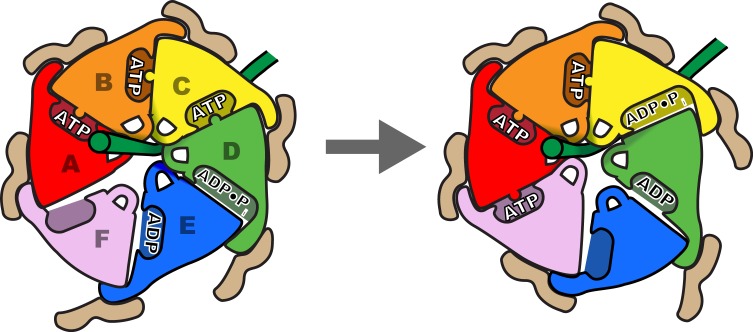
Figure 8. Schematic of one step in the translocation mechanism.
Left, Subunits A-D form a helical surface of pore loop 1 residues that binds substrate in a β conformation along or close to the helix axis. The helix is stabilized by Vta1VSL binding to adjacent subunits and by ATP binding at subunit interfaces. Right, next step in the cycle where subunit F has bound ATP and assembled on the growing end of the Vps4 helix, ATP has been hydrolysed at the C-D interface, and the nucleotide-binding site of subunit E has been opened to allow ADP·Pi release and rebinding of ATP.