Abstract
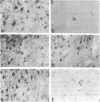
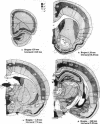
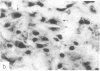
Cellular regulation by certain growth factor receptors and protooncogene products involves tyrosine kinase activity with the resultant tyrosine phosphorylation of protein substrates. In the present report we describe the distribution of phosphotyrosine-containing material detected by immunocytochemistry (ICC) in the rat forebrain. Specificity of the affinity-purified antibody against phosphotyrosine used in the ICC technique was demonstrated by the ability of phosphotyrosine and p-nitrophenyl phosphate but not phosphoserine, phosphothreonine, or L-tyrosine to inhibit the immunostaining reaction. With ICC, relatively high amounts of phosphotyrosine-positive material were observed in neurons in specific structures that included the supraoptic, paraventricular, and arcuate nuclei; the median eminence; medial habenula; subfornical organ; and piriform cortex. Moderate to high amounts were present in the cerebral cortical layers II-IV and in the pyramidal cell layer of the hippocampus. Small to moderate amounts were detected in a few other locations. Glial elements showed minimal staining. Other areas of the rat forebrain failed to react with this antibody. Importantly, the distribution of the areas positive for phosphotyrosine agreed to a remarkable extent with the distribution of the brain insulin receptor, which itself has tyrosine kinase activity. These findings suggest a relationship between the insulin receptor and the increased phosphotyrosine content of these neurons and support the concept that the brain insulin receptor is active in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir S., Shechter Y. Centrally mediated hypoglycemic effect of insulin: apparent involvement of specific insulin receptors. Brain Res. 1987 Aug 18;418(1):152–156. doi: 10.1016/0006-8993(87)90972-3. [DOI] [PubMed] [Google Scholar]
- Baskin D. G., Figlewicz D. P., Woods S. C., Porte D., Jr, Dorsa D. M. Insulin in the brain. Annu Rev Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- Baskin D. G., Wilcox B. J., Figlewicz D. P., Dorsa D. M. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988 Mar;11(3):107–111. doi: 10.1016/0166-2236(88)90155-5. [DOI] [PubMed] [Google Scholar]
- Bohannon N. J., Corp E. S., Wilcox B. J., Figlewicz D. P., Dorsa D. M., Baskin D. G. Localization of binding sites for insulin-like growth factor-I (IGF-I) in the rat brain by quantitative autoradiography. Brain Res. 1988 Mar 22;444(2):205–213. doi: 10.1016/0006-8993(88)90931-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp E. S., Woods S. C., Porte D., Jr, Dorsa D. M., Figlewicz D. P., Baskin D. G. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett. 1986 Sep 25;70(1):17–22. doi: 10.1016/0304-3940(86)90430-1. [DOI] [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P. R., Wood J. G., Freschi J. E., Kuo J. F. Immunocytochemical localization of protein kinase C in developing brain tissue and in primary neuronal cultures. Dev Biol. 1988 Mar;126(1):98–107. doi: 10.1016/0012-1606(88)90243-6. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Lesniak M. A., Pert C. B., Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986 Apr;17(4):1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Hirano A. A., Greengard P., Huganir R. L. Protein tyrosine kinase activity and its endogenous substrates in rat brain: a subcellular and regional survey. J Neurochem. 1988 May;50(5):1447–1455. doi: 10.1111/j.1471-4159.1988.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Hill J. M., Kiess W., Rojeski M., Pert C. B., Roth J. Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology. 1988 Oct;123(4):2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, LeRoith D. Tyrosine kinase activity of brain insulin and IGF-1 receptors. Biochem Biophys Res Commun. 1986 Jan 29;134(2):532–538. doi: 10.1016/s0006-291x(86)80453-3. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Sorge L. K., Fults D. W. An early developmental phase of pp60c-src expression in the neural ectoderm. Dev Biol. 1986 Sep;117(1):83–89. doi: 10.1016/0012-1606(86)90350-7. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M. Partial purification and characterization of a pp60v-src-related tyrosine kinase from bovine brain. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6025–6029. doi: 10.1073/pnas.82.18.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Okada M., Nakagawa H. Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem Biophys Res Commun. 1988 Jul 29;154(2):796–802. doi: 10.1016/0006-291x(88)90210-0. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A. Purification of the catalytically active phosphorylated form of insulin receptor kinase by affinity chromatography with O-phosphotyrosyl-binding antibodies. Arch Biochem Biophys. 1985 Oct;242(1):176–186. doi: 10.1016/0003-9861(85)90491-6. [DOI] [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hendricks S. A., Quarum M., Roth J. The insulin receptor of rat brain is coupled to tyrosine kinase activity. J Biol Chem. 1984 Mar 25;259(6):3470–3474. [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Unger J., McNeill T. H., Moxley R. T., 3rd, White M., Moss A., Livingston J. N. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31(1):143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Lustig A., Greengard P., Brugge J. S. Widespread distribution of the c-src gene product in nerve cells and axon terminals in the adult rat brain. Brain Res. 1988 Jun;427(3):215–222. doi: 10.1016/0169-328x(88)90044-7. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. Regional distribution of calcium- and cyclic adenosine 3':5'-monophosphate-regulated protein phosphorylation systems in mammalian brain. II. Soluble systems. J Neurosci. 1983 Feb;3(2):302–311. doi: 10.1523/JNEUROSCI.03-02-00302.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werther G. A., Hogg A., Oldfield B. J., McKinley M. J., Figdor R., Allen A. M., Mendelsohn F. A. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987 Oct;121(4):1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Wiestler O. D., Walter G. Developmental expression of two forms of pp60c-src in mouse brain. Mol Cell Biol. 1988 Jan;8(1):502–504. doi: 10.1128/mcb.8.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]