Summary
Autoimmune pancreatitis (AIP) is a rare and underdiagnosed fibrosclerosing inflammatory variant of chronic pancreatitis. Its true incidence and prevalence in the general population is still not confirmed despite advances in medicine. Differentiating it from pancreatic cancer is of paramount importance. In this imaging review, we highlight the imaging findings of this intriguing entity.
MeSH Keywords: Autoimmune Diseases, Magnetic Resonance Imaging, Multidetector Computed Tomography, Pancreas
Background
In 1961, Sarles et al. [1] coined the term idiopathic chronic pancreatitis in a patient who presented with obstructive jaundice and hypergammaglobulinaemia. Yoshida et al. [2] in 1995, introduced the term “autoimmune pancreatitis” (AIP). Today, AIP is recognised as a distinct form of rare fibroinflammatory subtype of chronic pancreatitis with typical imaging and histological findings. This disease entity has been associated with various names in the past, including sclerosing pancreatitis, tumefactive pancreatitis and non-alcoholic destructive pancreatitis. It has been most recently recognized as a IgG4-related disease. The purpose of this article is to acquaint the readers with the spectrum of imaging findings, both parenchymal and ductal changes, seen in AIP and enable them to differentiate it from entities that can mimic AIP or pancreatic malignancy.
Pathophysiology –
No definite aetiology has yet been identified for this mysterious entity. An association with HLA serotypes DRB1*0405 and DQB1*0401 has been reported in the Japanese literature [3]. An autoimmune mechanism is the culprit and initial stimulus for the Th2-cell immune response [3].
There are two subtypes of autoimmune pancreatitis – Type 1 and Type 2.
Type 1 –
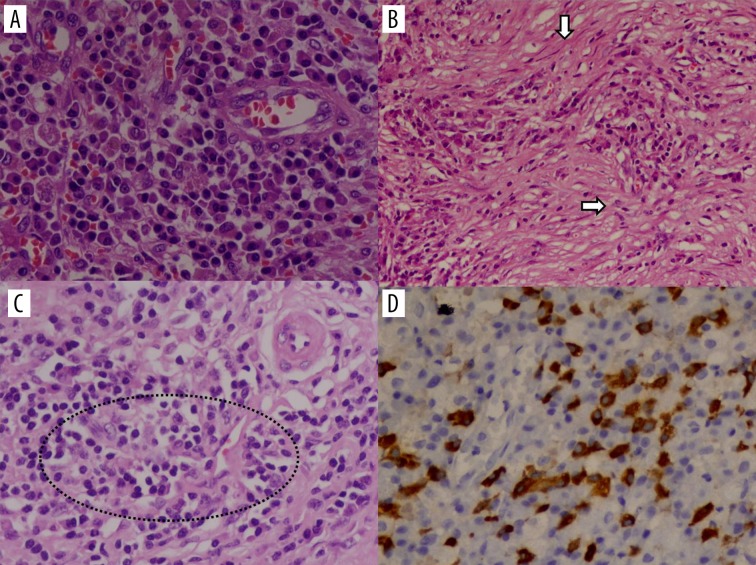
This is the more common type, seen generally in Asian populations. It is also known as IgG4-related lymphoplasmacytic sclerosing pancreatitis (LPSP). Type 1 AIP tends to affect older patients (>50 years of age). Clinically, patients present with obstructive jaundice and chronic, mild, recurrent abdominal pain. Significant weight loss and severe abdominal pain is not a feature of AIP. It is also associated with elevated serum levels of IgG4. Classical histological findings are dense lymphoplasmacytic infiltration consisting mainly CD4+ T lymphocytes, fibrosis without granulocytic infiltration, storiform fibrosis, obliterative phlebitis and more than 10 IgG4-positive cells (HPF) (Figure 1) [4–6]. Extrapancreatic organs are frequently involved at the time of initial presentation and they include the biliary tree (sclerosing cholangitis), chest (lung nodules, mediastinal fibrosis, adenopathy), retroperitoneum (retroperitoneal fibrosis, chronic periaortitis), salivary glands (sclerosing sialadenitis), kidneys (interstitial nephritis) and orbits (pseudolymphoma). Bowel involvement is rare. The response to steroids is poor with high relapse rates [3,7].
Figure 1.
Histopathology slides showing (A) sheets of plasma cells (HE, 400×) (B) storiform fibrosis (HE, 100×) (C) obliterative phlebitis (HE, 200×) (D) IgG4 immunostaining (Brown staining) of plasma cells (LSAB method, 400×).
Type 2 –
This is a relatively recently described form of AIP, seen in Europeans and Americans [8]. It tends to affect younger patients with no gender preponderance. It is also known as idiopathic duct-centric pancreatitis or AIP with a granulocytic epithelial lesion (GEL). Clinically, patients present with acute pancreatitis, abdominal pain or with features suggestive of inflammatory bowel disease. Histologically, the inflammation is centred on the ducts with neutrophilic infiltration, plasma cells and microabscesses being a characteristic feature [9]. This can ultimately obliterate the pancreatic duct. It is different from Type 1 AIP as there is less inflammation and vasculitis. Inflammatory bowel disease is more often associated with Type 2 AIP. Dramatic response to steroid therapy is seen and relapse rates are very lower.
Imaging
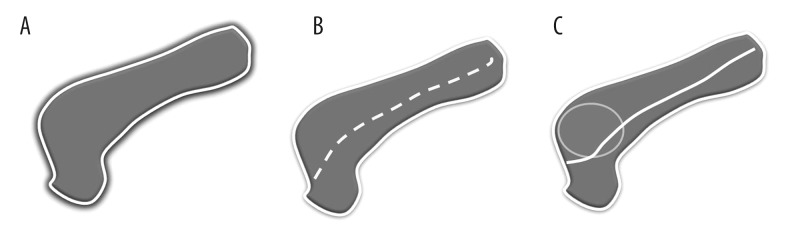
Contrast-enhanced computed tomography (CECT) is the investigation of choice to look for pancreatic parenchymal changes and exclude malignancy, while magnetic resonance cholangio-pancreatography (MRCP) or endoscopic cholangiography (ERCP) are useful in assessing the biliary and pancreatic duct abnormalities. However, in centres with expertise and experience in pancreatic imaging, one-stop imaging with contrast-enhanced MRI combined with MRCP can be done. The role of fluorodeoxyglucose-positron emission tomography (FDG-PET) in diagnosing AIP is of limited value. Reduced FDG uptake after a short course of steroid treatment, diffuse uptake, extrapancreatic uptake in other organs may be of some help in differentiating AIP from pancreatic cancer [10,11]. A pictorial diagram showing patterns of AIP is depicted in Figure 2.
Figure 2.
Pictorial diagram showing various types of autoimmune pancreatitis (A) sausage-shaped pancreas with loss of lobulation and peripancreatic halo (B) multifocal strictures without upstream dilatation (C) mass-forming AIP with a positive duct penetrating sign.
Pancreatic parenchyma –
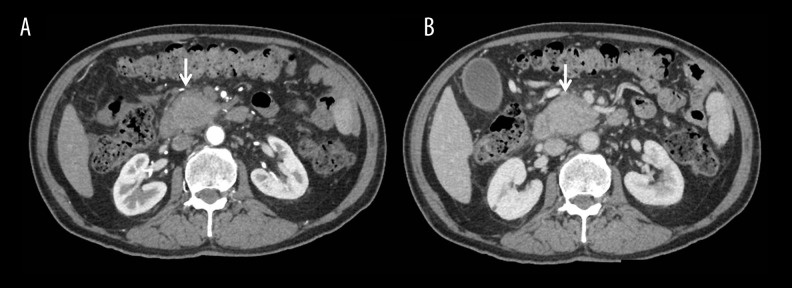
Three different forms of the disease process are recognised - diffuse, focal and multifocal disease. On imaging, diffuse involvement is seen as “sausage-shaped” enlargement with featureless and effaced normal lobular architecture of the pancreas (Figures 3–5). Post contrast, reduced enhancement is seen in early phase with homogeneous enhancement in delayed phase. A halo of soft-tissue is seen around the enlarged pancreas, which may show delayed enhancement due to fibro-inflammatory cell infiltration around the pancreatic tissue. This rim is hypointense on T1 and T2-weighted images. Peripancreatic inflammation, calcification and pseudocyst formation is not a usual feature of AIP. In case of delayed presentation and after steroid therapy, atrophy of the pancreatic parenchyma is seen, which represents the late burnt-out stage of the disease process.
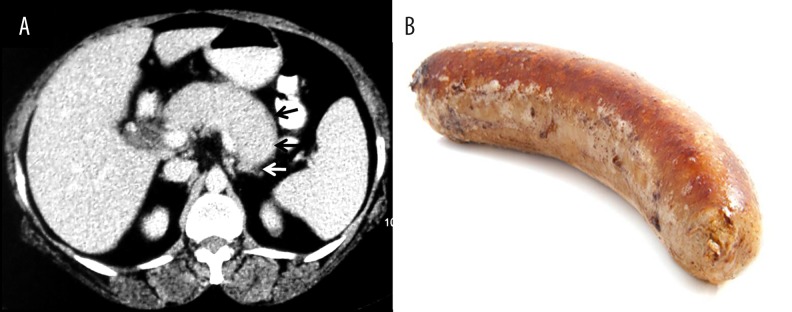
Figure 3.
AIP with halo: (A) Axial contrast-enhanced CT image showing sausage-shaped pancreas, loss of lobulation and a hypodense peripheral soft-tissue rim (arrows) with no peripancreatic stranding; (B) Picture showing the shape of a sausage.
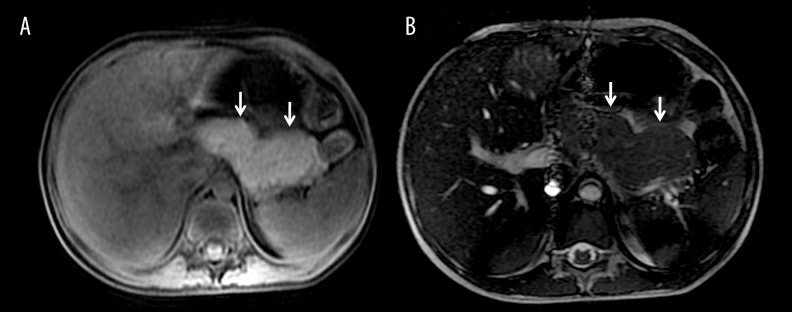
Figure 4.
Diffuse autoimmune pancreatitis: (A) Axial T1-weighted and (B) axial FIESTA MR image showing bulky pancreas with loss of lobulation (arrows) suggestive of AIP.
Figure 5.
Diffuse form of AIP: (A) Axial T2-weighted MR image showing bulky sausage-shaped, mildly hyperintense pancreatic parenchyma (dashed arrows) with a hypointense peripheral rim; (B) Diffusion-weighted MR image showing increased signal intensity (arrow) in the pancreas; (C) Apparent diffusion coefficient image showing low signal (arrow) suggestive of diffusion restriction.
Focal or mass-forming AIP shows homogeneous enhancement of the lesion in delayed phase due to retention of contrast with no peripancreatic fat infiltration, vascular invasion and internal cystic or necrotic portion (Figure 6). On arterial phase images, focal/mass forming AIP is hypovascular, similarly to pancreatic adenocarcinoma [12]. MRCP or EUS may show the main pancreatic duct penetrating through the mass lesion, suggestive of a positive duct penetrating sign in cases of focal AIP [13,14]. Capsule-like rim may or may not be seen in mass-forming AIP. Choi et al. have shown that apparent diffusion coefficient (ADC) value less than 0.9407×10−3 mm2/s on diffusion-weighted MRI may be useful in differentiating mass-forming AIP from focal ductal adenocarcinoma. The hypothesis behind this phenomenon is an increased cellularity due to a dense infiltration of lymphocytes and plasma cells, chronic fibroinflammatory processes and edematous change seen in AIP [15]. However, there may be scenarios in which dual pathology – AIP and ductal adenocarcinoma, coexist in a single patient [16]. Although previous studies have reported malignancies to be more common in IgG4-related diseases in comparison to the general population, cancer in AIP is either detected simultaneously or 3 to 5 years after the diagnosis of AIP [17–19]. Various malignancies that can be seen in association with AIP include non-Hodgkin lymphoma, including extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type (MALT lymphoma), as well as non-lymphoid breast tumours, colorectal, lung, renal, and prostate tumours [20–22].
Figure 6.
Mass-forming AIP: (A) Axial CECT pancreatographic phase showing hypovascular mass in the head of pancreas with a rim of soft tissue halo (arrow) around the lesion; (B) Delayed phase showing homogeneous enhancement of the mass lesion histopathologically confirmed to be mass-forming AIP.
Ductal involvement –
Long (>1/3 duct length) segment involvement or focal narrowing of the pancreatic duct or multiple strictures or substenoses in the affected segment without significant upstream dilatation (<5 mm), side branches arising from the strictured segment and the duct-penetrating sign are hallmark findings of AIP in MRCP/ERCP. Ductal dilatation in AIP is less severe as compared to neoplastic pathologies involving the pancreas. In the multifocal variant of AIP, the main pancreatic duct is either normal or shows substenoses in the affected segments (Figures 7–9).
Figure 7.

AIP with multifocal strictures: Coronal 2D-MRCP image showing multiple areas of strictures and substenoses (arrows) without upstream dilatation of the main pancreatic duct.
Figure 8.
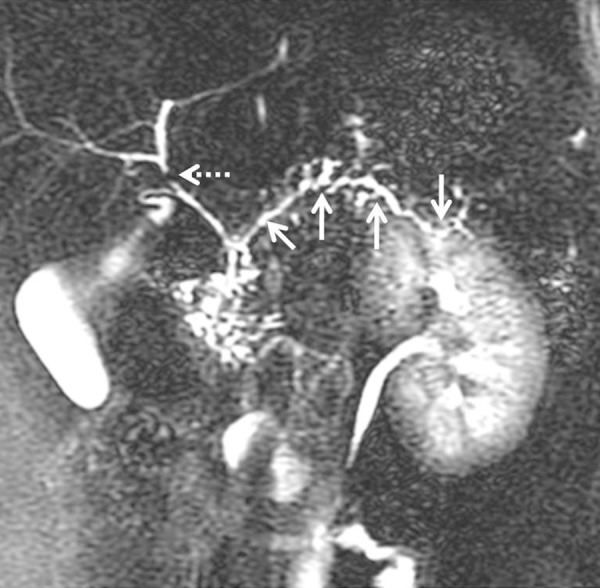
Ductal involvement in AIP: Coronal 2D-MRCP image showing multiple areas of stricture (arrows) and side-duct ectasia involving the main pancreatic duct without significant upstream dilatation. Vascular impression seen in the biliary tree at the hilum (dashed arrow).
Figure 9.
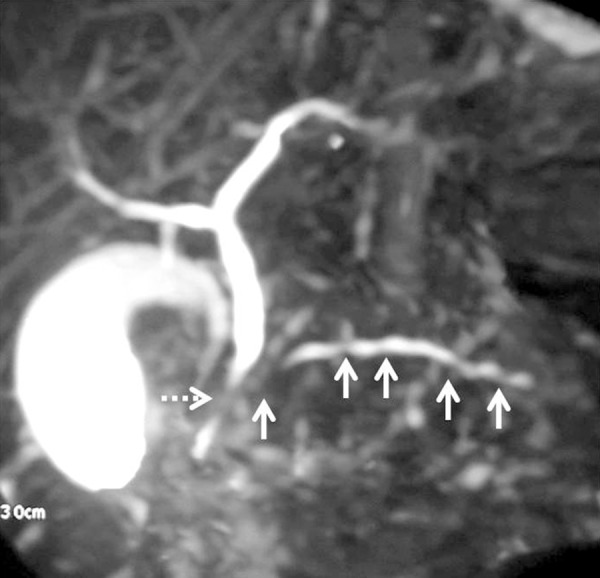
Ductal involvement in AIP: Coronal 2D-MRCP image showing multifocal strictures involving the main pancreatic duct (arrows) and a smooth stricture in the intrapancreatic portion of the common bile duct (dashed arrow).
The biliary tree can be simultaneously assessed in MRCP and ERCP. IgG4 sclerosing cholangitis is observed in a proportion of patients with AIP. The intrapancreatic portion of the common bile duct and proximal intrahepatic ducts are the most affected segments of the biliary tree. The affected segments of the biliary tree demonstrate irregular, long-segment, continuous strictures associated with contrast enhancement. The strictures may be confused with primary sclerosing cholangitis (PSC), but the strictures in PSC are typically short and band-like, multifocal and beaded in appearance with diverticula formation and peripheral pruning [23]. Extrapancreatic organs such as the biliary tree, liver, chest, retroperitoneum, salivary glands, kidneys and orbits may also be simultaneously involved and involvement of other organs may be a clue to the diagnosis of autoimmune aetiology (Figures 10–12). The differential diagnosis of diffuse AIP includes mainly diffuse ductal adenocarcinoma and interstitial pancreatitis. The differential diagnosis of focal mass forming AIP consists of ductal adenocarcinoma, lymphoma and metastasis. Distal pancreatic glandular atrophy, abrupt pancreatic duct cut-off, biliary and pancreatic ductal dilatation, vascular and perineural invasion and abdominal lymphadenopathy are more commonly seen in pancreatic adenocarcinoma. Pancreatic adenocarcinoma is hypovascular in all phases, whereas the focal type of AIP shows some degree of contrast retention in delayed phase due to severe inflammation and fibrosis. On MRI, pancreatic adenocarcinoma appears hypointense on T2-weighted images, while early AIP may show a subtle T2 hyperintense signal due to inflammation [12,27]. Diagnostic accuracy of the serum biomarker CA 19-9 is of limited value in differentiating focal AIP from pancreatic adenocarcinoma, as 9% of patients with AIP have elevated CA 19-9. However, patients with pancreatic cancer are more likely to have elevated CA 19-9 >100 U/ml than AIP patients (71% vs. 9%) [28]. Significant peripancreatic stranding is seen in acute interstitial oedematous pancreatitis. The presence of elevated serum IgG4 levels and imaging findings of diffuse, poorly enhancing and sausage-shaped pancreas, dotted enhancement during the pancreatic phase and delayed enhancement secondary to fibrosis, homogeneous T2-weighted signal intensity, associated renal involvement would favour a diagnosis of autoimmune pancreatitis over primary pancreatic lymphoma [24]. Pancreatic metastases have nonspecific imaging appearance and may be hypovascular or hypervascular (depending upon the primary tumor), but a history of a known primary malignancy may be helpful in the diagnosis.
Figure 10.
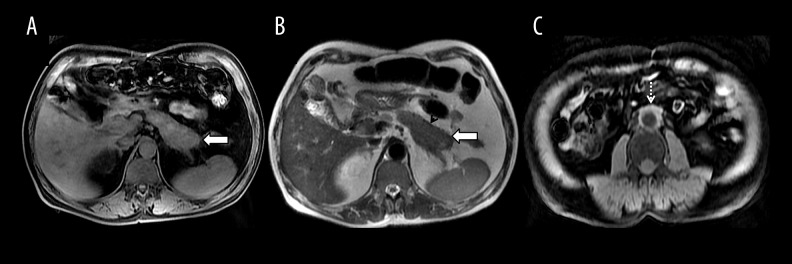
AIP with retroperitoneal involvement: (A) Axial T1-weighted MR image depicting altered T1 signal with a bulky pancreatic tail (arrow) and minimal peripancreatic stranding; (B) T2-weighted MR image showing hypointense pancreatic parenchyma (arrow) with a hypointense rim (arrowhead); (C) Axial T1-weighted MR image showing soft tissue proliferation around the aorta suggestive of periaortitis (dashed arrow).
Figure 11.
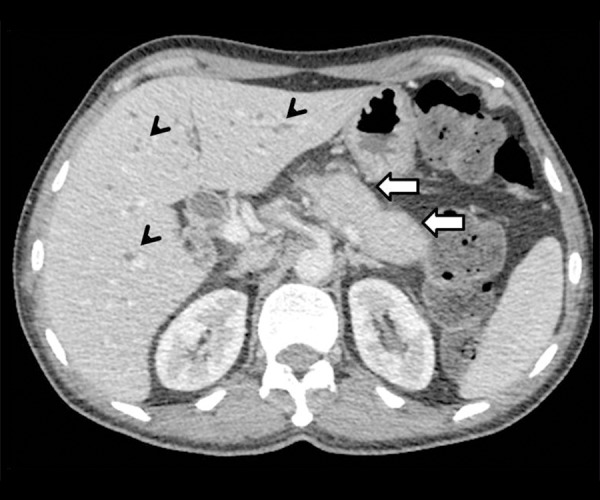
AIP with biliary involvement: Axial contrast-enhanced CT image showing sausage-shaped bulky pancreatic tail (arrow) and skip areas of biliary dilatation (arrowhead).
Figure 12.
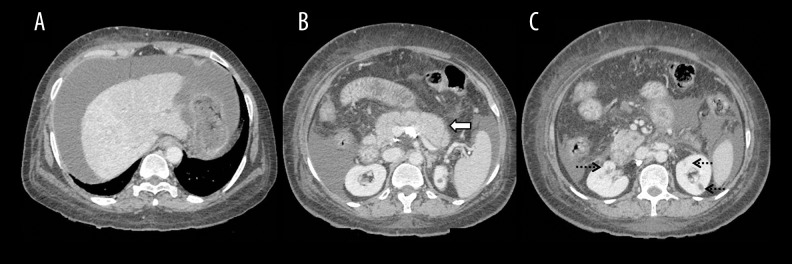
AIP with liver and renal involvement; (A) Axial contrast-enhanced CT image showing changes of autoimmune hepatitis with ascites; (B) Diffuse bulky enlargement of the pancreas (arrow); (C) Hypodense renal lesions (dashed arrows) in a case of IgG4-related systemic disease.
Diagnostic Criteria
Diagnosis of AIP is based on a multi-disciplinary approach with a combination of imaging, serological, histopathological and clinical variables. Moreover, there are disparities in diagnosing AIP worldwide. The Japanese/Asian guidelines, revised in 2009 by Okazaki et al. [25], include only three criteria - imaging, serology and histology. The United States HISORt criteria [26], developed at the Mayo clinic, are based on 5 main diagnostic criteria: histological findings, imaging, serology, other organ involvement and response to steroid therapy.
In 2010, the International Association of Pancreatology (IAP) developed international consensus guidelines to classify definitive and probable AIP type 1 and 2 [9]. The IAP guidelines have taken 5 factors into consideration for diagnosing AIP – imaging, serology, histology, extrapancreatic involvement and steroid responsiveness. For each criterion, there are two levels of evidence: typical or highly suggestive evidence (Level 1) and indeterminate/suggestive evidence (Level 2). Type 1 AIP can be confirmed with a variety of combinations of level 1 and level 2 evidence. Type 2 AIP requires imaging, histology or clinical history of inflammatory bowel disease [3,9]. EUS-guided biopsy is indicated in indeterminate cases.
It is challenging to differentiate radiologically the two types of AIP. However, the published literature shows that a capsule-like rim or halo is more frequently seen in Type 1 AIP. The diffuse type is more common than the focal type in both groups [29]. No statistically significant differences were seen in ductal changes between the two types. A characteristic feature of type 2 AIP, compared to type 1 AIP, is a low frequency of obstructive jaundice, which is related to less common lower bile duct strictures due to a lower prevalence of pancreatic head swelling [30]. Inflammatory bowel disease appears to be particularly associated with type 2 AIP, which is seen in up to 48% of cases [31,32]. Relapses are more common in type 1 and unusual in type 2. Extrapancreatic disease progression is more common in type 1 and unusual in type 2.
Recently, the Mayo Clinic has outlined strategies to divide patients into three categories to distinguish between AIP and pancreatic cancer. According to the Mayo Clinic strategy, patients can be divided into 3 groups: 1) highly suggestive of AIP, 2) indeterminate (supportive of AIP) and 3) highly suggestive of pancreatic cancer. All patients in groups 2 and 3 should undergo a work-up for pancreatic cancer (biopsy of the lesion, CA 19-9 level in serum, and metastasis evaluation) and also a work-up for AIP, because pancreatic cancer is more likely than AIP. The definitive diagnosis of AIP requires a pancreatic core biopsy, steroid trial and sometimes operative intervention [28,33–35].
Some patients may present with features of ordinary chronic, calcific pancreatitis on imaging. Kawa et al. [36], in their study of 51 patients, found a high serum IgG4 concentration in 7.4% of patients with ordinary chronic pancreatitis, suggesting that an advanced stage of AIP with high serum IgG4 may result in the development of this condition. The hypothesis explaining such a nature of chronic pancreatitis changes is increased intraductal pressure, pancreatic juice stagnation leading to stone formation and pancreatic atrophy [37]. Hirano et al. [38] studied isk factors for stone formation in AIP and reported that changes in the character of pancreatic juice due to high alcohol consumption may in part contribute to stone formation in AIP.
In future, serum MAPK-associated miRNAs could become a noninvasive biomarker for the differentiation between pancreatic adenocarcinoma and AIP [39].
Conclusions
AIP is a rare fibroinflammatory, mild variant of chronic pancreatitis. With the increasing use of imaging and improvements in patient care, a large number of patients are being diagnosed. Type 1 AIP is more common and is part of a IgG4-related, systemic disease. Type 2 AIP is difficult to diagnose and is more specific to the pancreas rather than being part of a systemic disease. Identifying imaging features supportive or suggestive of AIP may guide the clinician to making the correct diagnosis.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas – an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–98. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–68. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly DA, Malde DJ, Duncan T, et al. Review of the diagnosis, classification and management of autoimmune pancreatitis. World J Gastrointest Pathophysiol. 2014;5:71–81. doi: 10.4291/wjgp.v5.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klöppel G, Sipos B, Zamboni G, et al. Autoimmune pancreatitis: Histo- and immunopathological features. J Gastroenterol. 2007;42(Suppl 18):28–31. doi: 10.1007/s00535-007-2048-6. [DOI] [PubMed] [Google Scholar]
- 5.Zen Y. The pathology of IgG4-related disease in the bile duct and pancreas. Semin Liver Dis. 2016;36:242–56. doi: 10.1055/s-0036-1584319. [DOI] [PubMed] [Google Scholar]
- 6.Ardila-Suarez O, Abril A, Gómez-Puerta JA. IgG4-related disease: A concise review of the current literature. Reumatol Clin. 2016 doi: 10.1016/j.reuma.2016.05.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Ketwaroo GA, Sheth S. Autoimmune pancreatitis. Gastroenterol Rep (Oxf) 2013;1:27–32. doi: 10.1093/gastro/got011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki K, Tomiyama T, Mitsuyama T, et al. Diagnosis and classification of autoimmune pancreatitis. Autoimmun Rev. 2014;13:451–58. doi: 10.1016/j.autrev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–58. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 10.Shigekawa M, Yamao K, Sawaki A, et al. Is (18)F-fluorodeoxyglucose positron emission tomography meaningful for estimating the efficacy of corticosteroid therapy in patients with autoimmune pancreatitis? J Hepatobiliary Pancreat Sci. 2010;17:269–74. doi: 10.1007/s00534-009-0172-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee TY, Kim MH, Park DH, et al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. Am J Roentgenol. 2009;193:343–48. doi: 10.2214/AJR.08.2297. [DOI] [PubMed] [Google Scholar]
- 12.Negrelli R, Manfredi R, Pedrinolla B, et al. Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. Eur Radiol. 2015;25:359–67. doi: 10.1007/s00330-014-3371-y. [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa T, Sou H, Araki T, et al. Duct-penetrating sign at MRCP: Usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–16. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 14.Dillon J, Dart A, Sutherland T. Imaging features of immunoglobulin G4-related disease. J Med Imaging Radiat Oncol. 2016 doi: 10.1111/1754-9485.12511. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Choi SY, Kim SH, Kang TW, et al. Differentiating mass-forming autoimmune pancreatitis from pancreatic ductal adenocarcinoma on the basis of contrast-enhanced MRI and DWI findings. Am J Roentgenol. 2016;206:291–300. doi: 10.2214/AJR.15.14974. [DOI] [PubMed] [Google Scholar]
- 16.Sureka B, Bansal K. Dual Pathology? Autoimmune pancreatitis and ductal adenocarcinoma. Am J Roentgenol. 2015;205:W643. doi: 10.2214/AJR.15.15205. [DOI] [PubMed] [Google Scholar]
- 17.Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–7. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 18.Shiokawa M, Kodama Y, Yoshimura K, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108:610–17. doi: 10.1038/ajg.2012.465. [DOI] [PubMed] [Google Scholar]
- 19.Witkiewicz AK, Kennedy EP, Kennyon L, et al. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: Case report and review of the literature. Hum Pathol. 2008;39:1548–51. doi: 10.1016/j.humpath.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura Y, Iwamuro M, Ocho K, et al. A rare case of diffuse large B-cell lymphoma in a patient with IgG4-related autoimmune pancreatitis. Acta Med Okayama. 2016;70:279–83. doi: 10.18926/AMO/54504. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Voaklander R, Kasmin FE, et al. Autoimmune pancreatitis: a multiorgan disease presenting a conundrum for clinicians in the west. Gastroenterol Hepatol (NY) 2015;11:606–11. [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22:414–18. doi: 10.1007/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa T, Ohara H, Sano H, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004;60:937–44. doi: 10.1016/s0016-5107(04)02229-1. [DOI] [PubMed] [Google Scholar]
- 24.Anand D, Lall C, Bhosale P, et al. Current update on primary pancreatic lymphoma. Abdom Radiol (NY) 2016;41:347–55. doi: 10.1007/s00261-015-0620-8. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki K, Kawa S, Kamisawa T, et al. Japanese clinical guidelines for autoimmune pancreatitis. Pancreas. 2009;38:849–66. doi: 10.1097/MPA.0b013e3181b9ee1c. [DOI] [PubMed] [Google Scholar]
- 26.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–16. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi R, Frulloni L, Mantovani W, et al. Autoimmune pancreatitis: Pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011;260:428–36. doi: 10.1148/radiol.11101729. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmanova I, Gurlich R, Janik V, et al. Dilemmas in autoimmune pancreatitis. Surgical resection or not? Bratisl Lek Listy. 2016;117:463–67. doi: 10.4149/bll_2016_090. [DOI] [PubMed] [Google Scholar]
- 29.Song TJ, Kim JH, Kim MH, et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol. 2012;27:700–8. doi: 10.1111/j.1440-1746.2011.06934.x. [DOI] [PubMed] [Google Scholar]
- 30.Kawa S, Okazaki K, Notohara K, et al. Autoimmune pancreatitis complicated with inflammatory bowel disease and comparative study of type 1 and type 2 autoimmune pancreatitis. J Gastroenterol. 2015;50:805–15. doi: 10.1007/s00535-014-1012-5. [DOI] [PubMed] [Google Scholar]
- 31.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–76. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532–39. doi: 10.1159/000445234. [DOI] [PubMed] [Google Scholar]
- 33.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097–103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Kamisawa T, Imai M, Yui Chen P, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas. 2008;37:e62–67. doi: 10.1097/MPA.0b013e318175e3a0. [DOI] [PubMed] [Google Scholar]
- 35.Kawa S. Current concepts and diagnosis of IgG4-related pancreatitis (Type 1 AIP) Semin Liver Dis. 2016;36:257–73. doi: 10.1055/s-0036-1584318. [DOI] [PubMed] [Google Scholar]
- 36.Kawa S, Hamano H, Ozaki Y, et al. Long-term follow-up of autoimmune pancreatitis: Characteristics of chronic disease and recurrence. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S18–22. doi: 10.1016/j.cgh.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama M, Arakura N, Ozaki Y, et al. Type 1 autoimmune pancreatitis can transform into chronic pancreatitis: A long-term follow-up study of 73 Japanese patients. Int J Rheumatol. 2013;2013:272595. doi: 10.1155/2013/272595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano K, Tada M, Isayama H, et al. High alcohol consumption increases the risk of pancreatic stone formation and pancreatic atrophy in autoimmune pancreatitis. Pancreas. 2013;42:502–5. doi: 10.1097/MPA.0b013e31826b3984. [DOI] [PubMed] [Google Scholar]
- 39.Akamatsu M, Makino N, Ikeda Y, et al. Specific MAPK-associated MicroRNAs in serum differentiate pancreatic cancer from autoimmune pancreatitis. PLoS One. 2016;11:e0158669. doi: 10.1371/journal.pone.0158669. [DOI] [PMC free article] [PubMed] [Google Scholar]