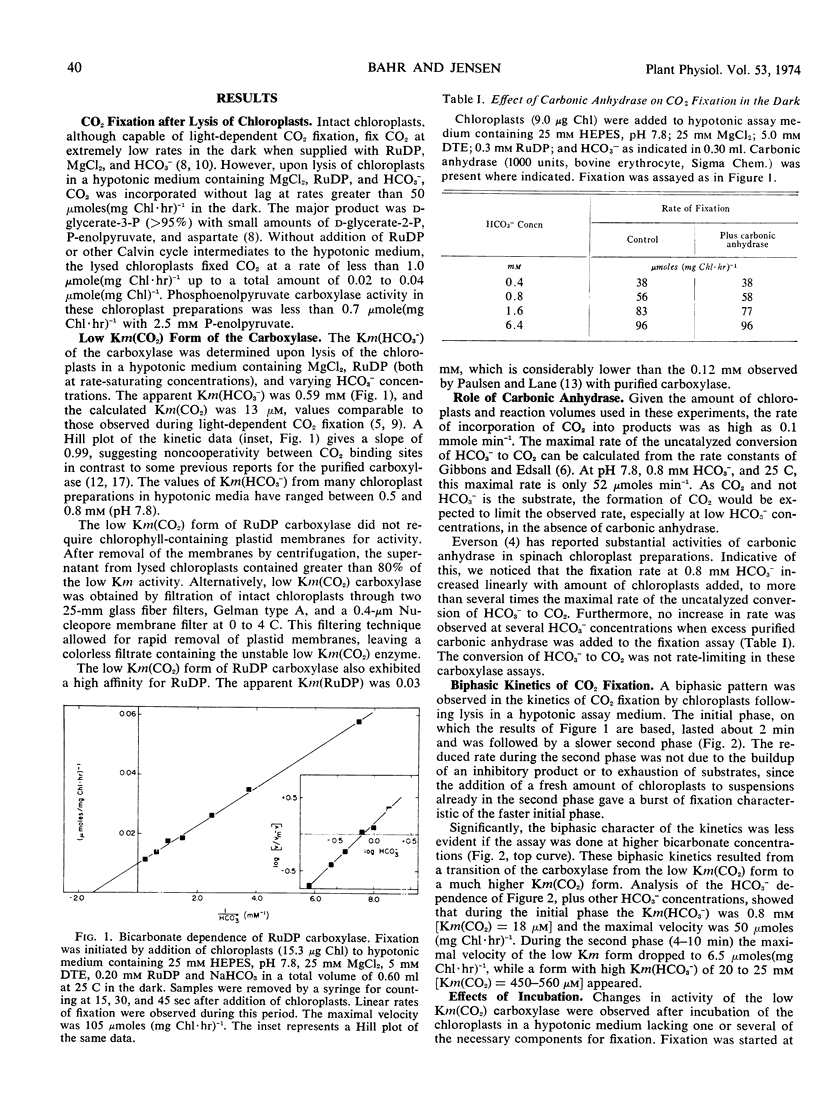
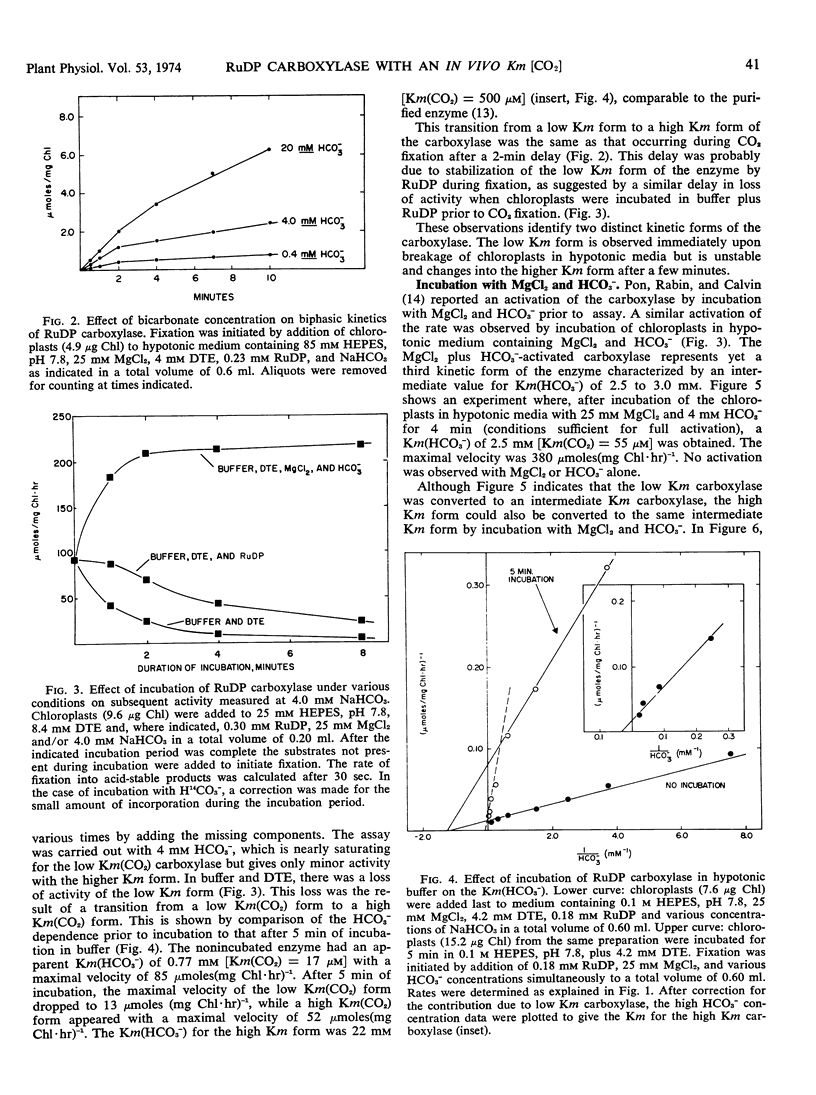
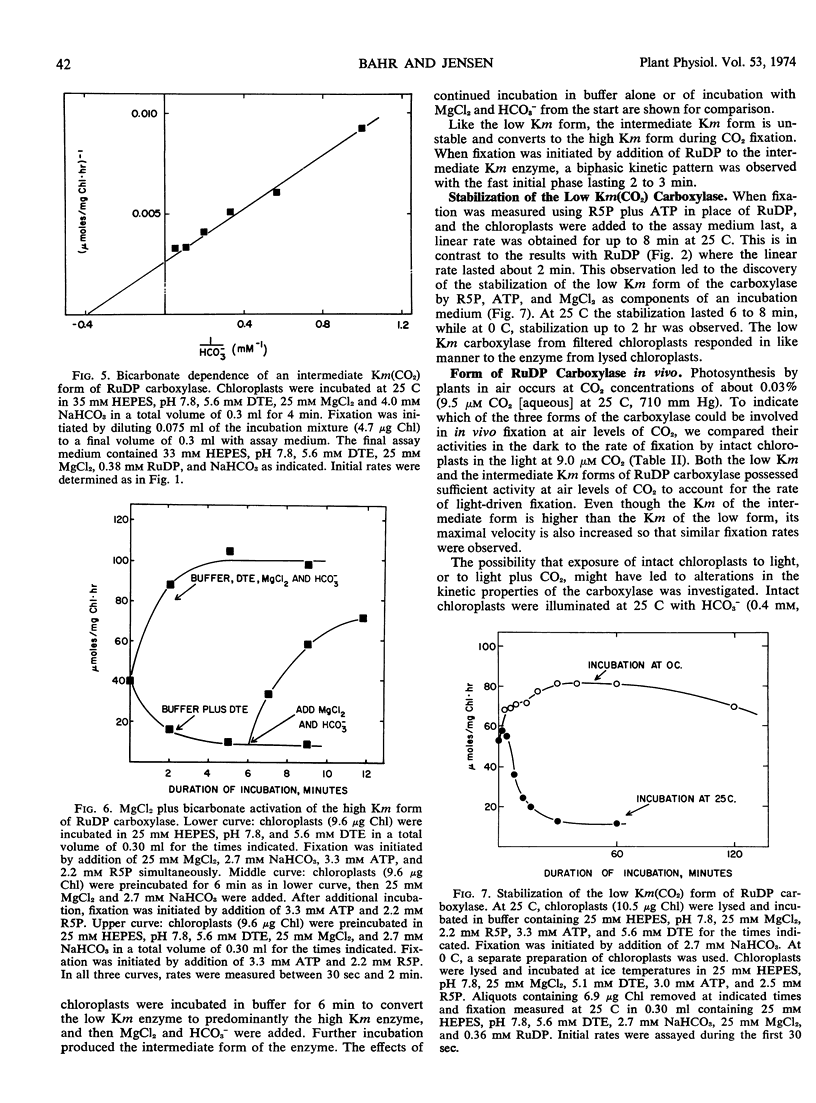
The properties of a form of ribulose diphosphate carboxylase having a high affinity for CO2 have been studied. Its apparent Km(HCO3−) of 0.5 to 0.8 mm (pH 7.8) and calculated Km(CO2) of 11 to 18 μm are comparable to the values exhibited by intact chloroplasts during photosynthesis. This form of the enzyme was released from chloroplasts in hypotonic media and was unstable, rapidly converting to a form having a high Km(HCO3−) of 20 to 25 mm similar to that for the purified enzyme. Incubation of the enzyme with MgCl2 and HCO3− yielded a third form with an intermediate Km(HCO3−) of 2.5 to 3.0 mm.
The low Km form had sufficient activity both at air levels of CO2 and at saturating CO2 to account for the rates of photosynthesis by intact chloroplasts. The low Km form could be stabilized in the presence of ribose 5-phosphate, adenosine triphosphate, and MgCl2, at low temperatures for up to 2 hours.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan B. B., Schürmann P. A regulatory mechanism for CO 2 assimilation in plant photosynthesis: activation of ribulose-1,5-diphosphate carboxylase by fructose 6-phosphate and deactivation by fructose 1,6-diphosphate. FEBS Lett. 1972 Jun 15;23(2):157–159. doi: 10.1016/0014-5793(72)80329-6. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. RATE OF HYDRATION OF CARBON DIOXIDE AND DEHYDRATION OF CARBONIC ACID AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3502–3507. [PubMed] [Google Scholar]
- Jensen R. G. Activation of CO 2 fixation in isolated spinach chloroplasts. Biochim Biophys Acta. 1971 Jun 15;234(3):360–370. doi: 10.1016/0005-2728(71)90203-9. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. 3. Light activation of the carboxylation reaction. Biochim Biophys Acta. 1968 Jan 15;153(1):227–234. doi: 10.1016/0005-2728(68)90164-3. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction-I protein. I. Effect of crystallization of fraction-I protein from tobacco leaves on ribulose diphosphate carboxylase activity. Biochim Biophys Acta. 1971 Jan 19;229(1):240–249. [PubMed] [Google Scholar]
- Murai T., Akazawa T. Homotropic effect of CO 2 in ribulose-1,5-diphosphate carboxylase reaction. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2121–2126. doi: 10.1016/0006-291x(72)90768-1. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakayama N., Akazawa T. Structure and function of chloroplast proteins. V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch Biochem Biophys. 1968 Sep 10;126(3):737–745. doi: 10.1016/0003-9861(68)90465-7. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]


