Abstract
Background
Neutrophil-lymphocyte ratio (NLR) is an indicator of the inflammatory state, and its increase has been shown to be a negative prognostic factor for many malignancies. The aim of this study was to determine whether there was a relationship between NLR and tumor aggressiveness in gastric cancer patients and to investigate the prognostic significance of NLR.
Material/Methods
The medical records of 189 patients with gastric cancer between January 2009 and January 2014 were examined for the presence of metastasis, tumor staging, tumor differentiation grade, and preoperative NLR value.
Results
Of the 189 patients, 61 were female (32.2%) and 128 were male (67.7%). Eight-eight patients had NLR values of four or higher. A comparison of the high NLR value group and the low NLR value group found no statistically significant difference for clinicopathological features of age, gender, type of operation, of degree of differentiation; differences ranged from 20.7% to 46.2%, p<0.001.
Conclusions
Increase in NLR has been associated with poor prognosis in univariate analysis and variations of this parameter have also been shown to be correlated with tumor progression. NLR values should be considered as a useful follow-up parameter.
MeSH Keywords: Lymphocyte Activation, Neutrophils, Stomach Neoplasms
Background
Gastric cancer is a common cancer type occurring in every region of the world. The most diagnosed cancers in the world are lung (13%), stomach (11.9%) and colon (9.7%), while death rates from different cancer have been reported to vary: lung (19.4%), liver (9.1%), and stomach (8.8%). The incidence of stomach (gastric) cancer is more common in males that females (male/female ratio 2/1), and usually increases in future years and peaks in the early 60 years of age [1,2].
Gastric cancer is a cancer that usually occurs with late clinical findings. It is usually asymptomatic until advanced stages. The most common symptoms in symptomatic patients are loss of appetite and weight loss. The five-year survival rate in patients who are diagnosed at an early stage cancer is reported to be improving, reaching up to 80% [3]. In countries where gastric cancer is frequently observed and routine screening programs are implemented, such as Japan, gastric cancer is more likely to be caught earlier, providing more chance of cure if possible [4]. Despite improvements in diagnosis and treatment, there are many unanswered questions about gastric cancer, including the boundaries of lymph node dissection, optimal surgical strategies (organ resections/borders, etc.) and variable chemotherapy regimens.
In addition, the role of neutrophils is of interest. Our blood has a liquid structure that consists of blood cells and plasma, and 90% of plasma is water. The remaining components consist of proteins and other soluble substances. Erythrocytes, leukocytes, and platelets form the blood cells. Leukocytes are divided into two forms “like pieced” (granulocytes, polymorphonuclear leukocytes) and “non-split” (agranulocytes, mononuclear leukocytes). Neutrophils, eosinophils, and basophils form the granulocytes group, while lymphocytes and monocytes form the agranulocytes group. The main functions of neutrophils are removing impurities which are harmful to the body through phagocytosis and destroying the impurities with its enzymes. Lymphocytes form the humoral immune response. The basic functions of this group are to recognize microorganisms and to create an antibody response against them. In normal white blood cells, the percentage of neutrophils is 62–65% and the percentage of lymphocytes is 30–35%; the neutrophil/lymphocyte ratio is approximately 2: 1 [5].
In recent years, the effect of NLR on prognosis has been investigated in bladder tumors, kidney tumors, and colorectal tumors. This study was designed to investigate the relationship between NLR, which is routinely preoperative measured in gastric cancer patients, and pathological tumor stage.
Material and Methods
Between January 2009 and January 2014, the clinical records of 189 patients with gastric cancer who had surgery in Tepecik Training and Research Hospital General Surgery Clinic/İzmir/Turkey were retrospectively reviewed. Patient data included age, gender, type of operation, presence of metastasis, tumor staging (TNM), tumor differentiation degree, and preoperative NLR value. In patients who had surgery due to gastric cancer, the relationship between postoperative pathologic staging and preoperative NLR values was investigated. The patients with insufficient file information were excluded from the study.
Data analysis was done by using SPSS/Windows 11.5 package program. Descriptive statistics are shown as mean ± standard deviation or median (minimum–maximum) for continuous and discrete numeric variables, and number of cases and (%) for categorical variables. The significance of the difference in median levels of NLR between the two groups was analyzed by Mann Whitney U test when the number of independent groups was two; and if the number was more than two it was analyzed by Kruskal-Wallis test. When the Kruskal-Wallis results were found important; condition(s) that caused the difference were determined with using Conover’s non-parametric multiple comparison tests. Whether there is a statistically significant correlation between age, T stage, N stage, and degree of differentiation with NLR values was investigated by using Spearman’s correlation test. Whether there was a statistically significant correlation between categorical variables and overall survival was evaluated by Kaplan-Meier survival analysis using log-rank test. In addition, 1–3 year overall survival rate, 5-year cumulative overall survival rate, the expected average life, and 95% confidence intervals (CI) for the duration were calculated for each variable. Whether there was a statistically significant effect on overall survival of continuous, discrete, and ordinal variables was evaluated by using univariate Cox proportional hazard regression analysis. Relative risk and 95% CI of each variable were calculated. As a result of univariate statistical analysis, when the correction was done according to all of the risk factors that had an effect on overall survival or that were considered to be effective clinically, then whether the impact of NLR on the outcome was continuing or not was investigated by Cox multivariate proportional hazard regression analysis. In univariate analysis variables that were identified as p<0.25 were included in multivariate models as risk factors. Results with p<0.05 were considered statistically significant.
Results
There was a total of 189 patients, 128 were male (67.7%) and 61 were female (32.3%). The male/female ratio was 2.09. The youngest patient was 29 years old, the oldest patient was 89 years old; the median age was 60.3 years of age. When examined by age groups, 10 patients (5.3%) were under 40 years of age, 30 (15.3%) were between 40 and 50 years of age, 51 (27%) were between 50 and 59 years of age, 46 (24.3%) were between 60 and 69 years of age, 43 (22.8%) were between 70 and 79 years of age, and 9 (4.8%) were between 80 and 89 years of age.
Of the 179 of the patients who had operable disease, 140 patients (74.1%) had total gastrectomy and 39 patients (20.6%) had subtotal gastrectomy. Ten patients (5.3%) had disease that was considered inoperable. In operable patients, pancreatic invasion (20 cases), invasion of the column (11 cases), spleen invasion (3 cases), and liver metastases (8 cases) were detected.
When the 179 surgical patients were evaluated according to the degree of pathological differentiation, 96 patients (53.6%) had less differentiated gastric carcinoma, 70 patients (39.1%) had moderate differentiated gastric carcinoma, and 13 patients (16.8%) had well differentiated gastric carcinoma.
The 179 cases (resection procedure applied) were staged according to the TNM staging system. When the T stage (stomach wall involvement) was analyzed, 20 patients (11.1%) were identified as T1, 17 (9.4%) as T2, 28 (15.6%) as T3, and 114 (63.9%) as T4. When the N stage (lymph node involvement) was analyzed, 55 patients (29.1%) were identified as NO, 18 (9.5%) as N1, 30 (15.9%) as N2, 46 (24.3%) as N3a, and 30 (21.2%) as N3b. In the 189 patients who had surgery, the absence of metastases was found in 171 cases; metastasis was seen in 18 cases.
When patients were grouped according to TNM, 34 patients (18%) were found to be stage I, 40 patients (21.2%) stage II, 100 patients (52.9%) stage III, and 15 patients (7.9%) stage IV (Table 1).
Table 1.
Distribution of gastric cancer patients (who underwent surgery) by pathological stage.
| Stage | n | % |
|---|---|---|
| IA | 20 | 10.5 |
| IB | 14 | 7.5 |
| IIA | 19 | 10.1 |
| IIB | 21 | 11.1 |
| IIIA | 26 | 13.7 |
| IIIB | 32 | 16.9 |
| IIIC | 42 | 22.3 |
| IV | 15 | 7.9 |
When the patients were evaluated according to NLR, the median value was found to be 3.8. In 13 patients (6.9%) NLR value was less than two, in 45 (23.8%) 2–3, in 43 (22.8%) between 3 and 4, in 25 (13.2%) between 4 and 5, and in 63 patients (33.3%) it was calculated as 5 and higher. When the reference value for NLR was taken as 4, in 101 patients (53.4%) NLR value was detected less than four and in 88 patients (46.6%) it was detected as 4 or higher (Table 2).
Table 2.
NLR level of patients who underwent surgery for gastric cancer.
| NLO levels | n | % |
|---|---|---|
| <2.00 | 13 | 6.9 |
| 2.00–2.99 | 45 | 23.8 |
| 3.00–3.99 | 43 | 22.8 |
| 4.00–4.99 | 25 | 13.2 |
| ≥5.00 | 63 | 33.3 |
| <4.00 | 101 | 53.4 |
| ≥4.00 | 88 | 46.6 |
In gastric cancer patients who underwent surgery, when median NLR levels were examined according to patient age, gender, type of surgery and pathologic differentiation degree, there were no statistically significant difference (p>0.05) (Table 3).
Table 3.
NLR level of patients who underwent surgery for gastric cancer according to their demographic and clinical characteristics.
| Variables | Median NLR value (min–max) | p value |
|---|---|---|
| Age groups | 0.626 | |
| <40 years | 3.5 (2.1–6.1) | |
| 40–49 years | 3.3 (1.0–8.0) | |
| 50–59 years | 3.8 (1.6–13.5) | |
| 60–69 years | 4.0 (1.7–8.2) | |
| 70–79 years | 3.7 (0.9–9.0) | |
| 80–89 years | 4.5 (2.2–11.0) | |
| Gender | 0.958 | |
| Male | 3.7 (1.0–13.5) | |
| Female | 3.8 (0.9–8.6) | |
| Operation type | 0.842 | |
| Total | 3.7 (0.9–11.0) | |
| Subtotal | 3.6 (1.5–13.5) | |
| Differentiation degree | 0.732 | |
| Little | 4.3 (0.9–13.5) | |
| Middle | 3.7 (1.1–8.3) | |
| Good | 3.5 (1.8–8.6) | |
When the patients were evaluated according to NLR levels considering tumor size (T), lymph node involvement (N), metastasis state (M), and pathological stages, for T stage there was statistically significant difference in median NLR values in T3, T4 compared with T1, and in T4 compared with T2 the median NLR levels were statistically significantly higher (p<0.05). There was no statistically significant difference between non-metastatic and metastatic groups (p=0.555). For stages, there was a statistically significant difference in median NLR values: in stage II, III, and IV compared with stage I; and in stage III and IV compared with stage II the median NLR levels were statistically significant higher (p<0.05) (Table 4, Figure 1).
Table 4.
The relationship between T, N, M, and pathologic stages with median NLR values in gastric cancer patients who underwent surgery.
| Variables | Median NLR (min–max) | p value |
|---|---|---|
| T | <0.001 | |
| I | 2.8 (1.5–6.7) | |
| II | 2.8 (1.1–7.8) | |
| III | 3.7 (2.1–7.8) | |
| IV | 4.4 (0.9–13.5) | |
| N | 0.005 | |
| 0 | 2.9 (1.1–8.0) | |
| 1 | 3.5 (1.6–7.8) | |
| 2 | 3.9 (1.5–11.0) | |
| 3A | 4.6 (1.7–13.5) | |
| 3B | 3.9 (0.9–8.2) | |
| M | 0.555 | |
| Positive | 3.5 (0.9–13.5) | |
| Negative | 4.4 (2.6–8.0) | |
| Stage | <0.001 | |
| I | 2.8 (1.1–6.7) | |
| II | 3.6 (1.5–8.0) | |
| III | 4.4 (1.6–13.5) | |
| IV | 4.8 (2.6–8.0) |
Figure 1.
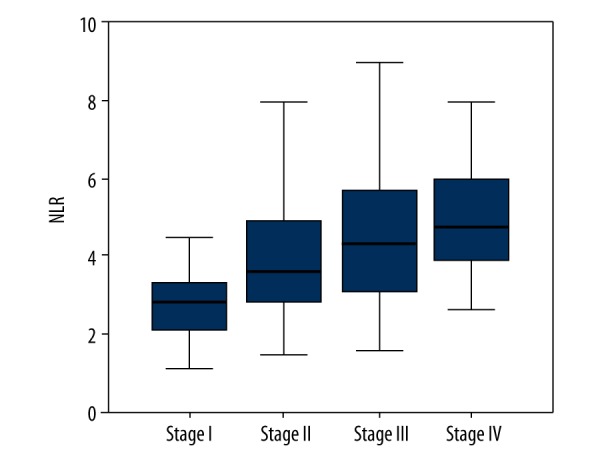
Distribution of NLR values according to the stages.
When overall survival levels (1–3 year survival rate and 5-year survival) and median survival times (month basis) were analyzed according to T, N, M, and pathologic stages in gastric cancer patients who underwent surgery, the overall survival levels differed statistically significantly (p<0.001) compared with T, N, and pathologic stage. In the metastatic group, the prognosis was statistically significantly worse than the non-metastatic group (p<0.001) (Table 5).
Table 5.
1–3 year and 5-year survival rates according to T, N, M and pathological staging.
| Variables | Survival rates | Median survival time | Log | p value | ||
|---|---|---|---|---|---|---|
| 1 year | 3 year | 5 year | ||||
| T | 53.30 | <0.001 | ||||
| I | 100.0 | 94.4 | 86.1 | 54.7 (50.2–59.1) | ||
| II | 92.0 | 86.9 | 79.2 | 50.1 (46.3–57.2) | ||
| III | 81.5 | 70.4 | 62.6 | 48.1 (38.4–57.7) | ||
| IV | 58.8 | 27.9 | 17.5 | 24.2 (20.1–28.2) | ||
| N | 56.29 | <0.001 | ||||
| 0 | 96.3 | 90.8 | 83.2 | 55.3 (51.1–59.6) | ||
| 1 | 88.9 | 54.7 | 46.3 | 37.3 (27.3–47.3) | ||
| 2 | 60.0 | 34.9 | 27.4 | 26.2 (18.8–33.7) | ||
| 3A | 50.0 | 24.0 | 16.5 | 22.2 (15.8–29.0) | ||
| 3B | 51.6 | 20.5 | 11.2 | 20.0 (14.0–26.0) | ||
| M | 12.94 | <0.001 | ||||
| Negative | 71.8 | 51.5 | 42.5 | 37.8 (33.6–42.0) | ||
| Positive | 40.0 | 10.0 | – | 13.2 (4.7–21.7) | ||
| Stage | 83.08 | <0.001 | ||||
| I | 100.0 | 92.0 | 86.0 | 53.3 (48.1–59.3) | ||
| II | 94.9 | 81.2 | 70.7 | 50.4 (44.0–56.8) | ||
| III | 53.6 | 26.2 | 15.5 | 24.6 (19.4–28.5) | ||
| IV | 26.7 | 7.7 | – | 10.4 (4.4–16.4) | ||
When overall survival rates of 1–3 year and 5-year survival, and median survival times (month basis) were analyzed according to NLR values in gastric cancer patients who underwent surgery, the overall survival levels differed statistically significantly (p<0.001). In the group with NLR value less than 4, the prognosis was statistically significantly worse than the group with NLR value ≥4 (p<0.001) (Figure 2, Table 6).
Figure 2.
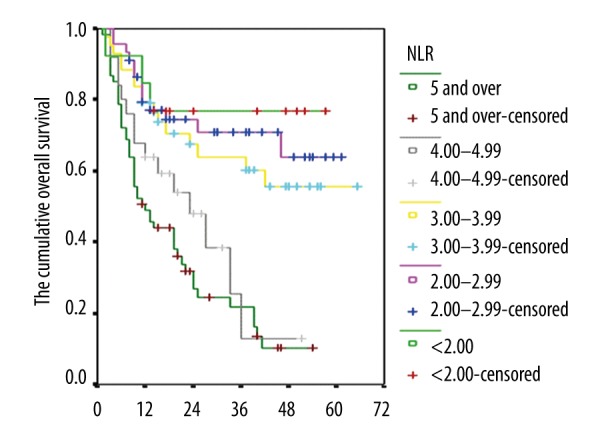
Kaplan-Meier curves that show the cumulative overall survival levels of the cases according to the NLR values.
Table 6.
General survival rates of gastric cancer patients who underwent surgery by NLR values.
| Variables | Survival rates | Median survival time | Log | p value | ||
|---|---|---|---|---|---|---|
| 1 year | 3 year | 5 year | ||||
| NLR | 40.47 | <0.001 | ||||
| <2.00 | 84.6 | 76.9 | 68.0 | 45.8 (34.7–57.0) | ||
| 2.00–2.99 | 79.5 | 70.8 | 63.7 | 45.5 (38.5–52.5) | ||
| 3.00–3.99 | 79.1 | 60.3 | 55.7 | 43.8 (35.7–51.9) | ||
| 4.00–4.99 | 64.0 | 12.8 | 6.2 | 23.5 (16.4–30.5) | ||
| ≥5.00 | 49.1 | 9.7 | 3.4 | 19.1 (14.8–23.5) | ||
| NLR | 36.66 | <0.001 | ||||
| <4.00 | 80.0 | 68.6 | 62.1 | 46.7 (41.5–51.9) | ||
| ≥4.00 | 53.4 | 21.7 | 11.6 | 20.7 (16.9–24.6) | ||
Discussion
Surgery is the only curative treatment for gastric cancer. Surgical treatment provides the best palliation and most accurate staging. In most patients with gastric cancer, gastric resection is required. Patients who have widespread metastasis and who cannot tolerate abdominal surgery are the exceptions. In some patients with symptomatic primary tumors with systemic metastasis, the decision for surgery is controversial. The palliation is usually not good for this group if resection operation cannot be done. Tumor staging alone does not provide sufficient information about the prognosis of the patients. It was found that there is a relationship between poor prognosis and some laboratory markers in gastric cancer patients. It is also believed that those markers are useful in prediction of early recurrence and bad prognosis.
One such marker that has been investigated is preoperative levels of NLR. In a study by Shimada et al. of 1,028 patients, the median NLR value was 2.62; in 32 cases it was <1.0; in 381 cases it was between 1 and 2; in 321 cases it was between 2 and 3; in 167 cases it was between 3 and 4; in 75 cases it was between 4 and 5; and in 52 cases it was greater than 5. In this study, when the threshold value for NLR was taken as 4.0, in 127 patients (12.4%) NLR was found to be ≥4.0, while in 901 patients (87.6%) it was found to be <4.0 [6]. In our study median NLR value was 3.8. In 13 cases (6.9%) NLR value was calculated as <2.0; in 45 cases (23.8%) NLR was between 2.0 to <3.0; in 43 cases (22.8%) it was 3.0 to <4.0; in 25 cases (13.2%) it was 4.0 to <5.0; and in 63 cases (33.3%) it was 5 or greater. In 101 cases (53.4%) NLR value was <4; while in 88 cases (46.6%) it was ≥4. A study by Lee et al. of 174 gastric cancer patients found no significant relationship between age and gender and NLR values (p=0.002) (7). However, another study of 220 gastric cancer patients, found increased NLR values were correlated with advanced age (≥60 years) (p=0.016); but there was no statistically significant relationship between male or female gender and NLR values [8]. Similarly, a study by Azab et al. found that while advanced age (≥60 years) was associated with high NLR values (p=0.01), women had statistically significantly lower NLR values than men (p=0.01) [9]. In our study, there was no significant correlation between gender and age of the patients or operations performed on patients based on NLR values.
In our study, there was a statistically significant difference in median NLR levels according to the T and N stage. But there was no significant statistical difference in terms of median NLR values between the metastatic and non-metastatic groups. In terms of stage, there was a significant difference in NLR values: increase of stage levels was correlated with median NLR values. In a study by Lee et al., a proportional relationship between increased NLR levels with TNM stages was shown (p=0.046) [7] similar to our study findings. In another study by Atila et al., a high level of NLR was shown to be a prognostic factor associated with metastasis of primary disease [10]. Shimada et al. found that in patients with T3/T4 stage disease, N1 and M1 stages had high NLR levels; similarly a progress in NLR levels from stage II to stage IV was shown [6]. In our study, there was a statistically significant difference in overall survival times according to T, N, and disease stage (p<0.001). Similarly, the prognosis of patients with metastasis was worse than patients without metastasis (p<0.001). When other studies in the literature were examined with this perspective, it has been shown that 1–3 year and 5-year life expectancy rates were higher in patients that had low stage disease with lower T and N levels and no metastasis [10–12].
It is known that chronic inflammation induces carcinogenesis, contributing to the onset or progression of cancer [13]. However, tumors do not only occur in areas of inflammation; for example, Helicobacter pylori infection is an agent considered a potential factor in the development of gastric cancer [14]. But still, chronic inflammation can trigger regional formation of immune response and release of inflammatory factors around tumors and this may result in a formation of an inflammatory microenvironment. As a result, an inflammatory process accompanies cancer progression contributing to tumorigenesis by secreting various cytokines that start angiogenesis and consequently tumor growth and metastasis, for example, vascular endothelial growth factor (VEGF), interleukin-18 (IL-18), and matrix metalloproteinases in the tumor microenvironment can contribute to tumorigenesis [15–17]. In recent years, several markers have been identified and put into practice for predicting the prognosis of gastric cancer. Carcinoembryonic antigen (CEA), human epidermal growth factor receptor-2 (HER-2) are pathological markers used for routine evaluation of gastric cancer. Ki-67, caspase-3, and p53 have also been reported to be related to survival in gastric cancer patients [18]. In addition, it is well known that microRNAs (miRNA) have many important regulatory functions in the pathogenesis of cancer [19]. However, because these biomarkers need to be studied in carcinogenic tissue, it is not possible to monitor the levels of these markers continuously throughout the progression of the disease. Whereas NLR can be analyzed easily in plasma or serum.
NLR is known to have a prognostic value in cancer patients. There are several possible mechanisms to explain the relationship between the poor prognosis and NLR in cancer patients. First, the increased number of neutrophils around the tumor area may suppress the anti-tumor responses of natural killer cells and activated T cells [20]. High NLR level reflects increased neutrophil-dependent inflammatory response as well as decreased lymphocyte-mediated anti-tumor immune reaction, and this contributes to aggressive tumor biology, cancer progression, and poor prognosis by weakening the lymphocyte mediated anti-tumor cellular immune response. Second, neutrophils can contribute to tumor growth and progression by producing cytokines such as tumor necrosis factor (TNF), IL-1, IL-6, angiogenic factors, and VEGF [21]. Third, reduction in the number of lymphocytes can weaken the lymphocyte-mediated anti-tumor cellular immune response. The number of neutrophils may not reflect the prognostic feature alone; likewise, low total lymphocyte counts may fail to show the effect on tumor growth. Therefore, high levels of NLR determined by the combined effect of neutrophilia and lymphocytopenia can provide more information about the tumor growth process and outcomes.
In this context, in our study the correlation between the NLR levels and the prognosis of disease was examined. According to our results, when the NLR classification was considered, the overall survival levels showed a statistically significant difference (p<0.001). In the group of patients with NLR level ≥4, the prognosis was worse than those with <4 (p<0.001). Similarly, Atila et al. investigated the prognostic importance of preoperative NLR level in gastric cancer patients and found that the 5-year survival rate was statistically significantly worse in patients with low levels of NLR compared to patients with high levels [10]. Shimada et al. reported that the prognosis was poor in patients with high NLR levels [6], as similar to our study. In addition, the significant correlation between the neutrophil count or NLR level and survival has been put forward in many other studies involving gastric cancer patients. A study by Lee et al. found that only the increased neutrophil count was specified as an independent risk factor associated with worse prognosis and decreased survival rate [8]. In the same study, while the increase in NLR level was related to the increase in the number of neutrophils or decrease in the number of lymphocytes or both, the authors emphasized that the increase in neutrophil counts had a more important place in the progression of cancer as a prognostic factor [8]. At various gastrointestinal tumors, prognostic markers have also been studied [22–25].
Conclusions
From our study and similar studies that we reviewed in the literature, we conclude that NLR is a valuable laboratory marker in gastric cancer patients to estimate the survival time and therefore the prognosis. NLR level is of critical importance for cancer prognosis, especially due to its easy clinical application in monitoring patients.
Footnotes
Source of support: Departmental sources
References
- 1.Forman D, Burley VJ. Gastric cancer: Global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–49. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.WHO IARC. Stomach cancer estimated incidence, mortality and prevalance worldwide in 2012. Globocan; 2012. [Google Scholar]
- 3.Lee JH, Choi MG, Min BH, et al. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg. 2012;99:1688–92. doi: 10.1002/bjs.8934. [DOI] [PubMed] [Google Scholar]
- 4.Li ZX, Kaminishi M. A comparison of gastric cancer between Japan and China. Gastric Cancer. 2009;12(1):52–53. doi: 10.1007/s10120-008-0495-2. [DOI] [PubMed] [Google Scholar]
- 5.National Blood and Blood Products Guide. 2011. [Google Scholar]
- 6.Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 13:170–76. doi: 10.1007/s10120-010-0554-3. 201. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotheraphy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DY, Hong SW, Chang YG, et al. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13(2):111–16. doi: 10.5230/jgc.2013.13.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azab B, Rivera MC, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atila K, Arslan NÇ, Sütçü MT, et al. Prognostic significance of preoperative neutrophil lymphocyte ratio in gastric cancer patients. 18. National Surgical Congress. 2012:95. [Google Scholar]
- 11.Hirashima M, Higuchi S, Sakamoto K, et al. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;124:329–34. doi: 10.1007/s004320050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 13.Xiao LJ, Zhao S, Zhao EH, et al. Clinicopathological and prognostic significance of Ki-67, Caspase-3 and p53 expression in gastric carcinomas. Oncol Lett. 2013;6(5):1277–84. doi: 10.3892/ol.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Zhang Q, Deng M, et al. Ananalysis of human microRNA and disease associations. PLoS One. 2008;3(10):e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;17325(16):1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 17.Webb NJ, Myers CR, Watson CJ, et al. Activated human neutrophils express vascular endothelial growth factor (VEGF) Cytokine. 1998;10(4):254–57. doi: 10.1006/cyto.1997.0297. [DOI] [PubMed] [Google Scholar]
- 18.Jablonska E, Puzewska W, Grabowska Z, et al. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30(3):93–99. doi: 10.1016/j.cyto.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-Free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–67. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shau H, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395–402. [PubMed] [Google Scholar]
- 21.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–22. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 22.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isik A, Okan I, Firat D, et al. A new prognostic strategy for gastric carcinoma: Albumin level and metastatic lymph node ratio. Minerva Chir. 2014;69:147–53. [PubMed] [Google Scholar]
- 24.Suzuki K, Araki H, Komoike Y, Takeuchi K. Permissive role of neutrophils in pathogenesis of indomethacin-induced gastric lesions in rats. Med Sci Monit. 2000;6(5):CR908–14. [PubMed] [Google Scholar]
- 25.Isik A, Peker K, Gursul C, et al. The effect of ozone and naringin on intestinal ischemia/reperfusion injury in an experimental model. Int J Surg. 2015;21:38–44. doi: 10.1016/j.ijsu.2015.07.012. [DOI] [PubMed] [Google Scholar]


