Abstract
Monocyte transendothelial migration is a multi-step process critical for the initiation and development of atherosclerosis. The chemokine monocyte chemoattractant protein-1 (MCP-1) is overexpressed during atheroma and its concentration gradients in the extracellular matrix (ECM) is critical for the transendothelial recruitment of monocytes. Based on prior observations, we hypothesize that both free and bound gradients of MCP-1 within the ECM are involved in directing monocyte migration. The interaction between a three-dimensional (3D), cell-free, collagen matrix and MCP-1; and its effect on monocyte migration was measured in this study. Our results showed such an interaction existed between MCP-1 and collagen, as 26% of the total MCP-1 added to the collagen matrix was bound to the matrix after extensive washes. We also characterized the collagen-MCP-1 interaction using biophysical techniques. The treatment of the collagen matrix with MCP-1 lead to increased monocyte migration, and this phenotype was abrogated by treating the matrix with an anti-MCP-1 antibody. Thus, our results indicate a binding interaction between MCP-1 and the collagen matrix, which could elicit a haptotactic effect on monocyte migration. A better understanding of such mechanisms controlling monocyte migration will help identify target cytokines and lead to the development of better anti-inflammatory therapeutic strategies.
Keywords: Atherosclerosis, Monocyte chemoattractant protein-1, Monocyte migration
1. Introduction
Atherosclerosis, the process of plaque formation and stenosis in the arteries, is the underlying cause of most cardiovascular diseases, including heart failure, stroke and coronary artery diseases; which continues to be one of the main causes of death in the western world [1, 2]. The initiation of atherosclerosis is caused by an inflammatory response elicited by lipoproteins retained to the arterial intima, which leads to the recruitment of inflammatory cells to the site of the insult [1, 3]. The processes by which the inflammatory cells, including monocytes, T and B cells, and lipid-laden macrophages [1, 4, 5] are recruited into the atherosclerotic plaques include the tethering and slow rolling of these cells on activated endothelial cells, followed by their adhesion to the endothelium, intravascular crawling, and ultimately their transmigration across the endothelial layer [3, 6]. Cellular adhesion molecules and chemokines play critical roles in these processes [3, 7].
Monocyte chemoattractant protein-1 (MCP-1) or chemokine ligand 2 (CCL-2), is a chemokine that is involved in the trafficking of monocytes across the endothelium [3, 8, 9]. MCP-1 is produced by a variety of cell types, including fibroblasts, endothelial and smooth muscle cells, astrocytes and monocytes: either constitutively or after induction by cytokines [8]. MCP-1 acts through interaction with chemokine receptor type 2 (CCR2) to regulate transendothelial migration of monocytes, memory T lymphocytes, and natural killer (NK) cells [8]. A number of reports show that MCP-1 is highly expressed in atherosclerotic lesions, and thus is the potential intervention point for the treatment of atherosclerosis [9–11]. Previous studies using knockout mice have shown that in the absence of either of MCP-1 or CCR2, there was a significant reduction in lipid deposition in the arteries, leading to a reduction in the formation of atherosclerotic lesions [4, 5, 12]. Many in vitro studies demonstrated that the migration of monocytes is directed by a chemotactic gradient of free MCP-1; however, information about the formation of such a gradient across the endothelial layer is limited [13–15]. It is known that MCP-1 is secreted from endothelial cells in a soluble form [15], and when these cells are stimulated the secretion of MCP-1 becomes non-polarized [14]. Based on these two findings, it was suggested that in vivo, MCP-1 secreted from the apical side of the endothelium was removed continuously by blood flow into the vascular lumen, while the MCP-1 secreted from the basal side diffused into the extracellular matrix (ECM), forming a transendothelial MCP-1 gradient [14, 15].
Previously, using a mathematical model, we have examined the formation of such MCP-1 concentration gradient in the ECM, in the form of a cell free, three dimensional (3D) collagen matrix [16]. We proposed that the free MCP-1 was capable of binding to the collagen matrix to form a bound MCP-1-collagen binding site complex, thus establishing a bound MCP-1 concentration gradient in the collagen matrix [16].
In the current study, we test the hypothesis that MCP-1 is indeed bound to the collagen matrix, and presents a haptotactic effect on the migration of monocytes. The objectives of this work were to examine the biophysical behavior of such interaction between the collagen matrix and MCP-1; and to examine the migration of monocytes in response to the MCP-1 bound to the collagen matrix. The results of this study will further our understanding of the interaction of MCP-1 with the ECM, and shed light on the role of MCP-1 on the recruitment of monocytes to the atherosclerotic plaques.
2. Materials and methods
2.1 Preparing the cell free 3D in vitro model
The collagen matrix was prepared using previously described protocols [16, 17]. Briefly, a 57.1 vol% type I bovine collagen solution was added to the wells of a solid well plate (Corning Life Sciences, Cambridge, MA) and incubated at 37°C, 5% CO2 (defined in the text as ‘standard conditions’) to form a gel. Complete endothelial cell growth medium (PromoCell, Heidelberg, Germany) was added to the top of the matrix and incubated overnight at standard conditions for equilibration of the matrix. The complete growth medium contained the basal medium plus supplements of fetal calf serum (2% v/v), endothelial cell growth supplement, epidermal growth factor, basic fibroblast growth factor, heparin, and hydrocortisone.
2.2 Determining the collagen sol-gel polymerization shrinkage
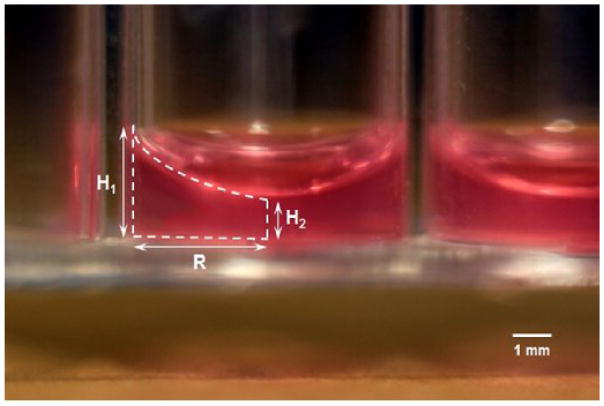
To measure the volume shrinkage percentage of the collagen matrix, 50, 75, and 100 μL collagen solutions were added to a 96-well plate and incubated at standard conditions to form matrices. Complete endothelial cell growth medium was then added to the matrices and samples were incubated at standard conditions overnight for equilibration. A side view image of the collagen matrix in a well is shown in Fig. 1. Thickness of the initial collagen solution and the final collagen matrix was measured at wall-contact and center of the well (marked as H1 and H2, respectively), using ImageJ (version 1.49t) software on an image of a standard thickness. Having the above-mentioned thicknesses (H1 and H2) and the radius of the well (R), the equations used to calculate the volume of the cylinder (Vcylinder) and the volume of half of the ellipse (Vhalf of ellipse) were used to calculate the volume of the collagen matrix (Vmatrix):
Figure 1.
Side view of the collagen matrix with an initial collagen solution volume of 75 μl (50× magnification). Representative image of triplicate samples.
Based on the initial collagen solution volume (Vsol) and the final collagen matrix (Vmatrix), the polymerization shrinkage percentage of the sol-gel collagen (PSPsol-gel) was determined using Eq. 1:
| Eq. 1 |
2.3 Characterizing the MCP-1 and collagen matrix interaction
2.3.1 Enzyme-linked immunosorbent assay (ELISA)
A collagen matrix was prepared in a 96-well plate with 75 μL collagen solution, as described above. Recombinant human CCL2/MCP-1 (R&D Systems, Minneapolis, MN; 50 ng/ml in 0.15 ml complete endothelial cell growth media; defined in the text as ‘complete media’) was added to the top of the matrix. The plate was incubated for 24 h under standard conditions, after which the matrix was washed ten times (10X) with complete media (1 h/wash). To quantify MCP-1 concentration in the collagen matrix, the matrix was digested after each wash with collagenase D (Roche Applied Science, Indianapolis, IN), and the MCP-1 concentration was measured using a Human MCP-1 sandwich ELISA kit (BD Biosciences, San Jose, CA) following the manufacturer’s protocol. To ensure that there was no loss of MCP-1 during the digestion of the collagen matrix, we also measured the amount of free MCP-1 in the wash solutions and used a mass balance to calculate the amount of MCP-1 in the collagen matrix.
2.3.2 Fluorescent Labeling
Recombinant human CCL2/MCP-1 CF (R&D Systems, Minneapolis, MN) was labeled with a fluorescent tag by using the Mix-n-Stain™ CF™ 555 protein labeling kit (Sigma-Aldrich, St. Louis, MO). The final concentration of labeled MCP-1 was measured by ELISA. Labeled MCP-1 (50 ng/ml, in 0.15 ml complete endothelial cell growth media) was added to the top of the collagen matrix samples and incubated for 24 h under standard conditions. After 24 h, some samples were washed 5X to remove any unbound MCP-1. Collagen matrices incubated with supplemented cell growth media without fluorescent MCP-1 (with and without washes) were used as controls. The fluorescent intensities of the collagen matrices containing MCP-1 (with and without five washes) were measured using a fluorescent microplate reader (Multimode DTX880, Molecular Devices, Sunnyvale, CA) at excitation and emission spectra of 535 and 565 nm and compared to the control samples.
2.3.3 Fourier transform-infrared (FT-IR) spectroscopy
Collagen matrices pretreated with MCP-1 (50 ng/ml in 0.15 ml complete endothelial cell growth media) samples were prepared in a 96-well plate format with or without six washes. Collagen matrices, incubated with endothelial cell growth medium with and without supplements, were used as negative controls. Samples were removed, mounted on slides, and air-dried overnight at room temperature. Infrared spectra were recorded with an FT-IR spectrometer (model Tensor 27, Bruker, Billerica, MA) equipped with a liquid nitrogen-cooled detector and an FT-IR microscope (model Hyperion 2000-ATR, Bruker, Billerica, MA) in the wavelength range 4,000 - 650 cm−1 at 16 different measurement positions for each sample. For each spectrum, 64 scans were collected with a 4 cm−1 resolution and a 64-scan background was recorded subsequently. Data manipulation was performed using the OPUS (version 7.2) software. Primary spectra were normalized followed by baseline correction for direct comparison between samples.
2.3.4 Thermogravimetric analyses (TGA)
Collagen matrices pretreated with MCP-1 (50 ng/ml in 1.5 ml complete endothelial cell growth media) were prepared in 12-well plates, as described above. Bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO) was used as a control sample. Samples were transferred to conical vials, frozen at −80 °C for 12 h, lyophilized for 48 h, and centrifuged (5,000 rpm, 5 min) to recover the dried powder. TGA of samples was performed in triplicate to investigate the thermal behavior of the binding between MCP-1 and the collagen matrix using a thermogravimetric analyzer (model Q50, TA Instruments, New Castle, DE). Samples were heated from room temperature to 950 °C at a heating rate of 20 °C/min under nitrogen flow (40 ml/min).
2.3.5 Differential scanning calorimetry (DSC)
Collagen matrices pretreated with MCP-1 were prepared as described above. DSC measurements of 5 mg samples, in triplicate, were analyzed using a Model Q2000 DSC (TA Instruments, New Castle, DE). Temperature-modulated differential scanning calorimetry (TMDSC) was performed under an atmosphere of nitrogen (flow rate 50 ml/min). Samples were incubated at −50 °C for 2 min, and then heated to 200 °C at the rate of 3 °C/min with a modulation amplitude and period of ±1.0 °C and 60 s, respectively. Empty pans were used as references. The denaturation enthalpy (ΔH) and the phase transition temperatures (Tm), which relates to the thermal denaturation temperature of samples, were determined.
2.4 Monocyte isolation
Blood was obtained from healthy donors from the Oklahoma Blood Institute (OBI) (Oklahoma City, OK). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden), a pan-monocyte isolation kit (Miltenyi Biotech, Auburn, CA) was used to negatively select monocytes from the PBMC population following the manufacturer’s protocol.
2.5 Monocyte adhesion and migration assay
To study the effect of free and collagen-bound MCP-1 on monocyte adhesion and migration, MCP-1 (25 ng/ml in 0.5 ml complete endothelial cell growth media) was added to the top of the collagen matrix and incubated for 24 h. The collagen matrix was washed 6 times with complete medium to remove the free MCP-1. Samples with and without total MCP-1 were used as positive and negative controls, respectively.
Complete media containing monocytes (1.5×105 cells/cm2 in 0.5 ml media) was added to the top of the collagen matrix and incubated at standard conditions for 2 h to allow for the monocytes to adhere to and migrate into the matrix. To count the number of attached and migrated monocytes, the samples were rinsed and then fixed with paraformaldehyde (4% v/v in PBS, Fisher Scientific, Pittsburgh, PA). The number of monocytes that adhered to and migrated into the collagen matrix was counted using phase contrast microscopy in five different fields of view, at 100× magnification.
The effect of bound MCP-1 on monocyte adhesion and migration was also studied by neutralizing the bound MCP-1 in the collagen matrix using a MCP-1 antibody. In this process, the matrix was washed 6 times and an excess of MCP-1 antibody (3 μg/ml in 0.5 ml complete endothelial cell growth media) was added. After incubating the matrix for 1 h, the neutralizing antibody (neutralizing Ab) was removed, and monocyte adhesion and migration assay was performed as described above.
2.6 Statistical analyses
All experiments were performed in triplicate and results expressed as mean ± standard deviation (SD). Differences between the experimental groups and control samples were determined using Student’s t-test with a p value < 0.05 considered significant.
3. Results
3.1 Collagen sol-gel polymerization shrinkage percentage (PSP)
Volume shrinkage occurs when collagen monomers in a solution crosslink at elevated incubation temperatures, forming a polymeric gel matrix. Measuring the final volume of the collagen matrix was critical for the calculation of the concentration of MCP-1 dispersed in the collagen matrix. As shown in Table 1, the collagen PSP did not change significantly with the volume of the initial collagen solution, suggesting that the collagen PSP is independent of the initial solution volume.
Table 1.
Polymerization shrinking percentage calculated for each soluble volume of collagen used to make the matrix.
| Collagen Type I | |
|---|---|
| Sol volume (μL) | Sol-Gel PSP (vol. %) |
| 50 | 19.06 ±4.63 |
| 75 | 18.94 ±1.57 |
| 100 | 19.20 ±2.53 |
3.2 MCP-1 Concentration in the collagen matrix
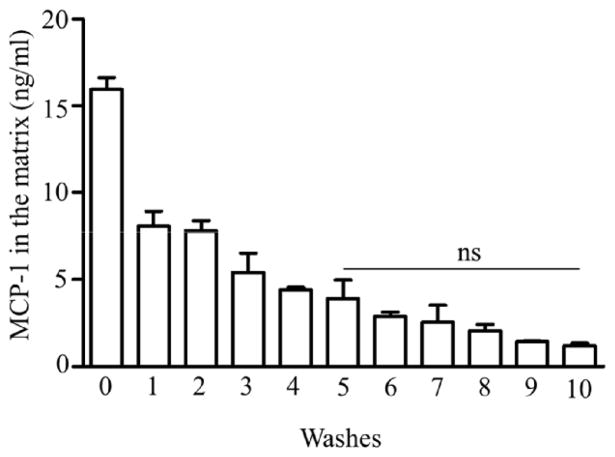
ELISA was used to determine the concentration of MCP-1 retained in the collagen matrix. After a 24 h incubation with MCP-1 and after each successive wash, the collagen matrix was digested and the amount of MCP-1 was measured by ELISA. Approximately 16 ng/ml MCP-1 diffused into the collagen matrix after 24 h incubation, prior to the wash steps. The results, represented in Fig. 2, show that the MCP-1 concentration in the collagen matrix decreased gradually with each wash, until the fifth wash. After the fifth wash, 5 ng/ml MCP-1 was retained in the collagen matrix, and no significant decrease in the retained MCP-1 concentration was observed with further washes (Fig. 2). This indicated retention of MCP-1 within the collagen matrix, possibly through an interaction between MCP-1 and collagen.
Figure 2.
The concentration of MCP-1 (ng/ml) retained in the collagen matrix after each wash. Collagen matrices were pretreated with MCP-1 and incubated for 24 h. The concentration of MCP-1 was determined by ELISA (as described in Materials and Methods). Values are presented as mean ± SD; n=3.
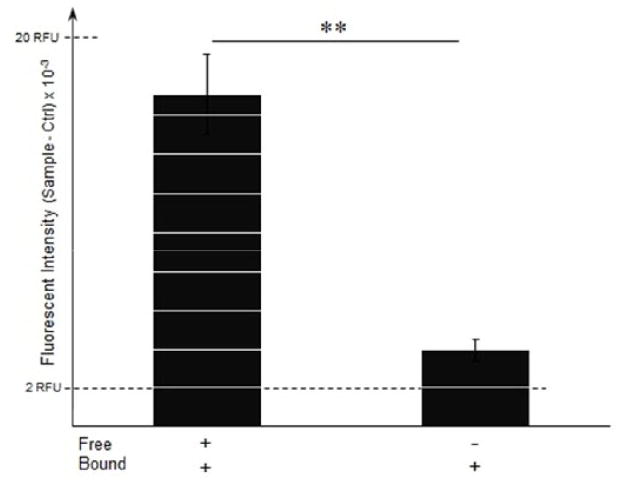
We verified the interaction of MCP-1 with the collagen matrix by using fluorescent labeled MCP-1. We measured the fluorescence intensities of collagen matrix samples that were incubated with labeled MCP-1, with and without five washes. Relative fluorescence unit (RFU) of samples were subtracted from the fluorescence intensities of control samples and shown in Fig. 3. Results show that the RFU of the matrices with free MCP-1 (without washes) was 4.4-fold higher when compared to the matrices with bound MCP-1 (after 5 washes; p < 0.01). These results also suggest that the labeled MCP-1 was retained in the collagen matrix, as its intensity was 1.6-fold higher than the control (p < 0.01). Taken together, these results clearly show that MCP-1 is retained within the collagen matrix, suggesting a possible binding between MCP-1 and the collagen matrix.
Figure 3.
Fluorescence measurements of labeled MCP-1 in the collagen matrix. Collagen matrices were pretreated with fluorescently labeled MCP-1 (50 ng/ml) and incubated for 24 h. Relative fluorescent units (RFU) of the collagen matrices at the end of the 24 h incubation and after five washes were measured at excitation/emission of 535/565 nm. Values are presented as mean ± SD of fluorescence intensities of samples subtracted from the background (only collagen); n=4; **p < 0.01 for change in fluorescence intensity of samples without (left) and with 5X washes (right).
3.3 Functional group characterization of MCP-1 and collagen binding
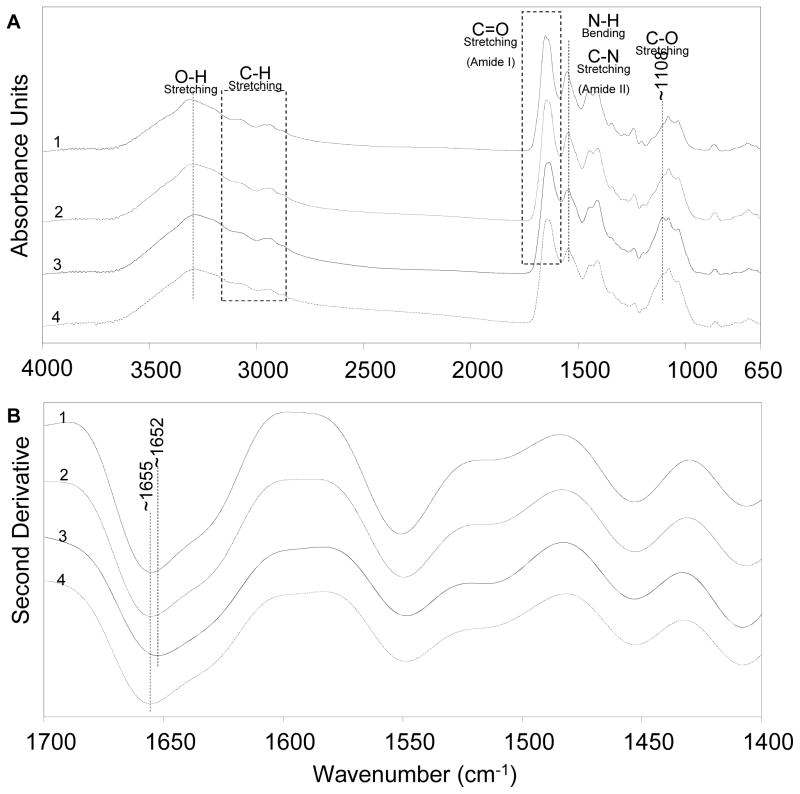
FT-IR spectra of collagen samples with or without serum (i.e., supplements); and total or bound MCP-1 are shown Fig. 4. The spectra are offset vertically for clarity. The FT-IR spectra in Fig. 4A shows major bands related to the O-H stretch (3200–3500 cm−1), the C-H stretch (2800–3200 cm−1), the amide I C=O stretch (1600–1700 cm−1), and the amide II N-H bend and C-N stretch (1500–1600 cm−1). These bands were observed in all the samples. However, the stretching vibration of the C-O peak was observed at ~1108 cm−1 for the collagen matrix containing total MCP-1, but not for the collagen matrix without MCP-1. The collagen matrix containing bound MCP-1 also shows a shoulder at the same wavelength as the C-O peak, but with lower intensity than the collagen matrix with total (bound and free) MCP-1.
Figure 4.
(A) Primary and (B) second derivative FT-IR absorption spectra of collagen matrices: (1) without supplements, (2) with supplements, (3), with supplements and bound MCP-1, and (4) with supplements and total MCP-1. Collagen matrices were pretreated with MCP-1 (50 ng/ml) and incubated for 24 h. For the MCP-1 treatment, the collagen matrix was collected from each well at the end of the 24 h incubation (total MCP-1) and after five washes (bound MCP-1). The spectra are the average of triplicate samples.
The second derivatives of the spectra for the collagen matrices are shown in Fig. 4B. The C=O peak for collagen matrices without MCP-1 was shifted 3 cm−1 to a lower frequency when the total MCP-1 was present in the collagen matrix. All other bands in the original and the second derivative spectra remained unchanged. The results show that presence of MCP-1 affects certain functional groups shown in the spectra for the collagen matrix, such as creating an intermolecular C-O interaction and formation of hydrogen bonding between O-H of MCP-1 and C=O of the collagen matrix (shift of the amide I band) within the samples.
3.4 Thermal characterization of MCP-1 and collagen binding
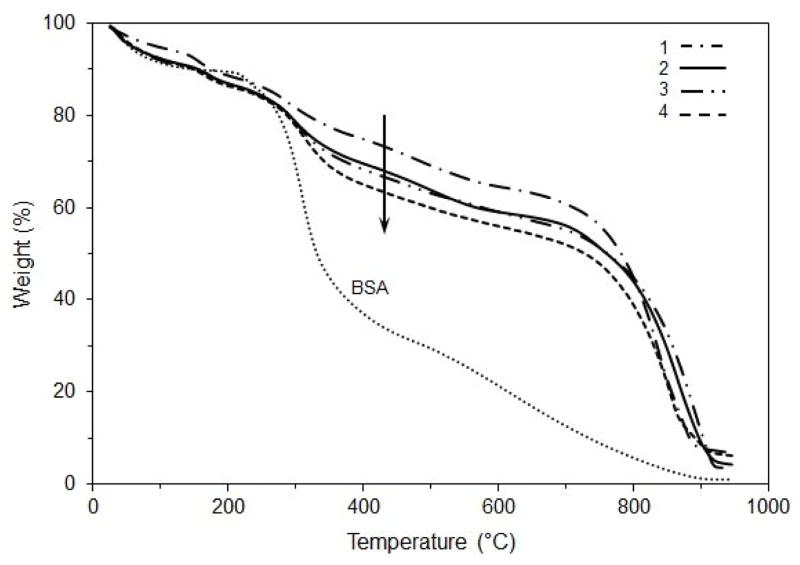
The TGA weight loss curves of a collagen matrix with bound MCP-1 and a matrix with total (bound and free) MCP-1 are shown in Fig. 5. A pure sample of BSA was used as a standard for comparison. The results show that the main decomposition step for BSA occurred in the range of 250–350 °C. Collagen matrices containing supplements (1, 2, and 3) show a decomposition step in the range of 250 – 300 °C that could be attributed to the loss of supplements. Collagen matrices containing MCP-1 (3 and 4) show a loss of MCP-1 in the 300–480 °C range. The collagen matrix containing only bound MCP-1 shows a decomposition profile between the control collagen matrix (without MCP-1 pretreatment) and the collagen matrix containing total MCP-1. All collagen matrices decomposed in the same range with the major step occurring between 700 and 900 °C. Taken together, these results clearly show that MCP-1 in the collagen matrix results in loss at lower temperatures that is distinctly different than for collagen alone.
Figure 5.
Thermogravimetric traces of BSA (bovine serum albumin) and the collagen matrices: (1) without supplements, (2) with supplements, (3) with supplements and bound MCP-1, and (4) with supplements and total MCP-1 for determining the effect of MCP-1 on denaturation of the collagen matrices. Collagen matrices were pretreated with MCP-1 (50 ng/ml) and incubated for 24 h. For MCP-1 treatment, collagen matrices were collected from each well at the end of the 24 h incubation (total MCP-1) and after five washes (bound MCP-1). The curves are the average of triplicate samples.
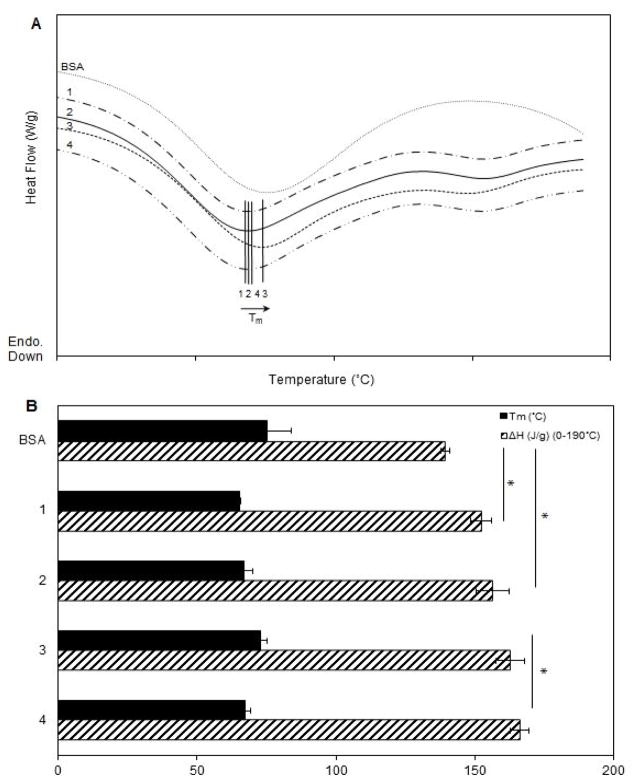
The DSC curves, Fig. 6A, show the endothermic peaks attributed to the denaturation of the samples. The thermograms were shifted vertically for clarity. The denaturation point (Tm) was obtained from the positions of the minima in the denaturation curves (Fig. 6A). By integrating the area under the peaks, the heats of fusion (ΔH) for each of the samples were calculated (Fig. 6B). The results show that the average Tm increased slightly for the collagen matrix containing total MCP-1, compared to the matrix with bound MCP-1 (1.1-fold, p < 0.05) and the matrix without MCP-1 (1.1-fold, p = 0.07). The collagen matrix containing bound MCP-1 had a larger melting enthalpy compared to the collagen matrix without MCP-1. The increase of enthalpy in the matrices containing either bound or total (i.e., bound and free) MCP-1 compared to the collagen matrices without MCP-1 support the attractive interactions between MCP-1 and collagen. The presence of MCP-1 enhances the thermal stability of the matrix.
Figure 6.
Differential scanning calorimetry thermograms (first heating) obtained with heating rate of 3°C/min. (B) Melting point temperatures and enthalpies of samples: BSA (bovine serum albumin) and the collagen matrices: (1) without supplements, (2) with supplements, (3) with supplements and bound MCP-1, and (4) with supplements and total MCP-1. Collagen matrices were pretreated with 50 ng/ml MCP-1 and incubated for 24 h. For MCP-1 treatment, collagen matrices were collected from each well at the end of the 24 h incubation (total MCP-1) and after five washes (bound MCP-1). Data are mean ± SD; n=3; * indicates p value of 0.05.
3.5 Determining the effect of free vs. bound MCP-1 on monocyte adhesion and migration assay
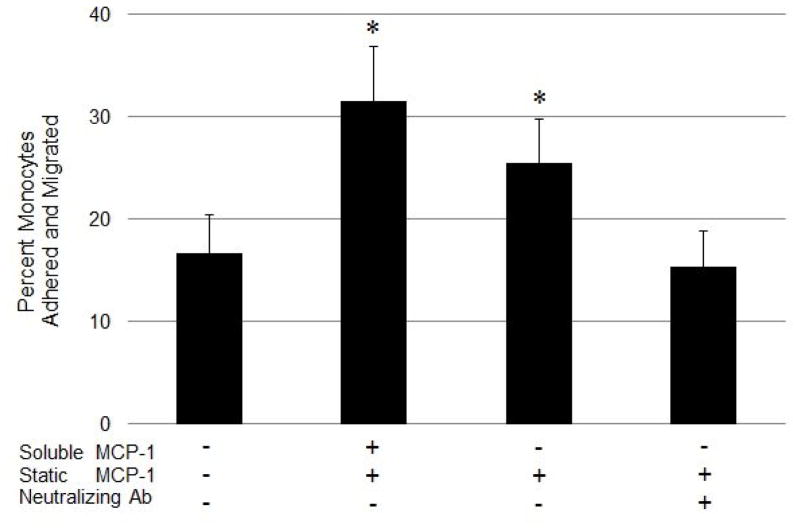
Monocytes were added to the top of the collagen matrix that was pre-treated with MCP-1, while collagen matrices without MCP-1 were used as control. The percentage of monocytes that adhered to and migrated into the collagen matrix 2 h post-addition is presented in Fig. 7. The results show that the matrices pre-treated with MCP-1 had a 1.9-fold higher number of adhered and migrated monocytes, when compared to the control matrices without MCP-1 (p < 0.05). Our results also show that the percentage of monocytes in the matrices with only the bound MCP-1 fraction was not significantly different from that of the matrices containing total (i.e., free and bound) MCP-1. Since we have established that free MCP-1 could be completely removed after five washes, this observation showed that the haptotactic (bound) gradient of MCP-1 had a similar effect on monocyte migration as the chemotactic (free as well as bound) gradient of MCP-1, with a significant increase (1.5-fold, p < 0.05) in the number of migrated monocytes compared to the control matrices without MCP-1.
Figure 7.
Monocyte adherence and migration into collagen matrix in response to MCP-1 gradients. Collagen matrices were pretreated with 25 ng/ml MCP-1 and incubated for 24 h. Samples with no wash (total MCP-1), washed 6X (bound MCP-1), or washed 6X and treated with an excess of MCP-1 neutralizing antibody (neutralizing Ab) were compared. A sample without the addition of MCP-1 was used as a negative control. Human monocytes (1.5*105 cells/cm2 in 0.5 ml media) were added on top of the collagen matrices and the wells were incubated at standard conditions for 2 h. At the end of the incubation time, the top surface was rinsed and the number of monocytes that adhered or migrated in the collagen matrix was counted. Values are presented as mean ± SD of the percent of attached and migrated monocytes normalized to the initial number of monocytes added to each well; n=3; *p < 0.05 for change in percentage of adhered and migrated monocytes compared to control samples lacking MCP-1 treatment.
We also studied the specificity of the MCP-1 gradient on monocyte migration by neutralizing the available MCP-1 with a specific MCP-1 antibody. The results show that there was no significant difference in monocyte migration between the antibody-treated matrices and the control matrices, which were not pre-treated with MCP-1. These results also show that the number of monocytes that migrated through the matrices treated with the MCP-1 antibody was significantly lower (1.6-fold, p < 0.05) than the matrices with bound MCP-1 that did not receive the antibody treatment. Our results clearly show that treatment with the MCP-1 antibody significantly abrogated the MCP-1-induced monocyte migration through the collagen matrix. This supports our hypothesis that the interaction between MCP-1 and the collagen matrix, and the possible resultant MCP-1 concentration gradient within the matrix, induce monocyte trafficking through the collagen matrix.
4. Discussion
Monocyte trafficking across the endothelium is a key event during early stages of atherosclerosis and is promoted by a concentration gradient of free MCP-1 [13, 14, 18]. However, the understanding of the formation of such a gradient in the subendothelial matrix is still limited. Previously, we have established a mathematical model of MCP-1 distribution in a collagen matrix [16]. Our current study has further probed the MCP-1-collagen interaction and the possible role of the haptotactic effect of MCP-1 on monocyte recruitment. Chemokines that are retained in tissue compartments have been shown to contribute to immune cell recruitment and adhesion. For example, IL-8 retained in the lung and skin contribute to neutrophil recruitment; while RANTES and MIP-1β retained on endothelial cells help in the adhesion of CD4+ T cells [19, 20]. Our results show that even after extensive washes, approximately 26 mass% of the total MCP-1 dispersed within the collagen matrix was retained in the collagen matrix. MCP-1 has been shown to remain stable under standard conditions for more than 24 h, and shows no significant degradation over a short term storage of less than four days [21]. Therefore, it may be assumed that the MCP-1 fraction retained in the collagen matrix in our experiments was stable and possibly bound to the matrix.
We have demonstrated the physical and thermal effects of MCP-1 interaction with collagen, by studying the biophysical changes occurring within the collagen matrix using FT-IR, DSC, and TGA. The initial concentrations of MCP-1 included in this study were physiologically relevant and were selected based on in vivo and in vitro experiments that measured the release of MCP-1 from cells [22–27], and the use of MCP-1 to direct cell migration [28–32]. With MCP-1 concentrations in this range, the techniques were used to both detect MCP-1 directly, and to characterize the physical and thermal effects of the interaction between MCP-1 and the collagen matrix.
We also used FT-IR to study the behavior of collagen upon MCP-1 interaction. There is no published FT-IR data for chemokines, such as MCP-1, due to the large amounts of protein required for the analysis. However, the FT-IR spectra for the collagen matrix without MCP-1 obtained from our study were consistent with the characteristic spectra of pure collagen reported by previous studies [33, 34]. FT-IR spectra obtained from our experiments demonstrated differences between samples with total and bound MCP-1.
We used DSC and TGA to study the thermal effects of MCP-1 interaction with collagen. There are several reports in which the thermal behavior of collagen have been studied using these techniques [35–38], but none with the addition of MCP-1. We observed higher denaturation temperatures in collagen matrices which were treated with MCP-1, suggesting the effects of binding sites and strong interactions, such as hydrogen bonding, on increasing the Tm and enthalpy. Previous reports have also shown that the denaturation temperature and crystallinity of the collagen complexes increase upon binding to other molecules (such as Ponceau SS), possibly due to an increase in the intermolecular crosslinking. Collagen has a structure with three left-handed helices, which unfold to make a random chain at higher temperatures [36]. Thus, the increased stability of collagen matrices upon MCP-1 binding could be due to the complexation of the collagen matrix by MCP-1, leading to resistance of the binding sites to unfolding. Miles and Baily have also shown that the only factor which determines the denaturation endotherm behavior of collagen is its rate of unfolding [36]. The fluorescent labeling of MCP-1 used in this study, did not affect its binding to the collagen matrix, as demonstrated by similar concentrations obtained from calculations of fluorescence intensities compared to the ELISA results (data not shown). Previous reports have also suggested that chemokine ligands modified for fluorescent labeling did not affect their binding to receptors [39, 40]. Chemokines are monomers at physiological concentrations in free solutions [41, 42]. In this study, the MCP-1 obtained after digesting the collagen matrix was able to bind to a capture antibody and be detected by ELISA, suggesting that the collagen-bound MCP-1 could be monomeric. In fact, the monomeric form of MCP-1 is known to bind to and activate CCR2 [43]; whereas its dimeric form cannot bind to CCR2 at concentrations up to 1 μM [44]. We did not observe any functional changes, as shown by the ELISA results, when we incubated nanomolar (i.e., monomeric) concentrations of MCP-1 (data not shown).
Our results show that the MCP-1 bound to the collagen matrix could elicit monocyte migration, and that this response was specifically due to MCP-1, was demonstrated as the administration of an anti-MCP-1 antibody led to a significant reduction in monocyte migration. We also observed that the collagen-bound MCP-1 elicited a larger monocyte migration response when compared to the free MCP-1. This clearly shows the presence of an active, haptotactic gradient of collagen-bound MCP-1, and implies that the MCP-1 bound to the collagen of atherosclerotic plaques may be responsible for the recruitment of monocytes to the site of inflammation in vivo. We are conducting further experiments in order to study the in vivo interaction of MCP-1 and collagen to get a better understanding of atherosclerotic inflammation.
Previous reports have shown that the N-terminal of MCP-1 was critical for its function and receptor specificity [45–47]; and as the N-terminals were “buried” in the dimer form, the dimerized CC chemokines do not participate in ligand-receptor interactions [44, 46]. In this light, it may be possible that the MCP-1 in the collagen matrix remains in its monomeric form with an active N-terminal. Furthermore, since the MCP-1 bound to the collagen matrix remained active, it is possible that it has similar conformation and receptor binding characteristics as free MCP-1. Our future work will include understanding the structure of the collagen-bound MCP-1, to further study its possible binding to the collagen matrix and the effect of the MCP-1 N-terminal on monocyte migration. In conclusion, our results show a definite biophysical interaction between MCP-1 and the collagen matrix, and demonstrate the haptotactic effect of the MCP-1 bound to the collagen matrix in directing monocyte migration.
Acknowledgments
This work was supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (1R15EB009527-01). The authors would like to thank Baron Vazindel and Dr. Thomas J. Tague at Bruker Optics Inc. (Fremont, CA) for their assistance during experimentation and data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1(2006):297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 1999. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Boring L, et al. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 5.Gu L, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat RevDrug Discov. 2010;9:141–53. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 8.Deshmane SL, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melgarejo E, et al. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Celll Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Kusano KF, et al. Significance of the level of monocyte chemoattractant protein-1 in human atherosclerosis. Circ J. 2004;68:671–6. doi: 10.1253/circj.68.671. [DOI] [PubMed] [Google Scholar]
- 11.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 12.Gosling J, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–8. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas MS, et al. Endothelial production of MCP-1: modulation by heparin and consequences for mononuclear cell activation. Immunology. 1997;92:512–8. doi: 10.1046/j.1365-2567.1997.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–8. [PubMed] [Google Scholar]
- 15.Weber KS, et al. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999;29:700–12. doi: 10.1002/(SICI)1521-4141(199902)29:02<700::AID-IMMU700>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Leemasawatdigul K, Gappa-Fahlenkamp H. Development of a mathematical model to describe the transport of monocyte chemoattractant protein-1 through a three-dimensional collagen matrix. Cardiovasc Pathol. 2012;21:219–28. doi: 10.1016/j.carpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gappa-Fahlenkamp H, Shukla AS. The effect of short-term, high glucose concentration on endothelial cells and leukocytes in a 3D in vitro human vascular tissue model. In Vitro Cell Dev Biol Anim. 2009;45:234–42. doi: 10.1007/s11626-008-9171-4. [DOI] [PubMed] [Google Scholar]
- 18.Sozzani S, et al. The signal transduction pathway involved in the migration induced by a monocyte chemotactic cytokine. J Immunol. 1991;147:2215–21. [PubMed] [Google Scholar]
- 19.Frevert CW, et al. Tissue-specific mechanisms control the retention of IL-8 in lungs and skin. J Immunol. 2002;168:3550–6. doi: 10.4049/jimmunol.168.7.3550. [DOI] [PubMed] [Google Scholar]
- 20.Gilat D, et al. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1 beta. J Immunol. 1994;153:4899–906. [PubMed] [Google Scholar]
- 21.Leemasawatdigul K, Gappa-Fahlenkamp H. Effect of storage conditions on the stability of recombinant human MCP-1/CCL2. Biologicals. 2011;39:29–32. doi: 10.1016/j.biologicals.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addabbo F, et al. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-kappaB/COX-2 signaling in human aortic endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E1143–54. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lemos JA, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–5. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 24.Ju Y, et al. Modulation of TNF-alpha-induced endothelial cell activation by glucosamine, a naturally occurring amino monosaccharide. Int J Mol Med. 2008;22:809–15. [PubMed] [Google Scholar]
- 25.Panicker SR, et al. Quercetin attenuates Monocyte Chemoattractant Protein-1 gene expression in glucose primed aortic endothelial cells through NF-kappaB and AP-1. Pharmacol Res. 2010;62:328–36. doi: 10.1016/j.phrs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Pasceri V, et al. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–4. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 27.Uriarte SM, et al. Effects of fluoroquinolones on the migration of human phagocytes through Chlamydia pneumoniae-infected and tumor necrosis factor alpha-stimulated endothelial cells. Antimicrob Agents Chemother. 2004;48:2538–43. doi: 10.1128/AAC.48.7.2538-2543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krankel N, et al. A novel flow cytometry-based assay to study leukocyte-endothelial cell interactions in vitro. Cytometry A. 2011;79:256–62. doi: 10.1002/cyto.a.21043. [DOI] [PubMed] [Google Scholar]
- 29.Luhrmann A, et al. The alveolar epithelial type I-like cell line as an adequate model for leukocyte migration studies in vitro. Exp Toxicol Pathol. 2007;58:277–83. doi: 10.1016/j.etp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Weber C, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 31.Weber C, et al. Downregulation by tumor necrosis factor-alpha of monocyte CCR2 expression and monocyte chemotactic protein-1-induced transendothelial migration is antagonized by oxidized low-density lipoprotein: a potential mechanism of monocyte retention in atherosclerotic lesions. Atherosclerosis. 1999;145:115–23. doi: 10.1016/s0021-9150(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 32.Weber C, et al. Role of alpha L beta 2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J Immunol. 1997;159:3968–75. [PubMed] [Google Scholar]
- 33.He L, et al. Modification of collagen with a natural cross-linker, procyanidin. Int J Biol Macromol. 2011;48:354–9. doi: 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007;67:1618–27. doi: 10.1016/j.chemosphere.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Engel J, Bachinger HP. Cooperative equilibrium transitions coupled with a slow annealing step explain the sharpness and hysteresis of collagen folding. Matrix Biol. 2000;19:235–44. doi: 10.1016/s0945-053x(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 36.Miles CA, Bailey AJ. Studies of the collagen-like peptide (Pro-Pro-Gly)(10) confirm that the shape and position of the type I collagen denaturation endotherm is governed by the rate of helix unfolding. J Mol Biol. 2004;337:917–31. doi: 10.1016/j.jmb.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Mitra T, et al. Preparation and characterization of malonic acid cross-linked chitosan and collagen 3D scaffolds: an approach on non-covalent interactions. J Mater Sci Mater Med. 2012;23:1309–21. doi: 10.1007/s10856-012-4586-6. [DOI] [PubMed] [Google Scholar]
- 38.Samouillan V, et al. The use of thermal techniques for the characterization and selection of natural biomaterials. J Funct Biomater. 2011;2(3):230–48. doi: 10.3390/jfb2030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen SJ, Hamel DJ, Handel TM. A rapid and efficient way to obtain modified chemokines for functional and biophysical studies. Cytokine. 2011;55:168–73. doi: 10.1016/j.cyto.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valente AJ, Rozek MM, Schwartz CJ, Graves DT. Characterization of monocyte chemotactic protein-1 binding to human monocytes. Biochem Biophys Res Commun. 1991;176:309–14. doi: 10.1016/0006-291x(91)90925-w. [DOI] [PubMed] [Google Scholar]
- 41.Hoogewerf AJ, et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–8. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 42.Paolini JF, et al. The chemokines IL-8, monocyte chemoattractant protein-1, and I-309 are monomers at physiologically relevant concentrations. J Immunol. 1994;153:2704–17. [PubMed] [Google Scholar]
- 43.Clark-Lewis I, et al. Structure-activity relationships of chemokines. J Leukoc Biol. 1995;57:703–11. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 44.Tan JH, et al. Design and receptor interactions of obligate dimeric mutant of chemokine monocyte chemoattractant protein-1 (MCP-1) J Biol Chem. 2012;287:14692–702. doi: 10.1074/jbc.M111.334201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong JH, Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J Exp Med. 1995;181:631–40. doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau EK, et al. Identification of the glycosaminoglycan binding site of the CC chemokine. MCP-1: implications for structure and function in vivo. J Biol Chem. 2004;279:22294–305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang YJ, Rutledge BJ, Rollins BJ. Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. Identification of a mutated protein that inhibits MCP-1-mediated monocyte chemotaxis. J Biol Chem. 1994;269:15918–24. [PubMed] [Google Scholar]