Abstract
Purpose/Objective
Recent analyses identify cardiac dose as an important predictor of overall survival (OS) following chemoradiation for locally advanced non-small cell lung cancer (NSCLC). However, the survival influence of cardiac dose following stereotactic body radiation therapy (SBRT) is unknown. We performed a dose volume histogram (DVH) analysis of patients treated with SBRT for early stage NSCLC to examine survival and cardiac toxicity.
Methods
We reviewed the charts of patients treated with SBRT for early stage NSCLC between 6/2007–6/2015 and documented cardiac DVH parameters including maximum and mean dose, V5, V10, V20, and V30. Biologically effective doses and 2 Gy equivalent doses were also calculated. DVH parameters were assessed as predictors of OS using Cox regression.
Results
We identified 102 patients with 118 treated tumors. At a median follow-up of 27.2 months (range: 9.8–72.5 months), the 2-year OS estimate was 70.4%. Cardiac DVH parameters include [median (range)]: maximum: 14.2 Gy (0.3–77.8 Gy); mean: 1.6 Gy (0–12.6 Gy); V5: 8.7% (0–96.4%). We identified no correlation between OS and any cardiac dose parameter. No patient developed acute (within 3 month) cardiac toxicity. Four patients died of cardiac causes, all with pre-existing heart disease.
Discussion
In our cohort, cardiac dose was not a predictor of OS following lung SBRT, despite a subset of patients receiving high maximum cardiac doses. Based on our limited cohort, high doses to small volumes of heart appear safe. Analyses of larger patient cohorts with longer follow-up are needed to better delineate safe cardiac DVH constraints for SBRT.
Keywords: Lung Cancer, Stereotactic Body Radiotherapy, Cardiac Dose
Introduction
Stereotactic body radiotherapy (SBRT) has emerged as a standard-of-care for early stage, medically inoperable non-small cell lung cancer (NSCLC), providing 3-year in-field control of greater than 90% in prospective trials [1, 2]. Early studies established elevated risks associated with SBRT over 3 fractions to centrally located tumors (defined as within a 2 cm margin of the proximal bronchial tree, or directly abutting/overlapping the mediastinal pleura) [3]. Subsequent studies using modestly protracted regimens of 50–60 Gy over 4–8 fractions have generally shown acceptable toxicity and good tumor control [4–6]. Toxicity studies evaluating the risks of SBRT have largely focused on the proximal bronchial tree, with rare treatment associated fatalities from hemoptysis or post-obstructive pneumonia [6–8], while fewer have focused on other central structures, particularly the heart.
The risks of radiation dose to the heart have long been understood in the context of patients treated for breast cancers and lymphomas [9, 10], with larger fields and relatively modest doses to large swaths of heart leading to long term cardiac disease years to decades later in often young patients with a relatively good oncologic prognosis. The role of cardiac dose in lung cancer treatment was largely ignored until a recent secondary analysis from the phase III Radiation Therapy Oncology Group (RTOG) 0617 identified cardiac dose volume metrics as major predictors of survival following concurrent chemoradiation for locally advanced NSCLC. Increased percentage volume of heart receiving ≥ 5 Gy (V5) and 30 Gy (V30) were strongly associated with a greater risk of death [11].
The cardiac dosimetry of SBRT is markedly different than typically found in stage III disease, with the potential for small volumes of heart to receive high biologic effective doses (BED), but typically lower mean and integral doses to the heart due to smaller treatment volumes. Furthermore, the patient population treated with lung SBRT is typically medically inoperable, with substantial pulmonary, cardiac, or other comorbidities, and often older on average than cohorts treated with chemoradiation for locally advanced disease. These patients’ pre-existing cardiac disease could theoretically pre-dispose them to an increased risk of radiation-induced cardiotoxicity. No previous studies have specifically evaluated the role of cardiac dosimetry on survival following SBRT for NSCLC. We conducted a retrospective survival analysis on a large cohort of patients treated with SBRT for early stage NSCLC at a single institution, with a detailed cardiac dose volume histogram (DVH) analysis to assess the impact of cardiac dose volume metrics on survival and cardiac toxicity.
Materials and Methods
Patients
Following Institutional Review Board approval, the charts of all patients treated with SBRT for early stage NSCLC between 6/2007–6/2015 at the University of California Davis were retrieved and reviewed. Eligible patients had a retrievable electronic treatment plan with DVH as well as a minimum follow-up of six months or until death. Standard follow up included history and physical examination and computed tomography of the chest every three months the first year following treatment, every 4–6 months the second year following treatment, and every 6–12 months thereafter until 5 years post-treatment. Treatment and disease parameters, as well as time to last follow-up or death were recorded. Cause of death (cardiac or non-cardiac) was identified from clinic and hospitalization notes. Cardiac events following SBRT were identified from follow up notes, primary care visits, cardiology visits, and hospitalization records, and were graded using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.03. Only grade 3 or higher cardiac events were captured due to challenges in identifying lower grade cardiac events reliably from the available records. Acute cardiac events were defined as those occurring within 3 months following SBRT.
Radiotherapy
Computed tomography (CT) simulation was performed with patients immobilized in the supine position using either the Elekta body frame (Elekta AB, Stockholm Sweden) or a long vacuum-lock bag on the Phillips Brilliance Big Bore 16 slice scanner (Royal Philips Electronics, Amsterdam, The Netherlands) with 2 mm slice thickness. Simulation was performed with abdominal compression using either a plate or belt, and fluoroscopic assessment was performed to ensure diaphragmatic excursion was limited to ≤1 cm. After December 2010, a ten phase four dimensional CT (4DCT) was obtained in addition to a free breathing CT, and use of abdominal compression with fluoroscopic verification was continued. The primary tumor was contoured using lung windows. An internal target volume (ITV) was generated based on a 10 phase 4DCT for patients with 4DCT simulation. An additional 5 mm planning target volume (PTV) margin was added for daily setup error following 4DCT simulation, and a 10 mm craniocaudal margin was used for patients without 4DCT simulation. Treatment plans were generated using Phillips Pinnacle treatment planning software (Royal Philips Electronics, Amsterdam, the Netherlands). Heterogeneity corrections using a superposition/convolution algorithm were applied starting in March of 2011, and cases prior were calculated without heterogeneity correction. 45 tumors were treated using static non-coplanar beams, 30 using fixed beam intensity modulated radiotherapy (IMRT), and 43 using volume modulated arc therapy (VMAT). The median prescribed dose was 50Gy (range: 40–60 Gy) over a median of 4 fractions (range: 3–8). Cardiac constraints were individualized for each case, depending on tumor proximity to the heart, but typically maximum point doses were limited to 105% of the prescription dose when the PTV overlapped the cardiac contour, and volumetric doses were kept as low as reasonably achievable. Treatment was delivered using an Elekta Synergy (Elekta AB, Stockholm Sweden). Image guidance was performed by onboard kV cone-beam CT (CBCT) imaging, with 3D anatomy match to the planning CT scan. Fluoroscopy was assessed prior to each fraction to ensure diaphragmatic excursion was limited to ≤1 cm.
The DVH was retrieved and reviewed for each patient. The cardiac contour was reviewed and edited if necessary. Consistent cardiac contouring was applied for each case in accordance with RTOG 1106 Atlases for Organs at Risk, extending from the inferior aspect of the pulmonary artery cranially to the apex caudally [12]. Cardiac maximum dose, mean dose, V5, V10, and V20 were retrieved for each case. Rigid registration was used to generate cumulative a cumulative DVH for patients with multiple treatment courses.
Statistical analysis
Overall survival (OS) was assessed with the Kaplan Meier method. To account for fractionation, we converted all maximum and mean cardiac doses to biologically effective dose (BED) assuming an alpha/beta ratio of 2 for the heart [13, 14], and a conversion to equivalent dose in 2 Gy fractions (EQD2/2) using the linear quadratic model. Both unconverted and converted values were analyzed. The influence of each cardiac DVH parameter on survival was assessed using a Cox regression model, with radiation dose as a continuous variable. Overall survival for the cohort as a whole, and for the patient cohorts with a cardiac maximum dose BED above and below the median, respectively, were estimated using the Kaplan-Meier method.
Results
We reviewed the charts of 118 patients treated with one or more courses of SBRT for early stage NSCLC. Nine patients lost to follow up within 6 months of treatment were excluded, as were two patients with prior conventionally fractionated radiotherapy to the lung and 5 patients with one or more SBRT plan that could not be retrieved, leaving 102 eligible patients. The median age was 76.3 years (range: 48.9–93.1 years), including 46 men and 56 women. Patients were treated with SBRT to 118 tumors with a median prescription dose of 50 Gy (range: 40–60 Gy). We included 13 patients treated for two or more synchronous (n=8) or metachronous (n=5) primaries. Thirty-two patients had at least one tumor classified as central based on the RTOG 0813 definition, located within 2 cm of the proximal bronchial tree or with PTV touching or overlapping the mediastinum [15], and 70 had peripheral tumors. Median follow-up for among surviving patients was 27.2 months (range: 9.8–72.5 months). The two-year OS estimate for the entire cohort was 70.4%. Patient and treatment characteristics are outlined in Table 1.
Table 1.
Patient and Treatment Characteristics
| Number (%) | |
|---|---|
| Median Age (range) | 76.2 (48.9–93.1%) |
| Gender | |
| Male | 46 (45.1%) |
| Female | 56 (54.9%) |
| T-stage | |
| T1a | 69 (58.5%) |
| T1b | 27 (22.9%) |
| T2a | 18 (15.3%) |
| T2b | 4 (3.4%) |
| Synchronous Primary | |
| 2 lesions | 7 (5.9%) |
| 3 lesions | 2 (1.7%) |
| Metachronous Primary | 6 (5.1%) |
| Ever Smoker? | |
| Yes | 74 (72.5%) |
| No | 28 (27.5%) |
| Medically inoperable? | |
| Yes | 83 (81.4%) |
| No | 19 (18.6%) |
| Location* | |
| Central | 32 (31.4%) |
| Peripheral | 70 (68.6%) |
| Histology | |
| Adenocarcinoma | 62 (52.5%) |
| Squamous cell Carcinoma | 32 (27.1%) |
| Other | 17 (14.4%) |
| Unbiopsied | 7 (5.9%) |
| Prescribed Dose | |
| 54 Gy in 3 fractions | 23 (19.5%) |
| 50 Gy in 4 fractions | 32 (27.1%) |
| 50 Gy in 5 fractions | 28 (23.7%) |
| Other | 35 (29.7%) |
| Treatment Technique | |
| Static non-coplanar beams | 45 (38.1%) |
| IMRT | 30 (25.4%) |
| VMAT | 43 (36.4%) |
Abbreviations: IMRT: Intensity modulated radiation therapy;
VMAT: volume modulated arc therapy
for patients with more than one treated tumor, if any tumor was central the patient was classified as central
The median PTV volume was 26.8 ml3 (range: 5.5—147.1 ml3). Converted to BED2/2, the median cardiac maximum EQD2/2 was 18.2 Gy2/2 (range: 0.2–341.4 Gy2/2), and the median cardiac mean EQD2 was 0.5 Gy2/2 (range: 0–10.8 Gy2/2). Thirteen patients (12.7%) had a maximum EQD2/2 exceeding 80 Gy, and 10 (9.8%) had a maximum EQD2/2 exceeding 150 Gy. Among the group of patients with an EQD2 exceeding 80 Gy, the median cardiac maximum dose expressed as EQD2 was 180.6 Gy (range: 80.4–299.4 Gy), and the median PTV volume was 37.1 cc (range: 11.5–139.4 cc). The median values for cardiac DVH parameters without correction for fractionation included: maximum dose: 14.2 Gy (range: 0.34–77.8); mean dose: 1.6 Gy (range: 0–12.6 Gy); V5: 8.7% (range: 0–96.4%.; V20: 0 (range: 0–17.0%); V30: 0 (range: 0–3.4%). Nine patients (8.8%) had a cardiac max dose >50 Gy. 3 patients (2.9%) had a mean cardiac dose exceeding 10 Gy. No patient exceeded the RTOG 0813 cardiac dose limit of 15cc to 32 Gy. The median cardiac max BED was 34.7 Gy (range: 0.3–682.8 Gy) for central tumors and 34.4 Gy2/2 (range: 0.4–598.7 Gy2/2) for peripheral tumors. The full, non-converted DVH analysis is shown in Table 2, and DVH statistics with all doses converted to BED2 and EQD2/2 are shown in Table 3.
Table 2.
Cardiac dose-volume parameters, unconverted
| Max (Gy) | Mean (Gy) | V5 (%) | V10 (%) | V20 (%) | V30 (%) | |
|---|---|---|---|---|---|---|
| Median (range) | 14.07 Gy (0.34–77.8 Gy) | 1.57 Gy (0–12.56 Gy) | 8.71% (0–96.4 Gy) | 1.12% (0–60.4% Gy) | 0% (0–17.0 Gy) | 0% (0–3.4% Gy) |
Table 3.
Cardiac dose-volume parameters converted to BED and EQD2/2
| Maximum point dose to heart (BED) | Heart Mean Dose (BED) | Maximum dose in 2 Gy equivalent fractions | Mean dose in 2 Gy equivalent fractions | |
|---|---|---|---|---|
| Median (range) | 37.2 Gy2(0.4–682.8 Gy2) | 1.1 Gy2 (0–12.6 Gy2) | 18.6 Gy2/2(0.2–341.4 Gy2/2) | 0.5 Gy2/2 (0–10.8 Gy2/2) |
Abbreviations: BED: Biologically Effective Dose
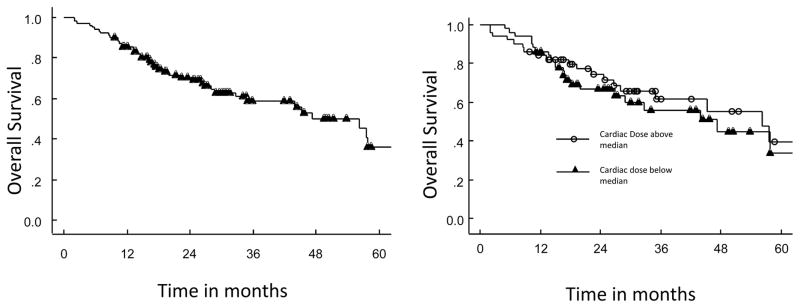
Our analysis yielded no statistically significant correlation between OS and any evaluated cardiac dose parameter (p>0.05 for all). Overall survival curves for patients with a cardiac point maximum BED above and below the median, respectively, are shown in Figure 1b. Thirteen patients (13%) had insufficient follow-up records available to confirm presence or absence of grade 3+ cardiac events during the entire follow-up period. No acute (defined as occurring within 3 months following SBRT) cardiac toxicity was identified in any patient. Among the 89 patients with sufficient follow-up to determined cardiac events, ten (11%) experienced a grade 3+ cardiac event, including four patients (4.5%) who died of cardiac causes (congestive heart failure in 3 patients and cardiac arrest in one), all with pre-existing cardiac disease. An additional 6 patients developed grade 3 cardiac events, including myocardial infarction (n=1), and chest pain leading to hospitalization and catheterization and/or stent placement (n=5). Three of the 4 patients with a cardiac cause of death had DVH parameters below the median for the cohort, and the median point max dose to the heart (9.4 versus 13.7 Gy), median BED point max (19.4 Gy2/2 versus 32.7 Gy2/2), and median cardiac mean BED (0.4 Gy2/2 versus 1.0 Gy2/2) were all lower among patients with cardiac events than among those without cardiac events.
Figure 1.
Figure 1a–b: Kaplan Meier curves showing overall survival for entire patient cohort (a) and cohort stratified by cardiac maximum dose converted to BED (b)
Discussion
Cardiac toxicity is a well-studied and feared complication following radiotherapy for Hodgkin’s disease, left sided breast cancers, and other malignancies that may result in significant radiation to the heart. Until publication of RTOG 0617, the cardiac risks following radiation for lung cancer were typically discounted in the face a malignancy with poor survival. However, the RTOG 0617 data demonstrated a remarkable impact on survival from cardiac dosimetry, specifically heart V5 and V30, despite relatively modest median follow-up of only 22.9 months [11]. These data suggest that cardiac dose may impact survival within 2 years of treatment, at least with the doses received during concurrent chemoradiation for locally advanced NSCLC. However, similar studies specific to early stage lung cancer treated with SBRT are lacking. The dosimetry of lung SBRT is markedly different than that encountered when treating locally advanced disease, with the potentially for very high maximum point doses for targets adjacent to the heart, but typically lower mean and volumetric parameters due to the small targets and highly conformal planning. The patient population treated with SBRT is typically medically inoperable, and hence expected to have a higher rate of significant comorbid cardiac disease as compared to patients with locally advanced disease fit enough to tolerate concurrent chemoradiation. While survival from stage I lung cancer is markedly superior to that of locally advanced disease. In our cohort, we identified cardiac maximum point exceeding 200 Gy2/2 in several patients when converted to EQD2/2, markedly higher than those achieved with conventionally fractionated radiotherapy. However, mean doses, V20, and V30 were typically low.
Prior studies focused on SBRT for centrally located tumors have identified increased risk of toxicity, particularly central airway injury [3, 7], but have not identified an excess of cardiac deaths. An early and widely cited dose escalation study using a 3 fraction regimen from Indiana University identified central tumor location (within 2 cm of the proximal bronchial tree) as a significant risk factor for high grade toxicity, and one of the fatalities attributed to SBRT in this study was a fatal pericardial effusion [3]. However, no cardiac dosimetry was provided for this case. Radiation Therapy Oncology Group (RTOG) 0813, a prospective phase I/II evaluation of 5 fraction SBRT for centrally located, early stage lung cancer allowed cardiac maximum doses of up to 105% of the prescription dose and suggested, but did not mandate, limiting the volume of heart receiving 32 Gy to <15 cc. With these constraints, no grade 4–5 cardiac toxicity was described in the initial presentation of results, although final results in full manuscript form are eagerly awaited [15, 16]. No published studies have yet specifically focused on the correlation between cardiac dose and survival among patients treated with SBRT for early stage lung cancer. A small, retrospective study evaluated changes in cardiac FDG uptake in the heart as a possible surrogate for myocardial injury following SBRT for tumors close to the heart. The investigators identified increased FDG uptake in the heart when more than 5 cm3 of the heart received 20 Gy [17]. However, these findings have not been validated in larger studies.
Our major finding in this study was that cardiac dose was not a predictor of overall survival in our patient population following SBRT for early stage NSCLC. This was true for each cardiac DVH parameter, including mean dose, max dose, V5, V10, V20 and V30. While the mean cardiac dose was generally extremely low, consistent with the dosimetry of SBRT, a subset of patients had high maximum cardiac EQD2/2 in excess of 80 Gy or even 150 Gy. Moreover, of the patients who died from known cardiac causes, all had preexisting cardiac disease and three of the four had cardiac dose parameters below the median of population as a whole. We also analyzed grade 3+ cardiac events during the follow-up period, and found no correlation between cardiac events and cardiac dose. This analysis was subject to the limitations of retrospectively identifying cardiac events from medical records, and from a lack of mandated follow-up cardiac testing such as routine stress testing, echocardiograms, or electrocardiograms. However, in aggregate, our results suggest delivery of high radiation doses to small volumes of the heart is relatively safe.
There are notable limitations to the present study. Given the significant medical co-morbidities in the medically inoperable population, competing causes of death could obscure a survival impact. Causes of death among the cohort varied and included metastatic disease, respiratory failure, and other primary neoplasms. In addition, the cause of death was not always clearly noted on patient’s electronic health record, particularly among long-term (>5 year) survivors. Our median follow-up of 27.2 months among surviving patients may be insufficient to detect an increase in cardiac deaths in this patient population. Our population of 102 patients may also have been too small to detect a subtle influence from cardiac dose. Contouring of and dose tracking to specific cardiac sub-structures may also prove useful in understanding the impact of high radiation doses to small volumes of the heart. Our patients were treated with a range of fractionation schedules, from 3–8 sessions, and the linear-quadratic model was used to convert to BED and EQD2/2. However, the applicability of the linear-quadratic model from large fraction sizes has been questioned [18], and the exact α/β ratio to use for calculations is poorly defined. However, in the absence of a clearly more robust, validated method of comparing fractionation schedules, we report actual delivered dose, BED, and EQD2/2. Finally, early plans were generated without heterogeneity corrections, leading to possible moderate errors in calculated dose in this patient subset.
Future studies should focus on a large patient cohort with longer follow-up to better delineate safe cardiac DVH constraints for SBRT, and should examine cardiac sub-structures in addition to the cardiac dose as a whole.
Conclusions
In conclusion, high radiation doses to small volumes of the heart appear relatively safe in the medically inoperable population treated with SBRT for NSCLC. This low rate of cardiac toxicity and death may be attributable to the typically low mean cardiac doses achieved in our cohort (median of 1.6 Gy), in keeping with the conformal dosimetry and typically small targets of SBRT. Our analyses did not identify a cardiac DVH parameter that predicted survival following lung SBRT. Our current institutional approach of limiting point maximum doses to <105% of the prescription dose and limiting volumetric constraints to “as low as reasonably achievable” does not appear to result in an excess of early cardiac toxicity. Larger analyses with longer follow up are needed.
Table 4.
Cardiac dose-volume parameters for four patients with a cardiac cause of death
| Patient | PTV volume | Central? | Dose and fractions | Max | Mean | V5 | V10 | V20 |
|---|---|---|---|---|---|---|---|---|
| 1 | 35.3 cc | No | 60/3 | 1.67 Gy | 0.34 Gy | 0 | 0 | 0 |
| 2 | 19.64 cc | No | 54/3 | 21.7 Gy | 3.8 Gy | 25.3% | 17.5% | 0.4% |
| 3 | 24.0 cc | Yes | 60/5 | 6.3 Gy | 0.6 Gy | 0.02% | 0 | 0 |
| 4 | 12.2 cc | No | 50/4 | 7.1 Gy | 0.4 Gy | 0.03% | 0 | 0 |
Clinical Practice Points.
The impact of cardiac dose-volume parameters on survival following SBRT for early stage lung cancer has not been well-studied.
In our cohort, cardiac dose metrics including maximum and mean dose and volumes receiving 5, 20, and 30 Gy were not predictive of survival.
No acute cardiac toxicity was identified in any patient in our cohort.
With a median follow-up of 27.2 months, cardiac death occurred in four patients with pre-existing cardiac disease and an additional 6 non-fatal cardiac events were identified.
Our dose volume analysis identified a subset of 13 patients with an EQD2 exceeding 80 Gy without cardiac fatality.
Further analyses should be performed on larger cohorts of patients, as our population may not be large enough to identify a small survival decrement associated with high cardiac dose.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: none
Presented in part at the 2016 Annual Meeting of the American Society of Radiation Oncology, Boston Massachusetts
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Timmerman R, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann P, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):967–71. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol. 2011;6(12):2036–43. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- 6.AB, et al. Primary Study Endpoint Analysis for NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC) International Journal of Radiation Oncology * Biology * Physics. 2016;94(1):5–6. [Google Scholar]
- 7.Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med. 2012;366(24):2327–9. doi: 10.1056/NEJMc1203770. [DOI] [PubMed] [Google Scholar]
- 8.Duijm M, et al. Dose and Volume of the Irradiated Main Bronchi and Related Side Effects in the Treatment of Central Lung Tumors With Stereotactic Radiotherapy. Semin Radiat Oncol. 2016;26(2):140–8. doi: 10.1016/j.semradonc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 10.Gagliardi G, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S77–85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 11.Bradley JD, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–99. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RTOG OAR Atlas. https://www.rtog.org/CoreLab/ContouringAtlases/LungAtlas.aspx. [cited 2016 06/14/2016]
- 13.Cosset JM, et al. Late toxicity of radiotherapy in Hodgkin’s disease. The role of fraction size. Acta Oncol. 1988;27(2):123–9. doi: 10.3109/02841868809090332. [DOI] [PubMed] [Google Scholar]
- 14.Gillette SM, et al. Late radiation response of canine mediastinal tissues. Radiother Oncol. 1992;23(1):41–52. doi: 10.1016/0167-8140(92)90304-d. [DOI] [PubMed] [Google Scholar]
- 15.Bezjak A, PR, Gaspar LE, et al. Primary Study Endpoint Analysis for NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2016;94(1):5–6. [Google Scholar]
- 16.Bezjak A, PR, Gaspar L, et al. NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiotherapy (SBRT) for Central Tumors - Adverse Events. 16th World Conference on Lung Cancer; 2015; Denver, Colorado. [Google Scholar]
- 17.Evans JD, et al. Cardiac (1)(8)F-fluorodeoxyglucose uptake on positron emission tomography after thoracic stereotactic body radiation therapy. Radiother Oncol. 2013;109(1):82–8. doi: 10.1016/j.radonc.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Park C, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):847–52. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]