In this issue, Deniset et al. provide new data that extend our knowledge on the mechanisms whereby Streptococcus pneumoniae is cleared by the spleen. The authors identify novel populations of murine splenic neutrophils that localize in the red pulp and the marginal zone. During the acute phases of S. pneumoniae infection, these populations of splenic neutrophils act in concert with specialized macrophage and B cell populations to provide very rapid innate immune protection.
Abstract
In this issue of JEM, Deniset et al. (https://doi.org/10.1084/jem.20161621) provide new data that extend our knowledge on the mechanisms whereby Streptococcus pneumoniae is cleared by the spleen. The authors identify novel populations of murine splenic neutrophils that localize in the red pulp and the marginal zone. During the acute phases of S. pneumoniae infection, these populations of splenic neutrophils act in concert with specialized macrophage and B cell populations to provide very rapid innate immune protection.

Insight from Patrizia Scapini and Marco A. Cassatella
Invasive infections by encapsulated bacteria, including Streptococcus pneumoniae, represent a major cause of morbidity and mortality worldwide (Blasi et al., 2012). These infections are extremely dangerous in asplenic or splenectomized patients, who can experience fatal sepsis within 24 h (Picard et al., 2003). Current knowledge on how the spleen clears S. pneumoniae relies on evidence showing thymus-independent (TI) antibody production by marginal zone (MZ) B cells and bactericidal activities by MZ macrophages as major protective mechanisms (Picard et al., 2003). In this issue, Deniset et al. show that specific depletion of MZ B cells and/or macrophages results in 40% mortality, but not earlier than 72 h after S. pneumoniae infection. Thus, the authors hypothesized that other innate immune mechanisms must rapidly take place to provide protection during the acute phase of the infection, before antibody production.
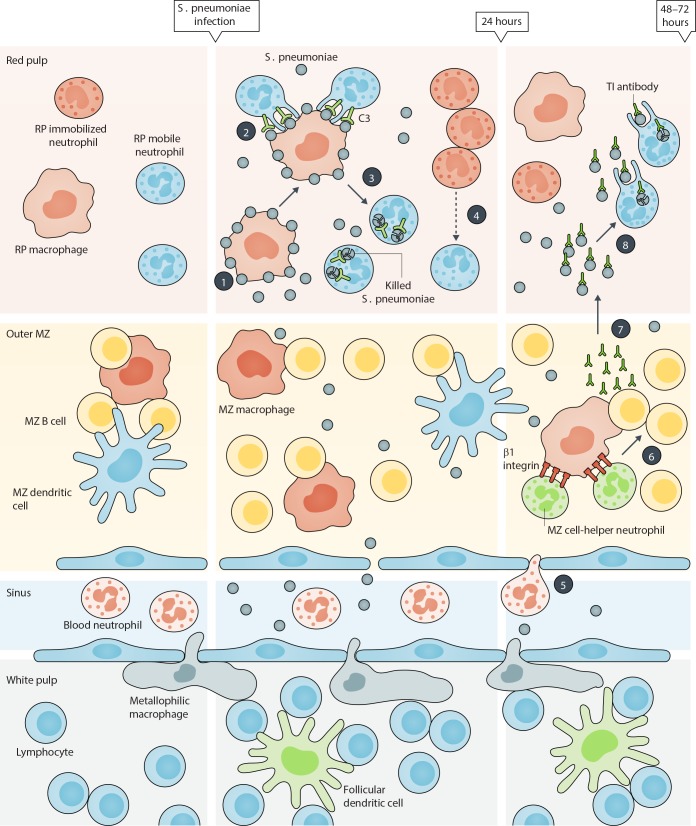
Using cutting-edge imaging technology to track the dynamic behavior of various splenic cell populations, the authors have initially identified two phenotypically distinct neutrophil populations that reside within the red pulp (RP) of the spleen under steady conditions (Deniset et al., 2017). More specifically, the mature Ly6Ghigh neutrophils, which crawl throughout the RP and scan the environment for pathogens (hence defined as “mobile” neutrophils), and the immature Ly6Gint neutrophils, which are mostly composed by band cells organized in large static colonies (defined as “immobilized” neutrophils). Unexpectedly, after S. pneumoniae infection, RP Ly6Ghigh, but not Ly6Gint, neutrophils were identified as the major cell players clearing bacteria during the initial 24 h. These RP Ly6Ghigh neutrophils were found to remove bacteria from the surface of RP macrophages and thereafter kill them through poorly defined mechanisms (phases 1–3 in figure). By taking advantage of parabiosis experiments, the authors further observed that, under basal conditions, RP Ly6Ghigh neutrophils were recruited from the general circulation. However, after the initiation of the infection, RP Ly6Ghigh neutrophils were found replenished by RP Ly6Gint neutrophils, which locally proliferate—likely as a consequence of an extramedullary hematopoiesis—and differentiate into RP Ly6Ghigh neutrophils (phase 4 in figure). The authors also observed that, between 24 and 48 h after S. pneumoniae infection, the MZ became markedly infiltrated by mature blood neutrophils interacting with MZ macrophages, in part via β1 integrin–mediated cell–cell interactions (phase 5 in figure). In turn, these newly recruited MZ neutrophils were found to assume the features of helper cells for B cells (phase 6 in figure) and to locally support TI antibody production by MZ B cells (phase 7 in figure). Notably, these latter TI antibodies enhanced the capacity of RP-resident mature Ly6Ghigh neutrophils to capture bacteria for ultimate clearance and eradication of the infection (phase 8 in figure).
Mechanisms whereby “specialized” neutrophil and macrophage populations fight S. pneumonia infection in the spleen of mice. The figure schematically illustrates the spleen’s anatomy. According to the results reported by Deniset et al. (2017), two main resident neutrophil populations locate in the RP of mouse spleen under steady-state conditions, mature Ly6Ghigh neutrophils (named as mobile neutrophils) and immature Ly6Gint neutrophils (named as immobilized neutrophils), respectively. The authors demonstrate that upon S. pneumonia infection, RP macrophages are responsible of the initial (e.g., occurring within 24 h) capturing of the bacteria (phase 1), which are then phagocytized by RP mobile neutrophils via complement-dependent mechanisms (phase 2) for partial clearance (phase 3). During this period, there is also a proliferation and expansion of RP-immobilized neutrophils, which locally differentiate to replenish the mobile neutrophil pool (phase 4). Blood neutrophils infiltrate the MZ of spleen between 24/48 h after S. pneumonia infection and establish firm interactions with MZ macrophages via β1 integrin–dependent mechanisms (phase 5). Later on, the newly recruited MZ neutrophils differentiate into B cell helper cells (phase 6) and sustain TI antibody production by MZ B cells (phase 7). TI antibodies, in turn, enhance the capacity of capturing the bacteria by RP mobile neutrophils for final pathogen clearance (phase 8).
The study by Deniset et al. (2017) further supports the notion that neutrophils exert a broader role in inflammation and immunity than previously thought (Tecchio et al., 2014). Moreover, the identification of specialized neutrophil populations that, depending on their preferential localization and maturation status, are temporally instructed to fight S. pneumoniae infection, enlarges the list of newly identified mature and immature neutrophil populations that appear under physiological or pathological conditions (Scapini et al., 2016). As such, neutrophils emerge as much more sophisticated innate immune cells than commonly thought. At the same time, Deniset et al. (2017) raise new questions that need clarification. For instance, the molecular events involved in the preferential RP or MZ homing by neutrophils remain unknown. RP and MZ macrophages are proposed to orchestrate neutrophil recruitment and education, but the mechanisms underpinning these effects need to be explored. Also unknown are the specific signals that, in the RP and MZ splenic areas, instruct mature neutrophils, therein arrived, to differentiate into either specialized phagocytes or B cell helper cells. Nonetheless, the finding by Deniset et al. (2017) that neutrophils acquire specific antimicrobial functions in a tissue location–dependent manner is remarkable, as it reconciles and further expands the knowledge from published observations (Balázs et al., 2002; Chorny et al., 2016). Early studies have in fact reported that circulating neutrophils capture heat-killed, but not viable, S. pneumonia, and thereafter carry this pathogen to the splenic MZ without enhancing local B cell responses (Balázs et al., 2002). This is consistent with the fact that only blood, but not splenic, neutrophils were tested for B cell helper properties (Balázs et al., 2002). More recently, Chorny et al. (2016) have reported that splenic perifollicular neutrophils enhance MZ B cell responses to canonical TI antigens, including S. pneumonia, through a mechanism involving pentraxin 3 (PTX3). However, these authors did not investigate whether perifollicular neutrophils additionally exert phagocytic capacity (Chorny et al., 2016). In such regard, the new findings by Deniset et al. (2017) suggest that this function is confined to RP neutrophils.
Another aspect that has been neglected by Deniset et al. (2017) is the characterization of the phenotype and transcriptional signature of RP and MZ neutrophils. In fact, the authors simply assumed that MZ splenic neutrophils display phenotypic and transcriptome properties similar to those of perifollicular neutrophils previously described (Puga et al., 2011; Magri et al., 2014; Chorny et al., 2016). In these latter studies, however, MZ neutrophils were found to specifically colonize the perifollicular area of the human, mouse, and rhesus macaque spleen, where they are instructed to acquire B cell helper properties under steady-state, but not infection, conditions, and therefore were called NBH (Puga et al., 2011; Magri et al., 2014; Chorny et al., 2016). Hence, it remains unclear whether the perifollicular neutrophils described by the Cerutti group (Puga et al., 2011; Magri et al., 2014; Chorny et al., 2016) and the MZ neutrophils described by Deniset et al. (2017) display comparable features. Moreover, it is worth mentioning that Puga et al. (2011) have described two different splenic NBH subsets displaying typical granulocytic morphology and phenotype, named NBH1 and NBH2. Because the NBH2 subset displayed immature features, it would be interesting to elucidate whether NBH1 and NBH2 neutrophils represent the human equivalent of splenic mature Ly6Ghigh and immature Ly6Glow neutrophils described by Deniset et al. (2017). Similarly, it would be important to address, under steady-state conditions, the impact on the RP versus MZ splenic topography of resident mature neutrophils by environmental factors, such as cytokines (i.e., GM-CSF) and mucosa-derived antigens (i.e., LPS in humans [Puga et al., 2011] or 16S rDNA in mice [Zeng et al., 2016]). Moreover, Hägglöf et al. (2016) have recently reported that, in mice treated with IL-18, neutrophils preferentially localize in the RP around CD1d-expressing MZ B cells. It is therefore likely that homeostatic and/or inflammatory stimuli differently condition the localization of neutrophils within specific splenic compartments. In this context, Puga et al. (2011) reported that, under infectious disorders, splenic human neutrophils lose their selective perifollicular topography and extensively infiltrate the follicular mantle and germinal center areas of splenic follicles. Therefore, there is the possibility that inflammatory conditions different from S. pneumoniae infection stimulate neutrophil migration into the follicles and subsequent neutrophil-dependent induction of follicular B cell responses. Finally, the identification of the immature Ly6Glow neutrophil pool resident in the splenic RP (Deniset et al., 2017) raises the question on its biological role. In this context, the concept that the spleen functions as a unique extramedullary reservoir of myeloid cells, including monocytes and neutrophils, is well established (Swirski et al., 2009; Summers et al., 2010). Therefore, a plausible possibility is that these RP immature Ly6Glow neutrophils may generate granulocyte-like myeloid derived suppressor cells (G-MDSC) and/or tumor-associated neutrophils (TANs) under cancer settings. Consistently, it has been recently reported that granulocyte/macrophage progenitors (GMPs) accumulate within the splenic RP of tumor-bearing mice, in turn giving rise to tumor-associated macrophages (TAMs) and TANs (Cortez-Retamozo et al., 2012). Similarly, an expansion of splenic GMPs has been also observed to occur in cancer patients (Cortez-Retamozo et al., 2012). Evidence that even the tumor microenvironment can modulate the B cell helper properties of splenic neutrophils also exists (Gätjen et al., 2016).
In sum, the study by Deniset et al. (2017) not only identifies novel, specialized, neutrophil populations within the murine spleen, but also highlights potential therapeutic avenues to promote the differentiation of neutrophils into better effectors for host protection and humoral immunity. However, to meet such a goal we need to (a) identify the factors responsible for these functional switches by neutrophils; (b) determine whether the findings by Deniset et al. (2017) are limited to the infection by S. pneumoniae (which is known to have a preferential splenic tropism), or are instead valid for other pathogens or pathological settings; and (c) clarify whether similar specialized neutrophil populations exist also in other organs, such as the liver and the lung.
References
- Balázs M., et al. Immunity. 2002 doi: 10.1016/S1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- Blasi F., et al. Clin. Microbiol. Infect. 2012 doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [Google Scholar]
- Chorny A., et al. J. Exp. Med. 2016 doi: 10.1084/jem.20150282. [DOI] [Google Scholar]
- Cortez-Retamozo V., et al. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1113744109. [DOI] [Google Scholar]
- Deniset J.F., et al. J. Exp. Med. 2017 doi: 10.1084/jem.20161621. [DOI] [Google Scholar]
- Gätjen, et al. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3486. [DOI] [Google Scholar]
- Hägglöf T., et al. Nat. Immunol. 2016 doi: 10.1038/ni.3583. [DOI] [PubMed] [Google Scholar]
- Magri G., et al. Nat. Immunol. 2014 doi: 10.1038/ni.2830. [DOI] [Google Scholar]
- Picard C., et al. Curr. Opin. Allergy Clin. Immunol. 2003 doi: 10.1097/00130832-200312000-00006. [DOI] [Google Scholar]
- Puga I., et al. Nat. Immunol. 2011 doi: 10.1038/ni.2194. [DOI] [Google Scholar]
- Scapini P., et al. Immunol. Rev. 2016 doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- Summers C., et al. Trends Immunol. 2010 doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., et al. Science. 2009 doi: 10.1126/science.1175202. [DOI] [Google Scholar]
- Tecchio C., et al. Front. Immunol. 2014 doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M.Y., et al. Immunity. 2016 doi: 10.1016/j.immuni.2016.02.006. [DOI] [Google Scholar]