Abstract
Black women have lower fracture risk compared with white women, which may be partly explained by improved volumetric bone mineral density (vBMD) and bone microarchitecture primarily within the cortical bone compartment. To determine if there are differences in trabecular microstructure, connectivity, and alignment according to race/ethnicity, we performed individual trabecular segmentation (ITS) analyses on high-resolution peripheral quantitative computed tomography (HR-pQCT) scans of the distal radius and tibia in 273 peri- and postmenopausal black (n = 100) and white (n = 173) women participating in the Study of Women’s Health Across the Nation in Boston. Unadjusted analyses showed that black women had greater trabecular plate volume fraction, plate thickness, plate number density, and plate surface area along with greater axial alignment of trabeculae, whereas white women had greater trabecular rod tissue fraction (p < 0.05 for all). Adjustment for clinical covariates augmented these race/ethnicity-related differences in plates and rods, such that white women had greater trabecular rod number density and rod-rod connectivity, whereas black women continued to have superior plate structural characteristics and axial alignment (p < 0.05 for all). These differences remained significant after adjustment for hip BMD and trabecular vBMD. In conclusion, black women had more plate-like trabecular morphology and higher axial alignment of trabeculae, whereas white women had more rod-like trabeculae. These differences may contribute to the improved bone strength and lower fracture risk observed in black women.
Keywords: white, black, individual trabecula segmentation, high-resolution peripheral quantitative computed tomography, trabecular microstructure
Introduction
Black women have a lower risk of fracture than white women,(1–3) and the reason for this difference is incompletely understood. Areal bone mineral density (aBMD) measured by dual-energy X-ray absorptiometry (DXA) is lower in white women than in black women(4,5) but does not fully explain observed differences in fracture rates between these two groups.(6) We previously performed a cross-sectional study analyzing high-resolution peripheral quantitative computed tomography (HR-pQCT) scans of the radius and tibia in white and black peri- and postmenopausal women participating in the Study of Women’s Health Across the Nation (SWAN) at the Boston site.(7) We found that black women had larger and denser bones than white women and that cortical microarchitecture was improved in blacks compared with whites. In contrast, most trabecular bone characteristics were similar between the two groups.
Individual trabecular segmentation (ITS) provides for the detailed analyses of trabecular bone morphology, characterizing the connectivity, axial alignment, and plate and rod-like qualities of individual trabeculae. This type of analysis may provide novel insights into bone strength, as plate-like morphology has been shown in experimental models to be stronger than a rod-like morphology. Furthermore, greater alignment of trabeculae with the predominant loading direction improves bone strength for that loading situation,(8) although not necessarily for other loading conditions. Studies in human cadavers have shown that greater plate-like and axially aligned trabeculae are associated with stronger bone.(9,10) ITS was initially developed for high-resolution images from microcomputed tomography(9) but has subsequently been adapted to HR-pQCT images from clinical studies.(11) For example, prior studies have reported differences in trabecular morphology between Chinese-American women and white women that may help to explain the clinical paradox of lower fracture rates among Chinese-American women despite concurrent lower aBMD.(12,13) Alterations in the plate and rod-like nature of trabecular bone may also explain skeletal fragility in other patient populations, including premenopausal women with idiopathic osteoporosis,(11) postmenopausal women with primary hyperparathyroidism,(14) amenorrheic female athletes,(15) postmenopausal women with prevalent fragility fracture,(16,17) and young adults with cystic fibrosis.(18) This new tool has not yet been utilized to evaluate trabecular microstructural differences between black and white women.
Thus, the goal of this study was to characterize the rod- and plate-like qualities, connectivity, and axial alignment of trabecular bone of the radius and tibia of peri- and postmenopausal black and white women and to determine whether ITS provides new information in addition to aBMD and standard HR-pQCT measures that may help explain differences in fracture risk according to race/ethnic origin. We also examined the contribution of ITS parameters to the prediction of bone strength from microfinite element analysis.
Subjects and Methods
Subjects and eligibility criteria
Subject eligibility and enrollment have previously been described.(7) In brief, this is a cross-sectional evaluation of women participating in the Study of Women’s Health Across the Nation (SWAN), a seven-site, longitudinal cohort study in community-based samples of women. Women participating in SWAN were initially recruited between 1996 and 1997 and were required to be 42 to 52 years old, have menstruated within the last 3 months, and belong to one of the site’s predesigned race/ethnic groups. SWAN eligibility criteria, cohort recruitment, and determination of menopause stage have been described previously in detail.(19) The Boston SWAN site recruited black and white women as per the predesignated race/ethnic groups. Ethnicity was determined by subject self-identification. Follow-up visits have occurred every 1 to 2 years for more than 15 years. For the present study, HR-pQCT and DXA scans were obtained at the Boston SWAN site during the 11th and 12th follow-up visits between 2010 and 2012. For this substudy, exclusion criteria included contraindication to DXA and/or HR-pQCT scanning, a history of solid organ transplant, or weight greater than 330 pounds (because of weight limits of the HR-pQCT equipment). The protocol was approved by the Partners Healthcare Institutional Review Board, and written informed consent was obtained from all participants.
Assessment of clinical covariates
Clinical characteristics were assessed prospectively at each study visit using standardized interviewer-administered and self-administered questionnaires as previously described.(7) Clinical covariates of interest included age, tobacco use, alcohol intake, medical diagnoses, medication use (including oral glucocorticoids, hormone-replacement therapy, and osteoporosis medications), reproductive history, menopause status, and physical activity.(20) Height was measured using a stadiometer and weight assessed using a balance scale.
Areal bone mineral density by DXA
Areal bone mineral density was assessed by DXA (QDR4500A, Hologic Inc., Bedford, MA, USA) at the posterior-anterior (PA) lumbar spine, total hip, femoral neck, and total body (excluding the head). For quality control, a Hologic anthropomorphic spine phantom was scanned daily. For reproducibility measures, 30 patients were scanned twice, with repositioning between scans. DXA precision (as calculated as root mean square CV) is 0.8% for PA spine, 1.7% for femoral neck, and 1.0% for total hip.
HR-pQCT scans
Trabecular and cortical bone volumetric density and microstructure were assessed using HR-pQCT scans (Xtreme CT, Scanco Medical AG, Bassersdorf, Switzerland) at the distal radius and tibia using the standard region of interest (ROI) placement of a fixed distance of 9.5 and 22.5 mm from the radial and tibial endplate, respectively, as previously described.(21–23) The manufacturer’s phantom was scanned daily for quality control. Scans were reviewed by investigators for motion artifact at the time of scanning and were repeated if significant motion artifact was present. Standard HR-pQCT outcomes were obtained using Scanco analysis software version V6.0. Extended cortical analyses and microfinite element analyses were performed as previously described.(24–28) These results have been previously reported.(7)
Individual trabecula segmentation analyses
ITS analyses were performed as previously described(9,11,29) after segmentation of the trabecular bone compartment from each HR-pQCT image using established methods.(24–26) The following trabecular microstructural parameters were obtained from the ITS algorithm: total trabecular bone volume fraction (BV/TV); plate and rod bone volume fraction (pBV/TV and rBV/TV); plate and rod tissue fraction (pBV/TV and rBV/TV); axially aligned bone volume fraction along the longitudinal axis (aBV/TV); trabecular plate and rod number density (pTbN and rTbN, 1/mm); mean trabecular plate and rod thickness (pTbTh and rTbTh, mm); mean trabecular plate surface area (pTbS, mm2); mean trabecular rod length (rTbl, mm); plate-plate, plate-rod, and rod-rod junction density (P-P JuncD, R-P JuncD, and R-R JuncD, 1/mm3). Same-day reproducibility (with repositioning) for ITS outcomes at the radius and tibia in our laboratory ranged from 1.2% to 9.4% for plate-like trabecular parameters; 0.9% to 17.1% for rod-like trabecular parameters; 1.0% to 7.7% for total and axially aligned bone volume fraction; and 4.1% to 28.5% for junction density measures.
Statistical analyses
We used independent samples two-sided t tests and/or chi-square tests to compare the clinical characteristics and mean values of DXA aBMD and ITS parameters of black and white women. Multivariable linear regression was then performed with the ITS parameters as the dependent variables, adjusting for clinical covariates known to affect bone health and those that were significantly different between groups by univariate analyses. Clinical covariates included age, weight, current tobacco and alcohol use, current physical activity score, diabetes, and history of systemic use of hormone-replacement therapy (HRT), osteoporosis medications (oral or intravenous bisphosphonates or raloxifene), and significant glucocorticoids (defined by self-report of glucocorticoid use >3 months at the baseline visit or report of use at ≥3 subsequent follow-up visits). Using an interaction term, we tested for effect modification by menopause duration (defined as years since final menstrual period) on racial differences in ITS outcomes. We also analyzed the above multivariate model with number of years since menopause substituted for age. Further analyses were performed with inclusion of either aBMD at the total hip or trabecular vBMD (from the HR-pQCT standard analyses) as additional covariates with the purpose of determining whether trabecular microstructural differences between racial groups identified by ITS provides information above and beyond what is available by standard measures. Total hip aBMD was chosen for these analyses because this is a standard DXA site recommended by the International Society for Clinical Densitometry for clinical use in the diagnosis of osteoporosis and represents a combination of both trabecular and cortical bone. Trabecular vBMD was chosen to determine if ITS identifies differences independent of that provided by the standard HR-pQCT analyses.
Within the full cohort, we determined associations between (a) ITS parameters and standard HR-pQCT parameters and (b) ITS parameters and bone strength estimates (failure load) by microfinite element analysis and using Pearson correlation coefficients. To determine which standard HR-pQCT and ITS variables were predictors of failure load at the radius and tibia, we utilized a general linear model with multiple predictors. In our previous publication,(7) we utilized an oblique component variable cluster analysis(30) to choose HR-pQCT variables to include to enter into the model. For the present analysis, we sought to determine whether trabecular plate volume fraction (pBV/TV) and rod volume fraction (rBV/TV) assisted in the predictive model of failure load. We thus replaced the trabecular structural outcome (ie, trabecular thickness) with pBV/TV and rBV/TV, while retaining the other variables from the model (ie, total cross-sectional area, trabecular vBMD, cortical vBMD, cortical thickness, cortical porosity, and race). Data are reported as mean ± standard deviation (SD) unless otherwise noted, and p values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Cohort characteristics and aBMD results
The clinical characteristics of the black (n = 100) and white (n = 173) subjects have been previously described in detail(7) and are summarized in Table 1. Participants were 59.9 ± 2.7 years old, and 93% were postmenopausal. Number of years since menopause was similar between races. White women weighed less and reported greater alcohol use and less tobacco use than blacks. Use of HRT, osteoporosis medications, and oral glucocorticoids were similar between groups. More black women had diabetes than whites. White women had significantly lower aBMD at the lumbar spine, total body, total hip, and femoral neck (p < 0.05 for all).
Table 1.
Clinical Characteristics and DXA aBMD Results of Study Cohort (Values Presented as Mean ± SD for Clinical Characteristics and DXA Results
| White (n = 173) | Black (n = 100) | p Value | |
|---|---|---|---|
| Age (years) | 60.0 ± 2.8 | 59.6 ± 2.6 | 0.21 |
| Weight (kg) | 76.4 ± 16.8 | 84.6 ± 19.1 | <0.01 |
| Height (cm) | 164.5 ± 5.9 | 163.7 ± 6.7 | 0.31 |
| Body mass index (kg/m2) | 28.2 ± 5.8 | 31.5 ± 6.5 | <0.01 |
| Postmenopausal (n, %) | 161 (93) | 94 (94) | 0.88 |
| Menopause duration (months) | 92.4 ± 41.7 | 96.0 ± 43.2 | 0.46 |
| Physical activity scorea | 8.5 ± 1.8 | 7.7 ± 1.9 | <0.01 |
| Tobacco, n (%) | 16 (9.3) | 18 (18) | 0.03 |
| Alcohol, n (%) | |||
| None | 19 (11.0) | 36 (36) | <0.01 |
| <2/day | 140 (75.1) | 63 (63) | |
| ≥2/day | 24 (13.9) | 1 (1) | |
| Significant glucocorticoid use, n (%) | 18 (10.4) | 13 (13) | 0.51 |
| Osteoporosis medication use, n (%) | 17 (9.8) | 9 (9) | 0.82 |
| Diabetes, n (%) | 10 (5.8) | 25 (25) | <0.01 |
| Confirmed fracture, n (%) | 22 (12.7) | 9 (9) | 0.35 |
| Systemic HRT use, n (%) | 62 (35.8) | 32 (32) | 0.52 |
| DXA aBMD results | |||
| Total body (g/cm2) | 1.077 ± 0.110 | 1.158 ± 0.124 | <0.0001 |
| Total hip (g/cm2) | 0.911 ± 0.135 | 1.006 ± 0.150 | <0.0001 |
| Femoral neck (g/cm2) | 0.761 ± 0.119 | 0.879 ± 0.140 | <0.0001 |
| Posterior-anterior spine (g/cm2) | 0.986 ± 0.164 | 1.065 ± 0.170 | 0.0002 |
Scores range from 3 to 9 with higher scores indicating increased physical activity.(20)
Racial/ethnic differences in ITS parameters
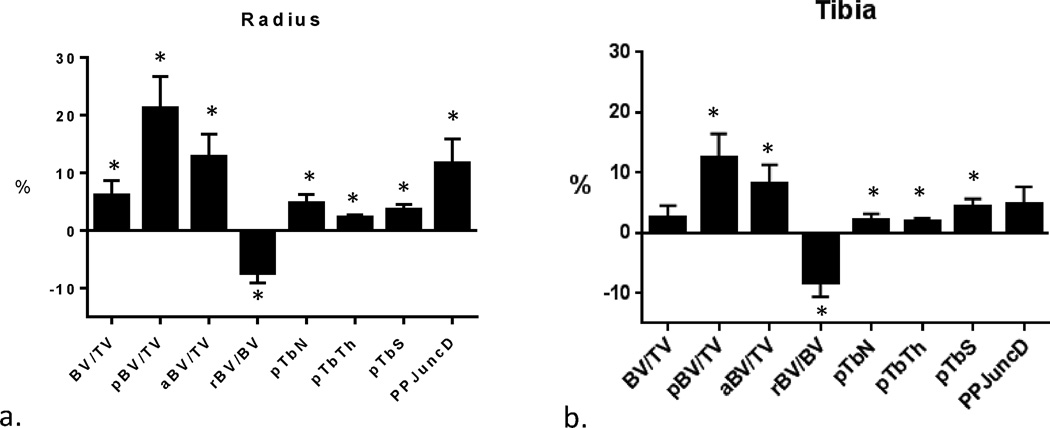
In unadjusted analyses, total trabecular bone volume (BV/TV) was 6% higher in black women at the radius and was similar between the two groups at the tibia (Table 2, Fig. 1). At both the radius and tibia, black women had greater trabecular plate volume fraction, plate thickness, plate number density, and plate surface area. Black women also had greater axial alignment of trabeculae at both the radius and tibia, along with greater plate connectivity at the tibia. White women, on the other hand, had greater trabecular rod tissue fraction at both the radius and the tibia along with greater rod thickness at the radius (Fig. 2). There was no significant effect modification by menopause duration on racial differences in ITS outcomes.
Table 2.
ITS Results of the Radius and Tibia in White and Black Women
| Unadjusted | Multivariable adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Radius | White | Black | p Value | White | Black | Clinical covariates p valuea |
Clinical covariates + hip aBMD p valueb |
Clinical covariates + Tb vBMD p valuec |
| Bone volume fraction (BV/TV) | 0.250 ± 0.004 | 0.265 ± 0.005 | 0.020 | 0.252 ± 0.004 | 0.261 ± 0.005 | 0.21 | 0.41 | 0.60 |
| Axial bone volume fraction (aBV/TV) | 0.094 ± 0.002 | 0.106 ± 0.003 | 0.002 | 0.094 ± 0.002 | 0.105 ± 0.003 | 0.006 | 0.23 | 0.002 |
| Plate bone volume fraction (pBV/TV) | 0.085 ± 0.003 | 0.103 ± 0.004 | <0.001 | 0.085 ± 0.003 | 0.102 ± 0.004 | 0.002 | 0.10 | <0.001 |
| Rod bone volume fraction (rBV/TV) | 0.165 ± 0.002 | 0.162 ± 0.003 | 0.47 | 0.167 ± 0.002 | 0.159 ± 0.003 | 0.054 | 0.001 | <0.001 |
| Plate tissue fraction (pBV/BV) | 0.329 ± 0.007 | 0.378 ± 0.010 | <0.001 | 0.328 ± 0.007 | 0.380 ± 0.010 | <0.001 | 0.003 | <0.001 |
| Rod tissue fraction (rBV/BV) | 0.671 ± 0.007 | 0.622 ± 0.010 | <0.001 | 0.672 ± 0.007 | 0.620 ± 0.010 | <0.001 | 0.003 | <0.001 |
| Trab plate number density (pTbN, 1/mm) | 1.36 ± 0.01 | 1.43 ± 0.02 | 0.002 | 1.37 ± 0.01 | 1.42 ± 0.02 | 0.021 | 0.56 | 0.007 |
| Trab rod number density (rTbN, 1/mm) | 1.87 ± 0.01 | 1.84 ± 0.02 | 0.26 | 1.88 ± 0.01 | 1.83 ± 0.02 | 0.020 | 0.001 | 0.001 |
| Mean trab plate thickness (pTbTh, mm) | 0.205 ± 0.001 | 0.209 ± 0.001 | <0.001 | 0.205 ± 0.001 | 0.209 ± 0.001 | <0.001 | 0.001 | <0.001 |
| Mean trab rod thickness (rTbTh, mm) | 0.212 ± 0.0004 | 0.214 ± 0.001 | 0.030 | 0.212 ± 0.001 | 0.214 ± 0.001 | 0.013 | 0.065 | 0.033 |
| Mean trab plate surface area (pTbS, mm2) | 0.154 ± 0.001 | 0.160 ± 0.001 | <0.001 | 0.154 ± 0.001 | 0.161 ± 0.001 | <0.001 | <0.001 | <0.001 |
| Mean trab rod length (rTbL, mm) | 0.662 ± 0.002 | 0.659 ± 0.003 | 0.45 | 0.660 ± 0.002 | 0.661 ± 0.003 | 0.64 | 0.032 | 0.014 |
| Rod-rod junction density (R-R JuncD, 1/mm3) | 3.01 ± 0.05 | 2.86 ± 0.08 | 0.13 | 3.05 ± 0.06 | 2.78 ± 0.08 | 0.006 | 0.001 | 0.001 |
| Plate-rod junction density (P-R JuncD, 1/mm3) | 3.64 ± 0.08 | 3.86 ± 0.10 | 0.09 | 3.69 ± 0.08 | 3.78 ± 0.11 | 0.53 | 0.16 | 0.08 |
| Plate-plate junction density (P-P JuncD,1/mm3) | 1.74 ± 0.05 | 1.95 ± 0.06 | 0.007 | 1.76 ± 0.05 | 1.92 ± 0.06 | 0.06 | 0.99 | 0.08 |
| Tibia | ||||||||
| Bone volume fraction (BV/TV) | 0.274 ± 0.004 | 0.280 ± 0.005 | 0.25 | 0.277 ± 0.003 | 0.275 ± 0.005 | 0.70 | 0.054 | 0.95 |
| Axial bone volume fraction (aBV/TV) | 0.118 ± 0.002 | 0.128 ± 0.003 | 0.009 | 0.119 ± 0.002 | 0.128 ± 0.003 | 0.038 | 0.31 | <0.001 |
| Plate bone volume fraction (pBV/TV) | 0.123 ± 0.003 | 0.138 ± 0.004 | 0.002 | 0.124 ± 0.003 | 0.136 ± 0.004 | 0.029 | 0.26 | <0.001 |
| Rod bone volume fraction (rBV/TV) | 0.151 ± 0.003 | 0.142 ± 0.004 | 0.055 | 0.153 ± 0.003 | 0.138 ± 0.004 | 0.002 | 0.001 | 0.002 |
| Plate tissue fraction (pBV/BV) | 0.444 ± 0.008 | 0.490 ± 0.011 | <0.001 | 0.443 ± 0.008 | 0.492 ± 0.012 | 0.002 | 0.010 | <0.001 |
| Rod tissue fraction (rBV/BV) | 0.556 ± 0.008 | 0.510 ± 0.011 | <0.001 | 0.557 ± 0.008 | 0.508 ± 0.012 | 0.002 | 0.010 | <0.001 |
| Trab plate number density (pTbN, 1/mm) | 1.49 ±0.01 | 1.52 ± 0.01 | 0.038 | 1.50 ± 0.01 | 1.51 ± 0.01 | 0.40 | 0.63 | 0.007 |
| Trab rod number density (rTbN, 1/mm) | 1.82 ± 0.01 | 1.78 ± 0.02 | 0.07 | 1.83 ± 0.01 | 1.76 ± 0.02 | 0.003 | 0.002 | 0.004 |
| Mean trab plate thickness (pTbTh, mm) | 0.217 ± 0.001 | 0.222 ± 0.001 | <0.001 | 0.217 ± 0.001 | 0.221 ± 0.001 | 0.005 | 0.024 | 0.001 |
| Mean trab rod thickness (rTbTh, mm) | 0.212 ± 0.0004 | 0.212 ± 0.001 | 0.60 | 0.212 ± 0.0005 | 0.212 ± 0.001 | 0.86 | 0.67 | 0.81 |
| Mean trab plate surface area (pTbS, mm2) | 0.165 ± 0.001 | 0.173 ± 0.002 | 0.003 | 0.165 ± 0.001 | 0.174 ± 0.002 | <0.001 | 0.001 | <0.001 |
| Mean trab rod length (rTbL, mm) | 0.651 ± 0.002 | 0.655 ± 0.002 | 0.12 | 0.649 ± 0.002 | 0.657 ± 0.002 | 0.006 | 0.001 | 0.003 |
| Rod-rod junction density (R-R JuncD, 1/mm3) | 2.65 ± 0.06 | 2.43 ± 0.09 | 0.052 | 2.70 ± 0.07 | 2.35 ± 0.09 | 0.004 | 0.004 | 0.004 |
| Plate-rod junction density (P-R JuncD, 1/mm3) | 4.01 ± 0.07 | 4.04 ± 0.10 | 0.79 | 4.09 ± 0.07 | 3.90 ± 0.09 | 0.13 | 0.007 | 0.047 |
| Plate-plate junction density (P-P JuncD,1/mm3) | 2.16 ± 0.04 | 2.26 ± 0.05 | 0.10 | 2.19 ± 0.04 | 2.21 ± 0.05 | 0.77 | 0.25 | 0.061 |
Multivariable-adjusted model included the following clinical covariates: age, weight, any HRT use, history of osteoporosis medication use, prior significant glucocorticoid use, current tobacco and alcohol use, current physical activity score, and diabetes. Values are presented as mean ± SE.
Bolded values indicate significantly higher values than the other racial group for unadjusted or clinical-covariate adjusted analyses.
Indicates significance at p < 0.05 with clinical covariate adjustment alone in the multivariable model.
Indicates significance at p < 0.05 when total hip aBMD was added to clinical covariates.
Indicates significance at p < 0.05 when Tb vBMD was added to clinical covariates.
Fig. 1.
Percent difference ± SEM in ITS parameters between white and black women (whites as reference) at the radius (A) and tibia (B). *Different from white at p < 0.05.
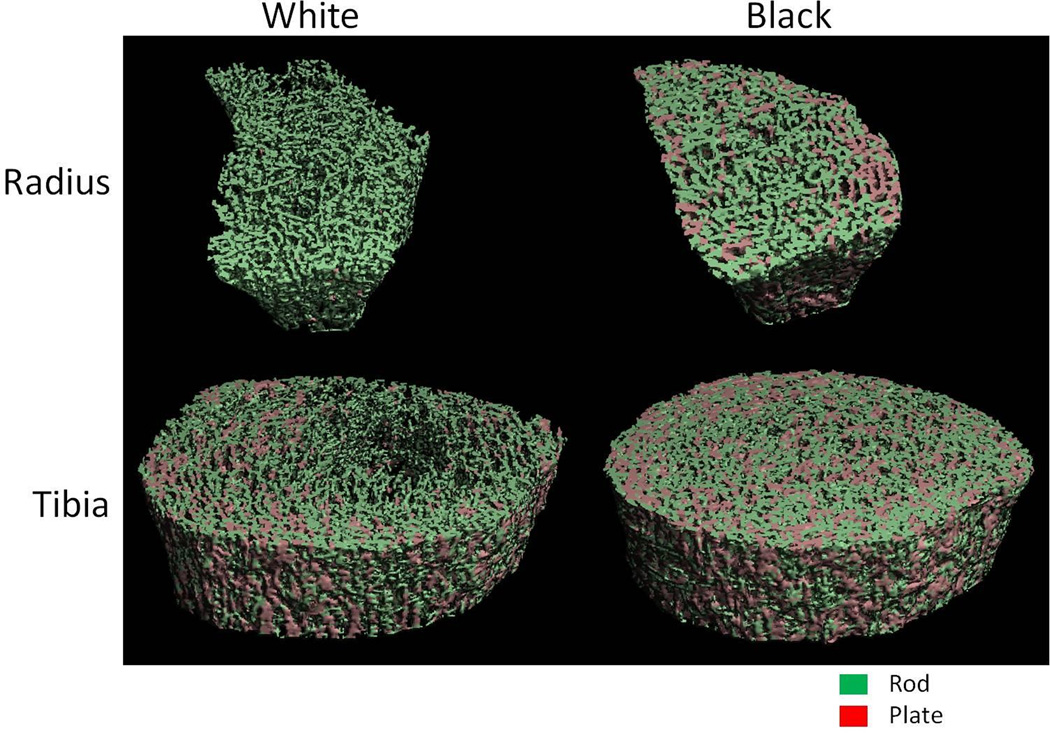
Fig. 2.
ITS images of the radius and tibia of a representative black woman and white woman.
Adjustment for clinical covariates augmented these racial differences in trabecular bone morphology (Table 2). After adjustment for clinical covariates, black women continued to have superior plate structural characteristics (plate volume fraction, plate thickness, and plate surface area) at both the radius and tibia and greater plate number density at the radius. Axial alignment of trabeculae also continued to be greater in black women at both sites after adjustment for clinical covariates. White women continued to exhibit greater trabecular rod number density and rod-rod connectivity at both the radius and the tibia, as well as greater trabecular rod volume fraction at the tibia. Adjusting for years since menopause instead of age in these analyses did not affect these results (Supplemental Table S1).
Results were further adjusted for areal BMD at the hip in addition to clinical covariates to determine if ITS analyses added additional information from what is already provided by DXA (Table 2). In these analyses, racial differences in trabecular plate volume fraction and axial alignment of both the radius and tibia were eliminated, but most other ITS parameters continued to remain significantly different between races at both sites. When trabecular vBMD was added to clinical covariates in the multivariable analyses, differences favoring plate-like trabecular networks in blacks and rod-like networks in whites persisted at both the radius and tibia (Table 2).
Correlation of ITS with standard HR-pQCT measures
We found that trabecular and whole bone outcomes from standard HR-pQCT analyses were generally significantly associated with those obtained from ITS analyses (Table 3). Strong correlations were noted between trabecular vBMD or trabecular thickness and ITS-derived plate volume fraction, plate number, plate connectivity, and axial alignment (r = 0.80 to 0.95, p < 0.05 for all). Conversely, ITS-derived outcomes reflecting rod-like properties (rod volume fraction, rod number, and rod connectivity) were strongly positively associated with trabecular number (r = 0.86 to 0.93, p < 0.05) and inversely associated with trabecular separation (r = −0.73 to −0.85, p < 0.05). These associations were similar at the tibia (Supplemental Table S2).
Table 3.
Correlation Coefficients (r) for Standard Whole Bone and Trabecular HR-pQCT Parameters and ITS Parameters at the Radius
| Standard HR-pQCT Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| ITS parameter | Total area |
Trab area |
Total vBMD |
Trab vBMD |
Trab number |
Trab thickness |
Trab separation |
Trab heterogeneity |
| Whole bone | ||||||||
| BV/TV | −0.18 | −0.23 | 0.69 | 0.99 | 0.78 | 0.67 | −0.79 | −0.74 |
| aBV/TV | −0.26 | −0.29 | 0.66 | 0.86 | 0.36 | 0.90 | −0.46 | −0.48 |
| Plate-like | ||||||||
| pBV/TV | −0.25 | −0.29 | 0.67 | 0.85 | 0.36 | 0.91 | −0.40 | −0.42 |
| pBV/BV | −0.25 | −0.29 | 0.52 | 0.57 | −0.06 | 0.90 | −0.05 | −0.13 |
| pTbN | −0.23 | −0.27 | 0.67 | 0.92 | 0.53 | 0.81 | −0.60 | −0.59 |
| pTbTh | −0.21 | −0.26 | 0.51 | 0.56 | 0.04 | 0.78 | −0.11 | −0.11 |
| pTbS | −0.22 | −0.23 | 0.23 | 0.07 | −0.54 | 0.64 | 0.43 | 0.29 |
| PPJuncD | −0.21 | −0.26 | 0.68 | 0.95 | 0.59 | 0.78 | −0.63 | −0.60 |
| RPJuncD | −0.17 | −0.22 | 0.64 | 0.94 | 0.80 | 0.56 | −0.78 | −0.70 |
| Rod-like | ||||||||
| rBV/TV | 0.001 | −0.03 | 0.34 | 0.62 | 0.92 | −0.01 | −0.85 | −0.74 |
| rTbN | 0.05 | 0.02 | 0.24 | 052 | 0.93 | −0.15 | −0.85 | −0.71 |
| rTbTh | −0.16 | −0.16 | 0.32 | 0.35 | −0.25 | 0.77 | 0.13 | −0.05 |
| rTbL | 0.005 | 0.04 | −0.45 | −0.80 | −0.85 | −0.33 | 0.85 | 0.77 |
| RRJuncD | 0.09 | 0.07 | 0.12 | 0.36 | 0.86 | −0.21 | −0.74 | −0.61 |
Bolded values indicate significance at p < 0.05.
ITS predictors of estimated bone strength
As previously published, estimated bone strength by microfinite analyses was greater in black women than whites.(7) In both races, ITS parameters were strongly correlated with failure load at both the radius and the tibia. Strong positive predictors of failure load included axial bone volume fraction, plate volume fraction, plate number density, and rod-plate and plate-plate junction density (r = 0.52 to 0.72, Supplemental Table S3). In the multivariable model combining standard HR-pqCT outcomes and ITS outcomes, variables were chosen from groups using cluster analysis from the following six categories: total cross-sectional area; trabecular structure (plate and rod bone volume); cortical area and volume (cortical thickness); cortical pore characteristics (cortical porosity); trabecular volumetric density; and cortical volumetric density. In this model, the independent predictors of estimated failure load at the radius were total cross-sectional area, plate bone volume, and cortical thickness. At the tibia, the independent predictors were similar and included total cross-sectional area, plate bone volume, cortical thickness, and trabecular vBMD (Table 4). Race was not a significant predictor of failure load apart from the other variables. These predictors together explained 89% of the variation in estimated failure load at the tibia and 91% at the radius.
Table 4.
Standardized Effects of the Predictors of µFEA-Estimated Failure Load at the Radius and Tibia
| Predictor | Radius | Tibia | ||
|---|---|---|---|---|
| Standardized effect | p value | Standardized effect | p value | |
| Total cross-sectional area | 0.61 | <0.0001 | 0.67 | <0.0001 |
| pBV/TV | 0.41 | 0.0008 | 0.27 | 0.027 |
| rBV/TV | 0.05 | 0.57 | 0.01 | 0.92 |
| Trab vBMD | 0.26 | 0.10 | 0.42 | 0.0015 |
| Ct thickness | 0.52 | <0.0001 | 0.52 | <0.0001 |
| Ct porosity | –0.04 | 0.29 | –0.09 | 0.06 |
| Ct vBMD | 0.09 | 0.08 | 0.11 | 0.07 |
| Race/ethnicity | 0.01 | 0.74 | 0.01 | 0.61 |
Discussion
In this study, ITS-based morphological analyses of HR-pQCT images revealed significant differences in trabecular bone morphology between black and white peri- and postmenopausal women. Black women had advantageous plate-like qualities and greater axial alignment of trabecular bone compared with white women, whereas white women had greater rod-like trabecular structural characteristics. Notably, these differences persisted after for clinical covariates and for hip BMD and trabecular vBMD. Thus, these alterations in trabecular morphology are likely not fully reflected by DXA and standard HR-pQCT analyses, suggesting that ITS may contribute important information regarding trabecular bone integrity and fracture risk. These differences in trabecular plate and rod-like qualities and orientation may help explain the greater estimated bone strength and reduced fracture risk observed in blacks compared with whites.
To our knowledge, this is the first study to use ITS to compare trabecular bone morphology between white and black women. Using standard analyses of HR-pQCT scans in this population, we previously found that black women had greater cortical thickness, volume, and area at both the radius and tibia along with greater cortical vBMD and lower cortical porosity at the tibia.(7) In contrast, most trabecular parameters were similar between the two groups, with the only differences being greater trabecular thickness at the radius and tibia and greater trabecular vBMD at the radius in black women. Notably, in the current study, ITS analyses revealed additional differences in trabecular morphology and alignment not reflected in standard analyses, namely that white women had significantly fewer and thinner plates with lower plate surface area along with a greater proportion of rod-like trabecular bone.
With age-related bone loss, intracortical and endosteal remodeling and fragmentation may occur as the cortical component gets smaller and the trabecular component increases in size. This remodeling could potentially overestimate the morphology of the trabecular compartment leading to higher measurements of trabecular density. Given that white women in our study had evidence of increased endosteal resorption,(7) this could increase measurements of trabecular density in white women relative to black women.
We also found that standard HR-pQCT measures may reflect different aspects of trabecular microstructure in this population. In comparing standard trabecular HR-pQCT parameters to ITS parameters, plate-like ITS measures were highly correlated with trabecular thickness and trabecular density, whereas rod-like measures had strong correlations with trabecular number and separation. Because many of the trabecular parameters from the standard HR-pQCT analyses were found to be similar between whites and black women, our findings of racial/ethnic differences in ITS parameters suggest that ITS may be more sensitive at identifying some trabecular trait differences between groups.
Skeletal advantages in black women may reflect improved bone acquisition during younger years and/or attenuation of bone loss in later years. Histomorphometric studies in younger women have shown that blacks have higher cortical and trabecular thickness than whites,(31–33) and studies in children of black race/ethnicity using pQCT suggest similar advantages in cortical and trabecular bone compared with whites.(34,35) With aging, plate-like trabecular network may convert to a more rod-like network.(13) In one histomorphometric study, African women had preserved trabecular number and separation with aging compared with white women.(32) Compared with women of other races, black women were also found to have the slowest decline in lumbar spine aBMD, a site reflecting mostly trabecular bone, over the menopausal transition.(5) Although we do not have measurements of bone marrow adiposity in our study subjects, a prior study of iliac crest biopsies reported lower bone marrow adiposity in blacks.(36) A lower bone marrow content could potentially contribute to a higher trabecular bone density, as assessed by X-ray attenuation. Together with our findings, this raises the possibility that black women may have inherent advantages in trabecular microstructure that persist despite aging. Future longitudinal studies will be necessary to better understand the etiology of the trabecular bone benefits observed in the peri- and postmenopausal women in this study.
Our findings are similar to previous studies that have reported that ITS analyses provide unique information about race- and ethnic-related differences in trabecular bone microstructure. For example, premenopausal Chinese-American women had higher plate bone volume fraction and plate number density along with greater plate connectivity than premenopausal white women.(12) Although these superior plate-like qualities were less apparent with aging, postmenopausal Chinese-American women continued to have a higher ratio of plate to rods and greater plate thickness than postmenopausal white women.(13) In the current study, we found that peri- and postmenopausal black women had advantages in plate-like trabecular qualities in addition to improved axial alignment compared with white women. Moreover, white women had a more rod-like trabecular network compared with blacks, in contrast to studies of Chinese-Americans who have similar rod-like features as their white counterparts.(12,13) Together, these studies suggest that there may be underlying race/ethnicity-based differences in trabecular microstructure favoring black women.
Alterations in trabecular morphology may have important clinical implications. Postmenopausal women with osteopenia and a history of fragility fracture have lower plate number and plate connectivity, along with reduced axial alignment of trabecular bone compared with osteopenic women without fractures, even though BMD T-scores were similar between the two groups.(16) In addition, preferential loss of plate-like and axially aligned trabeculae leading to a more rod-like trabecular network was found in postmenopausal women with fragility fractures.(17) Similarly, loss of plate-like trabecular bone qualities was noted in patient populations with conditions predisposing to osteoporosis and fracture, including postmenopausal women with primary hyperparathyroidism,(14) young adults with cystic fibrosis,(18) and patients taking chronic oral glucocorticoids.(37) Together, these studies suggest that plate-like and axially aligned trabeculae may be particularly important determinants of skeletal integrity, and advantages in these parameters in black women may be contributing to their reduced fracture risk compared with whites.
We previously reported that black women had greater estimated bone strength by microfinite analyses than whites(7) and that that total cross-sectional area, trabecular vBMD, and cortical thickness were the strongest HR-pQCT-derived predictors of failure load. In this study, we found that failure load was strongly associated with plate-like characteristics and axial alignment of trabeculae and less strongly associated with rod-like qualities. Moreover, when both standard HR-pQCT and ITS outcomes are considered as predictors, plate volume fraction is a significant predictor of failure load at both sites, independent of trabecular vBMD. These data suggest that the amount of plate-like trabecular bone is an important contributor to bone strength and may help to explain black women’s reduced fracture risk.
We previously found that adjusting standard HR-pQCT outcomes for total hip aBMD differences between black and white women led to more favorable trabecular microarchitecture in whites, including increased trabecular number and decreased trabecular separation at the radius and tibia and higher trabecular vBMD at the tibia. Despite these trabecular advantages, bone strength estimates were still greater in black women. In our current study, it becomes apparent that these trabecular improvements in whites after adjustment of DXA aBMD are primarily affecting rod-like trabecular bone, including rod bone volume, number, thickness, and connectivity. Because these rod-like characteristics contribute less to structural integrity than plate-like structures, this likely explains why bone strength continues to be stronger in black women after DXA aBMD adjustment. In addition, adjustment for DXA aBMD leads to loss of significance of plate bone volume and axial alignment at the radius and tibia along with plate number and thickness at the radius, suggesting that these trabecular characteristics are most reflected in DXA measurements.
Strengths of this study include the relatively large sample size along with the detailed clinical information regarding factors affecting skeletal health prospectively obtained over the past 15 years. Our study also has several limitations. The cross-sectional nature of this study cannot account for the dynamic changes in bone that occur with aging. In particular, we cannot discern whether the favorable plate morphology in black women is because of acquisition of favorable trabecular morphology in young adulthood or to better preservation of a “youthful” trabecular morphology with advanced age. Also, the microfinite element analyses used a fixed material property and therefore only reflect differences in bone structure. In addition, although height was similar between black and white women, it is possible that black women had longer extremities than white women,(38) which could lead to imaging of a more distal region when a fixed ROI is used. However, Boyd and colleagues showed that whereas cortical thickness decreases, there are minimal differences in trabecular vBMD with a slightly more distal scan site.(23) Consistent with this, Shanbhogue and colleagues reported that trabecular vBMD measures at the standard fixed site and a relative scanning site were strongly correlated,(39) suggesting that our findings of trabecular bone differences between blacks and whites of equal height are likely not attributable to artifacts due to use of a fixed scan location. Lastly, our results pertain to peripheral skeletal sites only, which may not necessarily be representative of trabecular microstructure at the axial skeleton.
In conclusion, black women had greater plate-like trabecular morphology and greater axial alignment of trabeculae, whereas white women had a more rod-like trabecular network. These trabecular differences found on ITS analyses were not fully captured by DXA and standard HR-pQCT measurements. Because a plate-like trabecular bone morphology contributes more to bone strength than rod-like structures, these racial differences in trabecular bone may contribute to the lower fracture risk observed in black women.
Supplementary Material
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009– present, Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark, NJ—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH program office: National Institute on Aging, Bethesda, MD—Winifred Rossi 2012–present, Sherry Sherman 1994–2012, Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program officers.
Central laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present, Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering committee: Susan Johnson, current chair; Chris Gallagher, former chair.
We thank the study staff at each site and all the women who participated in SWAN. We also thank X Edward Guo, PhD, and Yizhong Hu, PhD, at Columbia University for generously creating the ITS images.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study conduct and data collection: DL, KD, and JSF. Data analysis: EWY and MSP. Data interpretation: MSP, EWY, JSF, and MLB. Drafting manuscript: MSP, EWY, JSF, and MLB. Revising manuscript content: JSF and MLB. Approving final manuscript: all authors. MSP and EWY take responsibility for the integrity of the data analysis.
References
- 1.Griffin MR, Ray WA, Fought RL, Melton LJ., 3rd Black-white differences in fracture rates. Am J Epidemiol. 1992;136(11):1378–1385. doi: 10.1093/oxfordjournals.aje.a116450. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Barrett J, Malenka D, et al. Racial differences in fracture risk. Epidemiology. 1994;5(1):42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 7.Putman MS, Yu EW, Lee H, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013;28(10):2177–2185. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26(2):263–269. doi: 10.1002/jbmr.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XS, Sajda P, Saha PK, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23(2):223–235. doi: 10.1359/JBMR.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B, Liu XS, Wang J, Lu XL, Fields AJ, Guo XE. Dependence of mechanical properties of trabecular bone on plate-rod microstructure determined by individual trabecula segmentation (ITS) J Biomech. 2014;47(3):702–708. doi: 10.1016/j.jbiomech.2013.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu XS, Cohen A, Shane E, et al. Individual trabeculae segmentation (ITS)-based morphological analysis of high-resolution peripheral quantitative computed tomography images detects abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2010;25(7):1496–1505. doi: 10.1002/jbmr.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XS, Walker MD, McMahon DJ, et al. Better skeletal microstructure confers greater mechanical advantages in Chinese-American women versus white women. J Bone Miner Res. 2011;26(8):1783–1792. doi: 10.1002/jbmr.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker MD, Liu XS, Zhou B, et al. Premenopausal and postmenopausal differences in bone microstructure and mechanical competence in Chinese-American and white women. J Bone Miner Res. 2013;28(6):1308–1318. doi: 10.1002/jbmr.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013;28(5):1029–1040. doi: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell DM, Tuck P, Ackerman KE, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015;81:24–30. doi: 10.1016/j.bone.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein EM, Kepley A, Walker M, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res. 2014;29(5):1101–1109. doi: 10.1002/jbmr.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XS, Stein EM, Zhou B, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27(2):263–272. doi: 10.1002/jbmr.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putman MS, Greenblatt LB, Sicilian L, et al. Young adults with cystic fibrosis have altered trabecular microstructure by ITS-based morphological analysis. Osteoporos Int. 2016;27(8):2497–2505. doi: 10.1007/s00198-016-3557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 22.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Boyd SK. Site-specific variation of bone micro-architecture in the distal radius and tibia. J Clin Densitom. 2008;11(3):424–430. doi: 10.1016/j.jocd.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25(5):983–993. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25(4):882–890. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 27.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392–399. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 28.Vilayphiou N, Boutroy S, Szulc P, et al. Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res. 2011;26(5):965–973. doi: 10.1002/jbmr.297. [DOI] [PubMed] [Google Scholar]
- 29.Liu XS, Shane E, McMahon DJ, Guo XE. Individual trabecula segmentation (ITS)-based morphological analysis of microscale images of human tibial trabecular bone at limited spatial resolution. J Bone Miner Res. 2011;26(9):2184–2193. doi: 10.1002/jbmr.420. [DOI] [PubMed] [Google Scholar]
- 30.Harmon HH. Modern factor analysis. 3rd. Chicago: The University of Chicago Press; 1976. [Google Scholar]
- 31.Schnitzler CM, Mesquita JM. Cortical bone histomorphometry of the iliac crest in normal black and white South African adults. Calcif Tissue Int. 2006;79(6):373–382. doi: 10.1007/s00223-006-0053-z. [DOI] [PubMed] [Google Scholar]
- 32.Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE. Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner. 1990;10(3):183–199. doi: 10.1016/0169-6009(90)90261-d. [DOI] [PubMed] [Google Scholar]
- 33.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996;11(12):1967–1975. doi: 10.1002/jbmr.5650111219. [DOI] [PubMed] [Google Scholar]
- 34.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95(4):1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock NK, Laing EM, Taylor RG, et al. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab. 2011;29(1):44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- 36.Schnitzler CM, Mesquita J. Bone marrow composition and bone microarchitecture and turnover in blacks and whites. J Bone Miner Res. 1998;13(8):1300–1307. doi: 10.1359/jbmr.1998.13.8.1300. [DOI] [PubMed] [Google Scholar]
- 37.Sutter S, Nishiyama KK, Kepley A, Zhou B, Wang J, McMahon DJ, et al. Abnormalities in cortical bone, trabecular plates, and stiffness in postmenopausal women treated with glucocorticoids. J Clin Endocrinol Metab. 2014;99(11):4231–4240. doi: 10.1210/jc.2014-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogin B, Varela-Silva MI. Fatness biases the use of estimated leg length as an epidemiological marker for adults in the NHANES III sample. Int J Epidemiol. 2008;37(1):201–209. doi: 10.1093/ije/dym254. [DOI] [PubMed] [Google Scholar]
- 39.Shanbhogue VV, Hansen S, Halekoh U, Brixen K. Use of relative vs fixed offset distance to define region of interest at the distal radius and tibia in high-resolution peripheral quantitative computed tomography. J Clin Densitom. 2015;18(2):217–225. doi: 10.1016/j.jocd.2014.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.