Abstract
Though previous studies have demonstrated an increased fracture risk in females with anorexia nervosa (AN), fracture risk in males is not well characterized. The objective of this study was to examine sex differences in fracture risk and site-specific fracture incidence in AN. We performed a population-based retrospective cohort study using The Health Improvement Network. The median calendar year for the start of the observation period was 2004–5. We identified 9,239 females and 556 males <60 years of age with AN, and 97,889 randomly selected sex-, age-, and practice-matched participants without eating disorders (92,329 females and 5560 males). Multivariable Cox regression was used to estimate the hazard ratio (HR) for incident fracture. Median age at start of observation was 29.8 years in females and 30.2 years in males. The HR for fracture associated with AN differed by sex and age (interaction p = 0.002). Females with AN had an increased fracture risk at all ages (HR 1.59; 95% confidence interval [95% CI], 1.45–1.75). AN was associated with a higher risk of fracture among males >40 years of age (HR 2.54, 95% CI 1.32–4.90; p = 0.005) but not among males ≤40 years. Females with AN had a higher risk of fracture at nearly all anatomic sites. The greatest excess fracture risk was noted at the hip/femur (HR 5.59; 95% CI, 3.44–9.09) and pelvis (HR 4.54; 95% CI 2.42–8.50) in females and at the vertebrae (HR 7.25; 95% CI, 1.21–43.45) for males with AN. AN was associated with higher incident fracture risk in females across all age groups and in males >40 years old. Sites of highest fracture risk include the hip/femur and pelvis in females and vertebrae in males with AN.
Keywords: Anorexia nervosa, eating disorders, bone fractures, bone deficit, United Kingdom
INTRODUCTION
Anorexia nervosa (AN) is a condition characterized by intense fear of weight gain, severe body image distortion, and self-induced weight loss (1). Estimated lifetime prevalence of AN is 0.9% among women and 0.3% among men (2). AN is associated with a mortality rate between 5% and 10% after 10 years of onset (3). AN may contribute to bone deficits through several physiological mechanisms including low body mass index (BMI) and lean mass, hypogonadism with low estradiol and testosterone, resistance to growth hormone, low insulin-like growth factor 1 (IGF-1), and relative hypercortisolemia, which may all decrease surrogate markers of bone formation and bone resorption (4–7). In adolescents, both bone formation and resorption are suppressed, but studies have shown there is an uncoupling of bone formation and resorption in adulthood, with a decrease in formation and increase in resorption (8). Prior studies in women with AN have found significant deficits in bone mineral density (BMD) compared to healthy female controls, particularly at the lumbar spine (9–12). Although 5–15% of patients with AN are male (13), few studies have assessed BMD in males with AN (14–17). The limited existing studies in adolescent males with AN have found the most significant deficits in BMD at the hip, femoral neck, trochanter, and intertrochanteric regions, compared to healthy male controls (14,15).
AN has also been shown to be associated with an increased risk of fracture. One review pooled data from three prior fracture studies in AN (N=2,384) and reported a risk ratio of 2.6 for all fracture types, 5.3 for hip fractures, and 4.7 for spine fractures among patients with AN compared to controls (18–21). Though several studies have demonstrated an increased risk of fractures among females with AN (19–22), large population-based data is limited, and fracture risk in males with AN has not been characterized. Two studies examining fracture risk in AN were limited to females (21,22), one study did not stratify results by sex (19), and the only study to report a fracture risk for males had a small sample size (n=15 males) (20). Given increasing recognition of eating disorders among males (23), there is a critical need to determine the risk of fracture in this understudied population.
The Health Improvement Network (THIN) comprises a robust source of population-based data that has been used to characterize the risk of fracture associated with several chronic conditions in adults and children and to delineate age and sex effects on fracture risk (24–33). The objective of this large population-based study was to examine sex differences in fracture risk associated with AN. A secondary aim was to assess site-specific fracture incidence in AN and sex differences in site-specific fracture risk.
METHODS
Study Design/Data Source
We conducted a population-based retrospective cohort study using THIN (January 2012 version). THIN provides de-identified data including demographics, diagnoses, prescriptions, procedures, and select laboratory measures of over 10 million people from the electronic medical records of 553 general practices in the United Kingdom (UK). Medical diagnoses were recorded using the standard Read Code classification system in the UK (34). The study adhered to the Declaration of Helsinki, was approved by the THIN Scientific Review Committee, and determined by the Institutional Review Board of the University of Pennsylvania to meet eligibility criteria for IRB exemption authorized by 45 CFR 46.101, category 4.
Study Population
Participants with acceptable records for research based on checks performed by the data vendor and at least one Read code for AN (E271.00 “Anorexia nervosa”, Eu50000 “[X]Anorexia nervosa”, Eu50100 “[X]Atypical anorexia nervosa”, 1467.00 “H/O: anorexia nervosa”) were included as exposed. For each participant with AN, up to 10 randomly selected participants who did not have codes for AN or other eating disorders were included as unexposed after being matched on sex, age (3-year age groups up to 30 years and 5-year age groups thereafter), and practice. Data on participants ≥60 years of age or older were excluded. The timing of initial diagnosis of AN for all exposed participants was the date of their first entry of a code for AN. Participants with a first entry of a code for AN at <7 years of age were excluded (n=88). Covariates of interest included sex, age, age at initial diagnosis, observation time, country (England, Ireland, Scotland, or Wales), BMI (closest to but within two years of the start of the observation period), antidepressant use and alcohol abuse (defined by Read codes indicative of excessive alcohol consumption, alcohol abuse or alcoholism) within two years of the start of observation, and history of prior fracture.
As in our prior studies (32,33), fractures were identified using consistent Read diagnostic codes and classified according to anatomic site as follows: vertebra, skull/face, pelvis, rib/thorax, clavicle/scapula, humerus/elbow, forearm/wrist, hand, hip/femur, lower leg/ankle, and foot. We excluded surgically induced fractures and fractures attributed to birth trauma or metastatic bone disease. For the minority of first fracture events for which there were codes for ≥2 sites on the same date, the fracture was categorized as multiple sites. Fractures were categorized by the site-specific code if both site-specific and nonspecific codes were entered for the date of first fracture.
The start of the observation period for ascertainment of incident fracture was the latest of: the initial Read code for AN, 6 months after registration with the practice, and the date that the practice started using the Vision software as the electronic medical record. The requirement for the observation period to start after 6 months of registration and use of Vision software is standard practice for ascertainment of incident diagnoses in THIN and avoids inclusion of prevalent outcome events (32,33,35,36).
Analysis
Descriptive statistics were reported as median and inter-quartile range (IQR) for continuous variables and frequencies for categorical variables. Group differences in continuous variables were assessed using the Wilcoxon rank sum test, and categorical variables were assessed using the chi-square or Fisher’s exact test. Cox proportional hazards regression was used to compare the incidence of fracture in participants with AN to that of matched unexposed participants. We examined multiplicative interactions among age, sex, and AN. Stratified Cox regression models were used to estimate age- and sex-specific hazard rations (HRs) with 95% confidence intervals (95% CIs). A two-sided p value of <0.05 was considered statistically significant. Analyses were performed using STATA 13.0 (Stata Corporation, College Station, TX). Given a sample size of males with AN (N=556) and males without eating disorders (N=5,560), our study had statistical power (alpha=0.05, two-sided) of >80% to detect a HR for fracture of 1.30 in males. Given a sample size of females with AN (N=9,239) and females without eating disorders (N=92,329), our study had >80% power to detect a HR of 1.09 in females.
RESULTS
Cohort Characteristics
Our cohort comprised 9,239 females and 556 males with AN and 92,329 female and 5,560 male unexposed participants (Table 1). Consistent with the epidemiology of AN, there were more women with AN than men (ratio nearly 17:1). As expected, BMI was significantly lower in the AN group (p <0.001). The median observation period was shorter in the AN group compared to the unexposed group (p <0.001 in females, p = 0.06 in males). Among females, death was a censoring event for 126 (1.4%) AN participants compared to 436 (0.5%) unexposed participants (p <0.001). Among males, death was a censoring event for 19 (3.4%) AN participants compared to 54 (1.0%) unexposed participants (p <0.001). A history of fracture before the start of observation was more common among females with AN than unexposed females (p <0.001), but there was no statistically significant difference in prevalent fractures between males with AN versus unexposed males. Antidepressant use and alcohol abuse were more prevalent among both males and females with AN compared to those without eating disorders (all p <0.001).
Table 1.
Cohort characteristics
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Anorexia nervosa | No eating disorder | P | Anorexia nervosa | No eating disorder | P | |
| N | 9239 | 92,329 | 556 | 5560 | ||
| Age at start of observation, yearsa | 29.8 (22.8–38.6) | 29.8 (22.8–38.8) | 30.2 (22.1, 40.2) | 30.2 (21.8, 40.5) | ||
| Calendar year at start of observationa | 2005 (2001, 2008) | 2005 (2001, 2008) | 2004 (2001, 2008) | 2004 (2001, 2008) | ||
| Age at diagnosis, yearsa | 19.0 (16.0–24.0) | N/A | 19.5 (15.7, 25.0) | N/A | ||
| Country | ||||||
| England | 7474 (80.9%) | 74,687 (80.9%) | 454 (81.7%) | 4540 (81.7%) | ||
| Ireland | 253 (2.7%) | 2530 (2.7%) | 14 (2.5%) | 140 (2.5%) | ||
| Scotland | 987 (10.7%) | 9862 (10.7%) | 58 (10.4%) | 580 (10.4%) | ||
| Wales | 525 (5.7%) | 5250 (5.7%) | 30 (5.4%) | 300 (5.4%) | ||
| Observation time, yearsa | 3.4 (1.3, 7.0) | 3.7 (1.5, 7.3) | <0.001 | 3.5 (1.4, 7.3) | 4.0 (1.6, 7.9) | 0.06 |
| Body mass indexa,b | 20.0 (17.9–22.3) | 24.0 (21.4–28.2) | <0.001 | 21.4 (18.3, 24.2) | 25.9 (22.7, 29.1) | <0.001 |
| History of prior fracture | 1615 (17.5%) | 11,946 (12.9%) | <0.001 | 145 (26.1%) | 1381 (24.8%) | 0.52 |
| Anti-depressant use | 2942 (31.8%) | 11,924 (12.9%) | <0.001 | 151 (27.2%) | 352 (6.3%) | <0.001 |
| Alcohol abuse | 196 (2.1%) | 383 (0.4%) | <0.001 | 18 (3.2%) | 47 (0.9%) | <0.001 |
Median (inter-quartile range),
N=5994 for anorexia nervosa and 38,312 for no eating disorder
Fracture Incidence
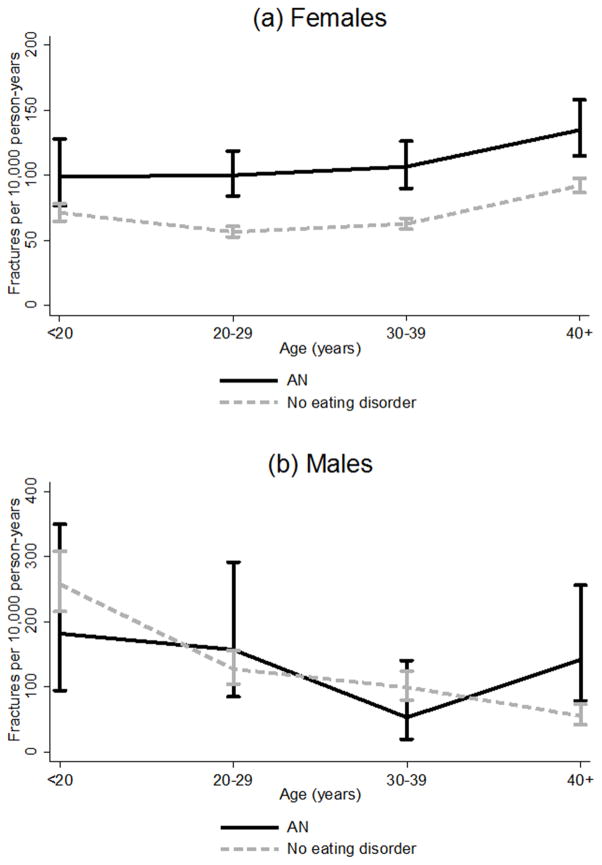
Over a median observation period of 3.4 and 3.7 years respectively, 507 incident fractures (112 per 10,000 person years) occurred in participants with AN compared with 3455 in those without eating disorders (73 per 10,000 person years). For participants with AN, the median time from the initial code for AN to the first incident fracture event was 12.2 years (IQR 5.0, 22.4). Figure 1 shows the incidence of first fracture in participants with AN versus participants without eating disorders. The fracture incidence in females with AN was higher than in unexposed females across all age groups. Of note, the incidence in males aged >40 years with AN was rate 142 per 10,000 person-years compared with rate 56 per 10,000 person years in their unexposed peers.
Figure 1.
Fracture incidence.
Cox Regression Analysis of Fracture Risk
Overall, the HR for incident fracture associated with AN was 1.54 (95% CI: 1.40, 1.69; p <0.001). However, a statistically significant three-way interaction was found among AN, sex, and age (interaction p = 0.002, Table 2). Therefore, all analyses were stratified on sex and age (Figure 2). Among female participants, there was a higher risk of fracture (HR 1.59) in those with AN compared to participants without eating disorders (95% CI: 1.44, 1.75; p <0.001), and this risk did not differ according to age (interaction p = 0.48). Among male participants, the risk of fracture associated with AN varied according to age (p for interaction with age >40 vs. ≤40 = 0.005). As shown in Table 2 and Figure 2, AN was associated with a significantly higher risk of fracture among males >40 years of age (HR 2.54, 95% CI 1.32 to 4.90; p = 0.005) but not among males ≤40 years (HR 0.82, 95% CI: 0.54, 1.26; p = 0.37). There were 11 incident fractures in males 40 years of age or older and 23 fractures in males ≤40 years of age. As shown in Table 2b, antidepressant use and alcohol abuse were independently associated with an increased risk of fracture in females. After adjustment for these covariates, AN remained associated with a higher risk of fracture: HR 1.31 (95% CI: 1.10, 1.56; p = 0.002) in females >40 years of age and HR 1.49 (95% CI: 1.32, 1.68; p <0.001) in females ≤40 years of age. In males >40 years of age, alcohol abuse was independently associated with a more than 6-fold higher risk of fracture, but did not substantially change model findings in terms of the higher risk of fracture associated with AN (HR 2.21, 95% CI: 1.11, 4.42; p = 0.02).
Table 2a.
Age- and sex-stratified Cox models of the HR for fracture associated with AN
| Females | Males | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age ≤40 years | 1.66 (1.47, 1.87) | <0.001 | 0.82 (0.54, 1.26) | 0.37 |
| Age >40 years | 1.46 (1.23, 1.73) | <0.001 | 2.54 (1.32, 4.90) | 0.005 |
Figure 2.
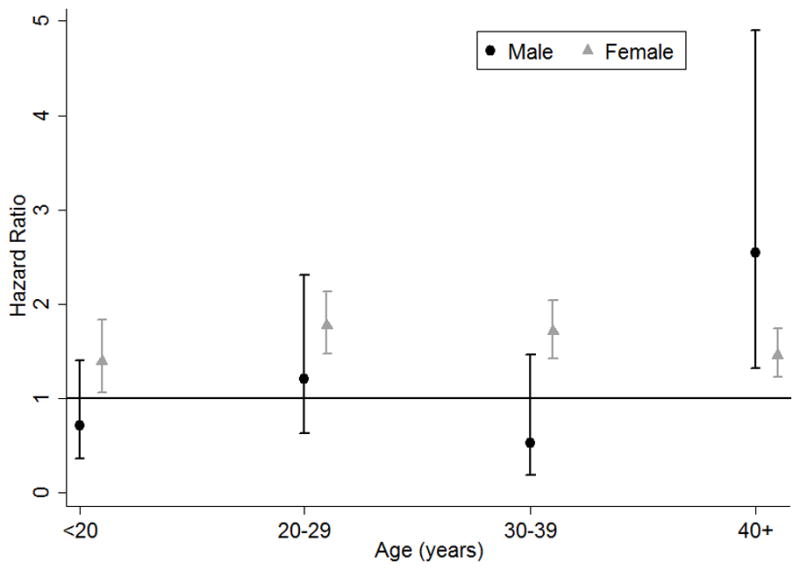
Hazard ratio for fracture associated with anorexia nervosa stratified by age and sex.
Table 2b.
Multivariable age- and sex-stratified Cox models of the HR for fracture associated with AN adjusted for antidepressant and alcohol use
| Females | Males | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) a | P | HR (95% CI) a | P | |
| Age ≤40 years | ||||
| AN | 1.49 (1.32, 1.68) | <0.001 | 0.74 (0.48, 1.15) | 0.18 |
| Antidepressant use | 1.51 (1.36, 1.68) | <0.001 | 1.47 (0.98, 2.20) | 0.06 |
| Alcohol abuse | 2.75 (1.90, 3.99) | <0.001 | 1.76 (0.65, 4.78) | 0.26 |
| Age >40 years | ||||
| AN | 1.31 (1.10, 1.56) | 0.002 | 2.21 (1.11, 4.42) | 0.02 |
| Antidepressant use | 1.46 (1.28, 1.67) | <0.001 | 1.20 (0.56, 2.55) | 0.64 |
| Alcohol abuse | 2.55 (1.72, 3.78) | <0.001 | 6.49 (2.26, 18.58) | <0.001 |
HR refers to fully adjusted model including AN, antidepressant use and alcohol abuse
We performed sex-stratified and age-adjusted Cox regression analysis of fracture risk in the subset of individuals with AN who had BMI data (n = 5994) and their matched individuals without an eating disorder who had available BMI data (n = 27,409). Among female participants, there was a higher risk of fracture (HR 1.46) in those with AN compared to participants without eating disorders (95% CI: 1.27, 1.69; p <0.001). Among male participants, AN was not associated with an increased risk of fracture, even in those 40 years of age or older. However, it should be noted that there were only 64 individuals and 3 fracture events in this age group. We also performed sex-stratified and age-adjusted Cox regression analysis of fracture risk restricting the entire cohort to those with a BMI <25 (normal or underweight, n = 5302 with AN/n = 14,192 with no eating disorder). Among female participants, there was again a higher risk of fracture (HR 1.54) in those with AN compared to participants without eating disorders (95% CI: 1.31, 1.83; p <0.001). Among male participants, AN was not associated with an increased risk of fracture. Among females, there was a significant interaction between AN and BMI (p = 0.04); higher BMI was protective in females with AN (HR 0.94, 95% CI: 0.90, 0.99; p = 0.02), but not in females without eating disorders. Indeed, among females with AN, being severely underweight (BMI <16) was associated with a HR for fracture of 1.71 (95% CI: 1.18, 2.48; p = 0.004).
Fracture Site
As shown in Table 3, females with AN had a higher risk of fracture at nearly all anatomic sites, with a two- to three-fold higher risk of vertebral, skull/face, clavicle/scapula, humerus/elbow, rib/thorax, and multi-site fractures. The greatest excess fracture risk (more than four-fold higher) was noted at the hip/femur (HR 5.59; 95% CI, 3.44, 9.09) and pelvis (HR 4.54; 95% CI 2.42, 8.50). Males with AN had a significantly increased risk of vertebral fractures (HR 7.25; 95% CI, 1.21, 43.45) compared to unexposed males, but not at other sites.
Table 3.
Age-adjusted hazard ratio (HR) for fracture associated with anorexia nervosa in females versus males
| Site | Number of events | Females | Males | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Females | Males | HR (95% CI) | P | HR (95% CI) | P | |
| Vertebra | 98 | 5 | 2.19 (1.30, 3.69) | 0.003 | 7.25 (1.21, 43.45) | 0.03 |
| Skull/face | 139 | 36 | 2.15 (1.39, 3.34) | 0.001 | 0.30 (0.04, 2.18) | 0.23 |
| Pelvis | 46 | 1 | 4.54 (2.42, 8.50) | <0.001 | ----- | ----- |
| Rib/thorax | 130 | 15 | 2.87 (1.89, 4.36) | <0.001 | 1.67 (0.38, 7.42) | 0.50 |
| Clavicle/scapula | 114 | 34 | 2.20 (1.36, 3.57) | 0.001 | 0.32 (0.04, 2.32) | 0.26 |
| Humerus/elbow | 244 | 17 | 2.17 (1.56, 3.02) | <0.001 | 1.39 (0.32, 6.10) | 0.66 |
| Forearm/wrist | 736 | 46 | 1.49 (1.19, 1.85) | <0.001 | 0.74 (0.23, 2.40) | 0.62 |
| Hand | 657 | 124 | 1.40 (1.11, 1.78) | 0.005 | 0.73 (0.35, 1.49) | 0.38 |
| Hip/femur | 72 | 7 | 5.59 (3.44, 9.09) | <0.001 | 1.89 (0.23, 15.74) | 0.56 |
| Lower leg/ankle | 554 | 48 | 1.28 (0.98, 1.68) | 0.07 | 0.71 (0.22, 2.28) | 0.57 |
| Foot | 714 | 62 | 1.36 (1.08, 1.71) | 0.008 | 1.81 (0.89, 3.67) | 0.10 |
| Multiple sites | 11 | 1 | 3.89 (1.03, 14.67) | 0.045 | ----- | ----- |
| Not specified | 723 | 57 | 1.91 (1.56, 2.33) | <0.001 | 1.26 (0.54, 2.94) | 0.59 |
| Overall | 3589 | 373 | 1.59 (1.45, 1.75) | <0.001 | 1.05 (0.74, 1.49) | 0.80 |
DISCUSSION
This is the first study to examine sex differences in fracture risk in AN using a large population-based cohort. We found that males with AN had an increased risk of fractures at the vertebrae and that males over 40 years of age with AN had increased overall fracture risk compared to males without an eating disorder in the same age group. Females with AN had increased fracture risk across anatomic sites and across all ages, with a particularly high risk of fracture at the hip/femur and pelvis.
Though 5–15% of patients with AN are male (13), little is known regarding fracture risk in males with AN. To our knowledge, only two previous studies have evaluated fracture risk in males with AN. One Danish retrospective cohort study included 2,149 patients with AN and found an increased risk for fractures compared to controls, but did not stratify results by sex (19). The only study to include a separate fracture risk for males with AN, a retrospective cohort from Minnesota, had a small sample size (n=15 males) and is dated (participants were diagnosed between 1935 and 1989 and the paper was published in 1999) (20). Our study therefore adds substantially to the literature on fracture risk among males with AN, finding an increased risk in age >40 years and at the vertebrae.
Fracture risk in AN was increased among males over 40 but not among adolescent or young adult males. The lack of increased fracture risk in this younger age group may be mediated by other factors. In the general population, adolescent boys have a significantly increased fracture risk compared to adolescent girls, thought to be secondary to injuries and activities such as high-impact contact sports (37,38). Males with AN may be less likely to participate in high-impact ball sports (such as football, rugby) that are associated with fractures, although eating disorders are associated with excessive exercise and are observed in higher proportions among athletes of endurance sports (such as running and cycling), aesthetic sports (such as figure skating, diving, and gymnastics), and sports with weight classes (such as rowing) compared to non-athletes (39,40). One prior study that assessed fracture risk in adolescent females did not find a significant association with increased physical activity and higher rates of fracture (22). In addition, exercise restrictions may be placed on many adolescents with AN (22). Older unexposed males may not participate as much in behaviors at high risk for fractures or injuries which may account for the increased fracture risk observed in the >40 year age group. The median age at start of observation in our study was around 30 years. Furthermore, males with eating disorders may be more likely than females with eating disorders to present with atypical AN; therefore, despite significant weight loss their weight and BMI may be above or within the normal range (41). Previous studies have demonstrated that prior obesity is a risk factor among males with restrictive eating disorders (41,42). This relatively larger BMI in males may also influence their fracture risk compared to females.
Males with AN had an increased risk of vertebral fractures. Vertebral fractures are a common type of fragility fracture and their presence indicates a substantial risk for subsequent fractures, independent of bone mineral density (43,44). Vertebral fractures may be underestimated, however, as they may be subclinical. The one previous study to include stratified data for males with AN did not find an increased vertebral fracture rate, but had a small sample size (0 incident vertebral fractures among 15 males) (20). One study of young women with AN followed with dual-energy X-ray absorptiometry found that 12.5% had incident vertebral fractures. Vertebral fractures, although generally asymptomatic, are associated with a five-fold increased risk of future symptomatic fracture independent of BMD (45). Although compared with a healthy population, vertebral fractures were higher in males with AN, when compared to females with AN, the males tended to have a higher occurrence of fractures at other sites such as the rib/thorax, hand, hip/femur, foot, and skull/face than of the vertebrae.
Previous studies on fracture risk mainly in females with AN demonstrated an increased fracture rate (incidence rate ratio [IRR] 1.98, 95% CI 1.60 to 2.44) with the highest increased fracture rate at the femoral neck (IRR 7.17, 95% CI 3.36 to 15.32) (18,19). One study from Minnesota found the greatest increased rates of fracture at the pelvis (standardized incidence ratio 4.8; 95% CI 1.3 to 12.0) (20). Our study confirms and expands upon these findings among females with AN, finding the highest risk sites for fracture at the hip/femur and the pelvis, sites that are traditionally associated with osteoporosis (20).
Females with AN may have a higher risk of hip or pelvis fractures due to a few potential mechanisms. Because of a low calorie intake, patients with AN may be at increased risk of orthostatic vital sign changes, falling, or syncopal episodes, with subsequent hip or pelvis fractures (18). In addition, severely malnourished patients with AN may have loss of the natural “fat cushion” on the hips, which makes the hip in particular more vulnerable in a fall (18). AN may be associated with increased fracture risk due to several physiologic mechanisms. Profound undernutrition in AN deprives the skeleton of vital building materials, such as calcium, vitamin D, and protein (18). Although approximately 30% of patients with AN who were not previously supplemented with vitamin D have 25-hydroxyvitamin D levels less than 20 ng/mL, the prevalence of vitamin D deficiency largely depends on whether or not patients were receiving supplementation for vitamin D at the time of testing (46–48). For instance, one study of adolescents with AN who were already in treatment for their eating disorder at a tertiary medical center where supplementation of a multivitamin containing vitamin D was the standard of care reported that only 2% of AN patients were deficient, compared to 24% of healthy controls (49). In females, AN is also associated with amenorrhea and estrogen deficiency which leads to loss of bone mass. Antidepressant use (50,51) and consuming more than two alcoholic drinks per day (52) have been previously shown to be associated with increased fracture risk. Our study confirms these associations in the AN population, particularly among females.
In the analysis of normal or underweight females, higher BMI was associated with a decreased fracture risk in those with AN. However, this association was not observed in females without eating disorders. The relationship between BMI and fracture risk is most pronounced at BMI levels below 20 kg/m2 (53,54). Only small decreases in fracture risk have been observed at BMI levels greater than 25 kg/m2 (53,54). The association between obesity and fractures reported in the literature may be site dependent; obese females have been shown to be at higher risk of proximal humerus fractures but at lower risk of pelvic and hip fractures compared to normal weight and underweight females (55).
Our study has several limitations. The THIN database does not include data on race. Although this is the largest study to date examining fractures and AN, particularly in males, there were relatively few fracture events in males; therefore, there were large confidence intervals in the HR for males. The diagnosis of AN was based on Read codes which have not been validated in THIN and as a result, there was potential for misclassification; however, any inaccuracies would be unlikely to create systematic bias and more likely to bias towards the null. THIN data are documented by the general practitioner and are thus reliant on this individual to accurately enter information from specialists, such as psychologists, psychiatrists, and eating disorder specialists for our secondary utilization of this data. The missing BMI data, though consistent with prior THIN studies (32,33), is a limitation of the study. It should be noted that the degree of missing data was considerably lower in individuals with AN (39%) than in those with no eating disorder (61%). Sensitivity analyses limited to normal or underweight individuals confirmed the increased risk of fracture in females with AN demonstrated in the full cohort and further demonstrated that having a lower BMI was a risk factor for fracture in females with AN but not in those without eating disorders, with a 71% higher risk in those with a BMI <16. Although AN was not found to be associated with an increased risk of fracture among the subset of male participants 40 years of age or older with BMI data, it should be noted that there were only 64 individuals and 3 fracture events in this age group with BMI data. This small subset was underpowered to detect an increased fracture risk in males 40 years of age or older. There were only five vertebral fractures reported in males with AN; thus, conclusions should be considered given this relatively small number of events. Vertebral fractures may also be underestimated given their propensity to be subclinical. Based on the coding, we were unable to discern whether reported fractures were traumatic or fragility fractures. Dietary intake and physical activity data were not available given the nature of the data. Finally, the data cannot establish a causal mechanism, although it can confirm associations. Although the THIN database has been shown to be representative of the UK, there is a possible lack of generalizability to other geographic regions.
Our study has several strengths that expand upon and fill critical gaps in the previous literature. First, the robust size of the THIN database allowed us to study over 9,500 patients with AN and compare them with over 95,000 unexposed participants without an eating disorder who were matched on sex, age, and practice. The only previous study in AN to include a separate fracture risk for males had a sample size of n=15 males (20), compared to the relatively larger sample size of n=556 males in this study. Second, we were able to delineate age and sex effects, as the longitudinal nature of the study allowed us to assess the risk of incident fracture in each decade of life. Third, we were able to discern more clearly differences in the distribution of fracture site. Finally, the THIN database has been shown to be representative of the larger UK population (56), making our findings generalizable to the UK.
CONCLUSIONS
AN and fractures remain formidable health problems with broad public health implications worldwide. This is the first large epidemiologic study to examine sex differences in fracture risk in AN. We found that males with AN had an increased risk of fractures at the vertebrae and that males over 40 years of age with AN had an increased overall fracture risk compared to males without an eating disorder. Females with AN had increased fracture risk across all ages and nearly all anatomic sites with a more than four-fold higher risk at the hip/femur and pelvis. Sex differences in fracture risk among adolescents with AN may be behaviorally mediated.
Acknowledgments
This project was supported by NIH Grants UL1 RR024134 and K23 DK093556 (MD) and 5R01HD082166-02 (NG). The funders had no role in the design, analysis, or results of the study. Authors roles: JN, NG, ML, LC, MD conceptualized the study; MD performed the data analysis; JN wrote the manuscript; JN, NG, ML, LC, MD substantially revised the manuscript.
Footnotes
Disclosure Statement: MRD has received research funding (outside the submitted work) from Genentech, Inc and Mallinckrodt Pharmaceuticals and has a consultancy agreement with Infiniti Medical. MBL has received funding from a NephCure Foundation/American Society of Nephrology Research Grant, Genentech Inc, and the National Kidney Foundation/Amgen Kidney Disease Outcomes Quality Initiative Research Fellowship. MBL has consultancy agreements with Amgen Inc, Johnson & Johnson, and Novartis. She is on the Scientific Advisory Board of Marodyne Medical and an NIH Data Safety and Monitoring Board. The remaining authors have no conflicts of interest to disclose.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker AE, Grinspoon SK, Klibanski A, Herzog DB. Eating Disorders. N Engl J Med. 1999 Apr 08;340(14):1092–1098. doi: 10.1056/NEJM199904083401407. 2014/04. [DOI] [PubMed] [Google Scholar]
- 4.Misra M, Klibanski A. Anorexia nervosa and bone. J Endocrinol. 2014 Jun;221(3):R163–76. doi: 10.1530/JOE-14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra M, Golden NH, Katzman DK. State of the art systematic review of bone disease in anorexia nervosa. Int J Eat Disord. 2016 Mar;49(3):276–292. doi: 10.1002/eat.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden NH, Kreitzer P, Jacobson MS, Chasalow FI, Schebendach J, Freedman SM, et al. Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr. 1994 Oct;125(4):655–660. doi: 10.1016/s0022-3476(94)70030-3. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003 Dec;88(12):5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 8.Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int. 2003;14( Suppl 3):S2–8. doi: 10.1007/s00198-002-1340-9. [DOI] [PubMed] [Google Scholar]
- 9.Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990 Sep;86(3):440–447. [PubMed] [Google Scholar]
- 10.Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004 Dec;114(6):1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 11.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002 Sep;87(9):4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 12.Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002 Jun;15(3):135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 13.Andersen AE, Holman JE. Males with eating disorders: challenges for treatment and research. Psychopharmacol Bull. 1997;33(3):391–397. [PubMed] [Google Scholar]
- 14.Misra M, Katzman DK, Clarke H, Snelgrove D, Brigham K, Miller KK, et al. Hip structural analysis in adolescent boys with anorexia nervosa and controls. J Clin Endocrinol Metab. 2013 Jul;98(7):2952–2958. doi: 10.1210/jc.2013-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra M, Katzman DK, Cord J, Manning SJ, Mendes N, Herzog DB, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008 Aug;93(8):3029–3036. doi: 10.1210/jc.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata JM, Golden NH, Peebles R, Long J, Leonard MB, Carlson JL. Assessment of sex differences in bone deficits among adolescents with anorexia nervosa. International Journal of Eating Disorders. 2016 doi: 10.1002/eat.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata JM, Golden NH, Peebles R, Long J, Murray SB, Leonard MB, et al. Assessment of Sex Differences in Body Composition Among Adolescents with Anorexia Nervosa. Journal of Adolescent Health. 2017 doi: 10.1016/j.jadohealth.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Patients with eating disorders. A high-risk group for fractures. Orthop Nurs. 2003 Sep-Oct;22(5):325–331. doi: 10.1097/00006416-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders--a nationwide register study. Int J Eat Disord. 2002 Nov;32(3):301–308. doi: 10.1002/eat.10101. [DOI] [PubMed] [Google Scholar]
- 20.Lucas AR, Melton LJ, 3rd, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999 Oct;74(10):972–977. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 21.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. 1991;265:1133–1138. [PubMed] [Google Scholar]
- 22.Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014 Jul;47(5):458–466. doi: 10.1002/eat.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domine F, Berchtold A, Akre C, Michaud PA, Suris JC. Disordered eating behaviors: what about boys? J Adolesc Health. 2009;44:111–7. doi: 10.1016/j.jadohealth.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y. Diuretic initiation and the acute risk of hip fracture. Osteoporos Int. 2013 Feb;24(2):689–95. doi: 10.1007/s00198-012-2053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins GS, Mallett S, Altman DG. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651. doi: 10.1136/bmj.d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez L, Roskell N, Castellsague J, et al. Clinical burden and incremental cost of fractures in postmenopausal women in the United Kingdom. Bone. 2012;51:324–31. doi: 10.1016/j.bone.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez L, Roskell N, Castellsague J, et al. Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ. 2011;14:99–107. doi: 10.3111/13696998.2010.547967. [DOI] [PubMed] [Google Scholar]
- 28.Orton E, Kendrick D, West J, Tata LJ. Independent risk factors for injury in pre-school children: three population-based nested casecontrol studies using routine primary care data. PLoS One. 2012;7:e35193. doi: 10.1371/journal.pone.0035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujades-Rodriguez M, Smith CJ, Hubbard RB. Inhaled corticosteroids and the risk of fracture in chronic obstructive pulmonary disease. QJM. 2007;100:509–17. doi: 10.1093/qjmed/hcm056. [DOI] [PubMed] [Google Scholar]
- 30.Schelleman H, Pollard JR, Newcomb C, et al. Exposure to CYP3A4-inducing and CYP3A4-non-inducing antiepileptic agents and the risk of fractures. Pharmacoepidemiol Drug Saf. 2011;20:619–25. doi: 10.1002/pds.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Staa TP, Kanis JA, Geusens P, Boonen A, Leufkens HG, Cooper C. The cost-effectiveness of bisphosphonates in postmenopausal women based on individual long-term fracture risks. Value Health. 2007;10:348–57. doi: 10.1111/j.1524-4733.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 32.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9:2133–40. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN) Diabetes Care. 2015 Oct;38(10):1913–1920. doi: 10.2337/dc15-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chisholm J. The Read clinical classification. BMJ. 1990 Apr 28;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denburg MR, Jemielita TO, Tasian GE, et al. Assessing the risk of incident hypertension and chronic kidney disease after exposure to shockwave lithotripsy and ureteroscopy. Kidney Int. 2016 Jan;89(1):185–92. doi: 10.1038/ki.2015.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005 Jul;14(7):443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 37.Blimkie CJ, Lefevre J, Beunen GP, Renson R, Dequeker J, Van Damme P. Fractures, physical activity, and growth velocity in adolescent Belgian boys. Med Sci Sports Exerc. 1993 Jul;25(7):801–808. doi: 10.1249/00005768-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 39.Rosendahl J, Bormann B, Aschenbrenner K, Aschenbrenner F, Strauss B. Dieting and disordered eating in German high school athletes and non-athletes. Scand J Med Sci Sports. 2009 Oct;19(5):731–739. doi: 10.1111/j.1600-0838.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- 40.Thiemann P, Legenbauer T, Vocks S, Platen P, Auyeung B, Herpertz S. Eating disorders and their putative risk factors among female German professional athletes. Eur Eat Disord Rev. 2015 Jul;23(4):269–276. doi: 10.1002/erv.2360. [DOI] [PubMed] [Google Scholar]
- 41.Carlat DJ, Camargo CA, Jr, Herzog DB. Eating disorders in males: a report on 135 patients. Am J Psychiatry. 1997 Aug;154(8):1127–1132. doi: 10.1176/ajp.154.8.1127. [DOI] [PubMed] [Google Scholar]
- 42.Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health. 2003 Dec;33(6):427–435. doi: 10.1016/s1054-139x(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 43.Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, et al. Vertebral Fracture Assessment: the 2007 ISCD Official Positions. J Clin Densitom. 2008 Jan-Mar;11(1):92–108. doi: 10.1016/j.jocd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Burger H, van Daele PL, Algra D, Hofman A, Grobbee DE, Schutte HE, et al. Vertebral deformities as predictors of non-vertebral fractures. BMJ. 1994 Oct 15;309(6960):991–992. doi: 10.1136/bmj.309.6960.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divasta AD, Feldman HA, Gordon CM. Vertebral fracture assessment in adolescents and young women with anorexia nervosa: a case series. J Clin Densitom. 2014 Jan-Mar;17(1):207–211. doi: 10.1016/j.jocd.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modan-Moses D, Levy-Shraga Y, Pinhas-Hamiel O, Kochavi B, Enoch-Levy A, Vered I, et al. High prevalence of vitamin D deficiency and insufficiency in adolescent inpatients diagnosed with eating disorders. Int J Eat Disord. 2015 Sep;48(6):607–614. doi: 10.1002/eat.22347. [DOI] [PubMed] [Google Scholar]
- 47.Gatti D, El Ghoch M, Viapiana O, Ruocco A, Chignola E, Rossini M, et al. Strong relationship between vitamin D status and bone mineral density in anorexia nervosa. Bone. 2015 Sep;78:212–215. doi: 10.1016/j.bone.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Veronese N, Solmi M, Rizza W, Manzato E, Sergi G, Santonastaso P, et al. Vitamin D status in anorexia nervosa: A meta-analysis. Int J Eat Disord. 2015 Nov;48(7):803–813. doi: 10.1002/eat.22370. [DOI] [PubMed] [Google Scholar]
- 49.Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int. 2008 Mar;19(3):289–294. doi: 10.1007/s00198-007-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moura C, Bernatsky S, Abrahamowicz M, Papaioannou A, Bessette L, Adachi J, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS) Osteoporos Int. 2014 May;25(5):1473–1481. doi: 10.1007/s00198-014-2649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Brand MW, Pouwels S, Samson MM, van Staa TP, Thio B, Cooper C, et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos Int. 2009 Oct;20(10):1705–1713. doi: 10.1007/s00198-009-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Jr, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008 May;121(5):406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005 Nov;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 54.Compston J. Obesity and bone. Curr Osteoporos Rep. 2013 Mar;11(1):30–35. doi: 10.1007/s11914-012-0127-y. [DOI] [PubMed] [Google Scholar]
- 55.Prieto-Alhambra D, Premaor MO, Fina Aviles F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012 Feb;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 56.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]