Abstract
Objective
Depression is often associated with disruptions in sleep and circadian rhythms. We aimed to confirm these relationships via actigraphic assessment in a large, population-based sample, and test whether sex moderates these relationships.
Methods
418 participants (age 35–85, mean 57.04, SD = 11.47) completed questionnaires and one week of actigraphy, used to calculate sleep and rest-activity statistics including mesor (mean activity level), amplitude (height of rhythm), and acrophase (time of day that rhythm peaks).
Results
Depressive symptoms, assessed via Center for Epidemiologic Studies Depression Scale (CES-D), were associated with disrupted sleep and rest-activity rhythms. Furthermore, men demonstrated longer sleep onset latency (SOL; B = −13.28, p < .001), longer wake time after sleep onset (B = −6.26, p < .01), lower sleep efficiency (B = 5.91, p < .001), and lower total sleep time (TST; B = 33.16, p < .001) than women. Sex moderated the relationship between depression and SOL, TST, mesor, and amplitude; sex-stratified models revealed higher depression scores were associated with greater SOL (B = 1.05, p < .001) and less TST (B = −0.87, p < .10) for women with higher depressive symptoms, but lower mesor (B = −1.75, p < .01) and amplitude (B = −1.94, p < .01) for men with higher depressive symptoms.
Conclusions
Depressive symptoms were related to disrupted sleep continuity and rest-activity rhythms in this population-based sample; however, these relationships differed by sex. Women with greater depressive symptoms exhibited difficulty with sleep continuity, whereas men with greater depressive symptoms demonstrated disruption throughout the 24-hour rhythm.
Keywords: depression, sex differences, actigraphy, sleep continuity, rest-activity rhythms
Disturbances in the sleep-wake cycle and rest-activity rhythms are consistently associated with unipolar depression as risk factors for developing depression, symptoms during episodes, and lingering symptoms after remission that predict recurrence (1–3). Depressed individuals demonstrate disrupted circadian and rest-activity rhythms, including delayed melatonin secretion (4), diurnal mood variation with worse mood in the morning and delayed activity peak (5, 6), and less robust and stable rest-activity rhythms (7–9). Sleep difficulties in depression are characterized most frequently by insomnia, but also by hypersomnia in some individuals (10). Overall, disruptions include reduced total sleep time, lower sleep efficiency, longer time to fall asleep, more awakenings during the night, shorter rapid eye movement (REM) sleep latency, and prolonged first REM sleep period duration (11, 12).
The relationship between depression and impaired sleep and rest-activity patterns may vary by sex. Women in general experience greater lifetime incidence of depression (13, 14) and higher insomnia rates than men (15). However, studies of sleep and activity patterns in depressed subjects present mixed findings. Some actigraphy studies demonstrated that men had shorter sleep duration, more sleep fragmentation, and less stability in rest-activity rhythms over several days than women (7, 16, 17) whereas polysomnographic findings indicated that men generally had longer total sleep time and more slow wave and REM sleep but longer sleep latencies than women, although effect sizes were small (18). One study that focused on sex differences in depressive symptom severity found that, among other symptoms, depressed women reported more sleep complaints than depressed men (19). Considering the important role that sleep and activity level play in precipitating and maintaining depressive symptoms, it is possible that the sex differences associated with depression, sleep, and activity level may lead to differences in the relationship between depression and sleep and rest-activity rhythms.
In this study, we assessed the relationship between depressive symptoms and disturbances in sleep and rest-activity rhythms in a large, population-based sample using actigraphy, whereas previous research has often considered single-sex, medical, or depressed samples (2, 3, 6, 8). We hypothesized that higher depression scores would be related to more disrupted sleep continuity and rest-activity rhythms. We further examined the role of sex in moderating the relationship between depressive symptoms and sleep and rest-activity rhythms. Because the majority of studies have indicated that women are more vulnerable to developing depression, and depressed women have more significant sleep disruptions such as insomnia, we expected that sleep continuity and rest-activity disturbances would be more severe in women with higher levels of depressive symptoms than men with higher levels of depressive symptoms.
Methods
Participants
Participants were 440 individuals who completed the Biomarker Project, a subsample from the MIDUS II (Midlife in the United States) survey. MIDUS is a longitudinal, national survey that included a population-based sample of 7108 adults age 25 to 75 at study start in 1995. Of this sample, a geographic subset (n = 1255) participated in the Biomarker Project, which included several physiological assessments described elsewhere (20). Relevant to the current study, actigraphy was completed by 440 participants living near Madison and Milwaukee, WI, along with other self-report and interview measures. Participants were removed from the present analyses if they were nightshift workers (n = 5) or if they had poor actigraphy compliance, defined as having less than 5 days (71%) usable data (n = 17). The final sample was 418 participants. This subsample of the Biomarker Project differs from the larger MIDUS sample at study start as follows: older age as expected with a longitudinal cohort (t (5916) = 3.2, p = .001), more female representation (60% versus 54%), and more non-White representation due to intentional recruitment of African Americans living in an urban center (Milwaukee, WI) with the larger Biomarker Project (32% versus 19%); however, this subsample did not differ from the larger sample in terms of education level.
Procedure
Participants provided informed consent and underwent procedures described elsewhere (20). Relevant to this study, participants completed a phone interview, physical examination, questionnaires, and one week of actigraphy. The University of Wisconsin Health Sciences Institutional Review Board approved study procedures.
Measures
Participants provided demographic (i.e., age, sex, race) and socioeconomic (i.e., education level) information via phone interview and reported whether they exercised at least 20 minutes three times per week and currently smoked cigarettes. Height and weight were collected during an in-person physical exam to calculate body mass index (BMI).
Participants also completed the Center for Epidemiologic Studies Depression Scale (CES-D) (21) and Pittsburgh Sleep Quality Index (PSQI) (22). The CES-D is a 20-item scale that assesses depressive symptoms in the past week; it shows good convergence with other measures of depression and adequate internal consistency (alpha was 0.73 in this sample). Scores above 16 indicate clinically-significant levels of depression (23). Current use of sleep medications was determined via the PSQI medication subscale, which consists of one item assessing whether participants use sleep medications not at all (0), less than once per week (1), once or twice per week (2), or three or more times per week (3).
Actigraphy
All participants completed one week of actigraphy via Mini Mitter Actiwatch®-64 along with a sleep diary indicating time that participants went to sleep and got up the following morning. Participants wore the actigraph continuously on their non-dominant wrist for seven consecutive days and were asked to press the event marker button on the actigraph when attempting sleep and waking up for the day. Data were collected in 30-second epochs and downloaded to Philips Actiware for a total possible number of 20,160 epochs per participant. Rest intervals used to compute sleep statistics were manually defined as indicated in the participant’s sleep diary. If a sleep diary value was not available, event markers were used to determine rest intervals, which occurred in setting rest intervals for 28 participants (6.2% of the sample). If event markers were not available, imputations were used (i.e., for weekdays, the average of the other weekdays with at least 3 weekdays needed for imputation; for weekends, the value from the other weekend day), which occurred in setting rest intervals for 22 participants (5.3% of the current sample). Also, all data were inspected to exclude unusable segments (i.e., when the watch was off wrist by participant report). For the current sample, the average amount of usable epochs was 18,201 (SD = 1,039), so on average about 10% of epochs (about 0.7 days) were unusable due to the watch being removed. Then, sleep onset latency (SOL), wake time after sleep onset (WASO), sleep efficiency (SE), and total sleep time (TST) were computed by the Actiware program algorithms based on the rest intervals. Data for the sleep indices were aggregated across the seven days of data collection using mean values.
Additionally, the raw actigraphy data were converted to 1-minute epochs and exported to SAS 9.3 (SAS Institute Inc.) A traditional cosinor analysis was used to simultaneously fit 12- and 24-hour rhythms to epoch-by-epoch activity counts averaged by the minute over the 24-hour period for all days (24). This model yields three statistics that describe an individual’s rest-activity rhythm. Mesor refers to the intercept of the model, or the mean activity level for a participant, considering the cosine model. Amplitude indicates the difference between the peak or trough and the mean of the circadian rhythm wave, providing a measure of the height of the rhythm. Finally, acrophase indexes the time of day that the peak of the rhythm occurs.
Statistical Analyses
Multiple linear regression was used to test for main effects of depressive symptoms and sex in their association with sleep and rest-activity rhythm variables. We added an interaction term to test whether sex moderated the relationship between depressive symptoms and sleep and rest-activity rhythm variables. For all regression models, the depression score was treated as a continuous variable and all independent variables were centered at their mean (sex: women = 0.5, men = −0.5). In each model, we adjusted for age, race, education level, smoker status, exercise, BMI, and use of sleep medications. We further explored all models where the interaction between depressive symptoms and sex was significant at p < .10 by running sex-stratified simple regressions to test for a main effect of depressive symptoms as they relate to the sleep or rest-activity rhythm variable. All reported regression weights refer to standardized coefficients.
Results
Participant characteristics are provided in Table 1. There were significant differences between men and women for race (Χ2 (1, N = 418) = 9.27, p = .002), with women more likely to identify as non-White than men. No other sex differences emerged, including no sex differences in CES-D scores (t (412) = .19, p = .85), with 17.5% of men and 15.9% of women scoring above the clinical significance cut off. Unadjusted correlations between depressive symptoms and sleep and rest-activity indices revealed significant relationships between higher levels of depressive symptoms and longer SOL, greater WASO, lower SE, and later acrophase (all r’s ranged from +/− 0.2–0.3, p < 0.01). Higher levels of depressive symptoms demonstrated trending relationships with less TST and lower amplitude (both r’s = −0.1, p = 0.07). There was no significant relationship between depressive symptoms and mesor at the bivariate level (r = −0.01, p = 0.8).
Table 1.
Sample Descriptive Characteristics
| Overall | Men | Women | ||||
|---|---|---|---|---|---|---|
| Mean (SD; range) |
N (%) | Mean (SD; range) |
N (%) | Mean (SD; range) |
N (%) | |
| Age | 57.0 (11.5; 35–85) |
58.2 (11.6; 37–85) |
56.3 (11.3; 35–84) |
|||
| Sex (% female) | 252 (60.3) | |||||
| Race | ||||||
| White | 284 (67.9) | 127 (76.5) | 157 (62.3) | |||
| Black and/or African American | 88 (21.1) | 27 (16.3) | 61 (24.2) | |||
| Native American or Alaska Native | 1 (0.2) | 0 (0) | 1 (0.4) | |||
| Asian | 2 (0.5) | 1 (0.6) | 1 (0.4) | |||
| Multiracial | 38 (9.1) | 9 (5.4) | 29 (11.5) | |||
| Other | 4 (1.0) | 2 (1.2) | 2 (0.8) | |||
| Don’t know | 1 (0.2) | 0 (0) | 1 (0.4) | |||
| Years of education | 14.6 (2.7; 8–21) |
14.8 (2.7; 7.5–22) |
14.4 (2.8; 7.5–22) |
|||
| Body mass index | 30.6 (7.1; 17.1–65.1) |
30.0 (5.7; 20.5–49.8) |
31.0 (7.9; 17.1–65.1) |
|||
| Exercise (% current exerciser) | 300 (71.8) | 121 (72.9) | 179 (71) | |||
| Smoker status (% current smoker) | 61 (14.6) | 30 (18.1) | 31 (12.3) | |||
| PSQI-Sleep medications | ||||||
| Not during the past month | 316 (75.6) | 134 (80.7) | 182 (72.2) | |||
| Less than once a week | 32 (7.6) | 9 (5.4) | 23 (9.1) | |||
| Once or twice a week | 15 (3.6) | 3 (1.8) | 12 (4.8) | |||
| Three or more times a week | 55 (13.2) | 20 (12.0) | 35 (13.9) | |||
| CES-D | 8.9 (8.3; 0–54) |
9.0 (8.6; 0–54) |
8.9 (8.1; 0–37) |
|||
PSQI = Pittsburgh Sleep Quality Index, CES-D = Center for Epidemiologic Studies Depression Scale.
Multiple regression models are reported in Table 2, and all models are adjusted for the aforementioned covariates. Depressive symptoms were significantly associated with the sleep and rest-activity rhythm variables SOL, SE, and acrophase, indicating that higher depression scores were related to longer time to fall sleep, less efficient sleep, and a more delayed rest-activity pattern (Model 1). Sex was a significant main effect, independent of depressive symptoms, for SOL, WASO, SE, and TST with men demonstrating longer SOL, longer WASO, lower SE, and lower TST than women (Model 2).
Table 2.
Multiple Regressions with Actigraphic Sleep Continuity and Rest-Activity Variables
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| B | t | B | t | B | t | |
| Sleep Onset Latency | ||||||
| CES-D | 0.56 | 2.82** | 0.51 | 2.59* | 0.46 | 2.34* |
| Sex | −13.28 | −4.44*** | −12.99 | −4.35*** | ||
| CES-D*Sex | 0.66 | 1.85† | ||||
| Adjusted R2 | 0.02** | 0.06*** | 0.06*** | |||
|
Wake Time After Sleep Onset |
||||||
| CES-D | 0.28 | 1.87† | 0.26 | 1.70† | 0.24 | 1.56 |
| Sex | −6.26 | −2.70** | −6.14 | −2.65** | ||
| CES-D*Sex | 0.27 | 0.98 | ||||
| Adjusted R2 | 0.01† | 0.02** | 0.02** | |||
| Sleep Efficiency | ||||||
| CES-D | −0.14 | −2.23* | −0.12 | −1.93† | −0.11 | −1.76† |
| Sex | 5.91 | 6.38** | 5.85 | 6.31*** | ||
| CES-D*Sex | −0.13 | −1.21 | ||||
| Adjusted R2 | 0.01* | 0.09*** | 0.09*** | |||
| Total Sleep Time | ||||||
| CES-D | −0.15 | −0.34 | −0.01 | −0.01 | 0.12 | 0.28 |
| Sex | 33.16 | 5.20*** | 32.41 | 5.10*** | ||
| CES-D*Sex | −1.71 | −2.26* | ||||
| Adjusted R2 | 0.00 | 0.06*** | 0.07*** | |||
|
Mesor (mean activity level) |
||||||
| CES-D | −0.52 | −1.14 | −0.51 | −1.12 | −0.66 | −1.43 |
| Sex | 2.56 | 0.36 | 2.85 | 0.40 | ||
| CES-D*Sex | 1.70 | 2.02* | ||||
| Adjusted R2 | 0.00 | 0.00 | 0.01* | |||
|
Amplitude (height of the rest- activity rhythm) |
||||||
| CES-D | −0.74 | −1.68† | −0.73 | −1.64† | −0.89 | −1.98* |
| Sex | 7.06 | 1.01 | 7.38 | 1.06 | ||
| CES-D*Sex | 1.86 | 2.27* | ||||
| Adjusted R2 | 0.003† | 0.003† | 0.01* | |||
|
Acrophase (peak of the rest- acitvity rhythm) |
||||||
| CES-D | 0.03 | 2.54* | 0.03 | 2.58* | 0.03 | 2.51* |
| Sex | 0.17 | 0.89 | 0.17 | 0.89 | ||
| CES-D*Sex | 0.00 | 0.19 | ||||
| Adjusted R2 | 0.01* | 0.01* | 0.01* | |||
p < .10;
p < .05;
p < .01;
p < .001.
CES-D = Center for Epidemiologic Studies Depression Scale.
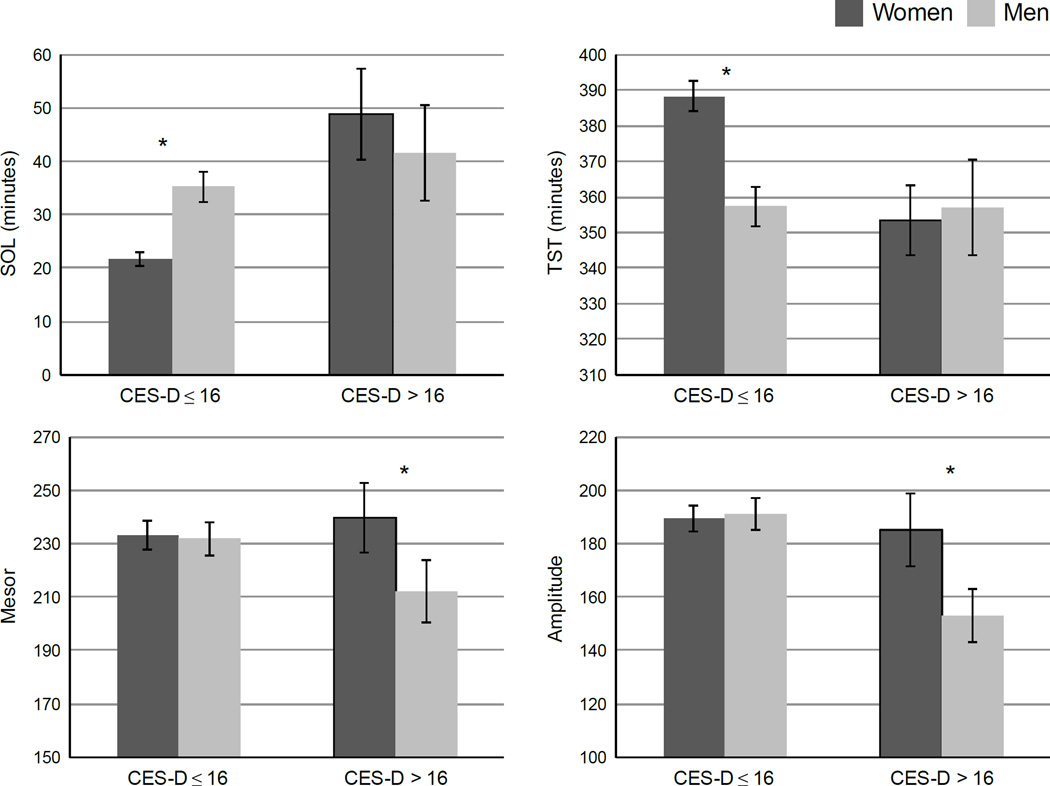
Sex moderated the relationship between depressive symptoms and SOL, TST, mesor, and amplitude (Model 3). Because sex was a significant moderator for these variables, these models were run as sex-stratified simple regressions (Table 3). To visualize these findings, Figure 1 depicts the means for SOL, TST, mesor, and amplitude for men and women scoring above or below the CES-D cut-off score for clinically significant levels of depression. For the sleep continuity variables, higher depression scores were associated with greater SOL and less TST (trend, p = .095) for women, whereas these relationships were non-significant in men. Independent-samples t-tests confirmed that women with high depressive symptoms showed greater SOL (t (236) = −5.92, p < .001) and less TST (t (236) = 3.20, p = .002) than women with low depressive symptoms, but did not differ in SOL or TST as compared to men either with high depressive symptoms (SOL, t (58) = −.59, p = .56; TST, t (58) = .23, p = .82) or low depressive symptoms (for TST only, t (160) = .34, p = .74; SOL marginally higher among women with high depressive symptoms, t (160) = 1.91, p = .058). In contrast, for the rest-activity rhythm variables, higher depression scores were associated with lower mesor and lower amplitude for men, with no significant main effects among women. Follow-up independent t-tests confirmed that men with high depression scores demonstrated lower amplitude than women with low depression scores (t (239) = 2.71, p = .007), women with high depression scores (t (67) = −1.81, p = .075), and men with low depression scores (t (164) = 2.70, p = .008), with mesor trending in the same direction.
Table 3.
Sex-Split Simple Regressions
| Women | Men | |||
|---|---|---|---|---|
| B | t | B | t | |
| Sleep Onset Latency | ||||
| CES-D | 1.05 | 4.76*** | −0.14 | −0.39 |
| Adjusted R2 | 0.08*** | 0.00 | ||
| Total Sleep Time | ||||
| CES-D | −0.87 | −1.67† | 0.95 | 1.39 |
| Adjusted R2 | 0.01† | 0.01 | ||
|
Mesor (mean activity level) |
||||
| CES-D | 0.48 | 0.76 | −1.75 | −2.81** |
| Adjusted R2 | 0.00 | 0.03** | ||
|
Amplitude (height of the rest- activity rhythm) |
||||
| CES-D | 0.25 | 0.42 | −1.94 | −2.97** |
| Adjusted R2 | 0.00 | 0.04** | ||
p < .10;
p < .05;
p < .01;
p < .001.
CES-D = Center for Epidemiologic Studies Depression Scale.
Figure 1.
Sex moderates the relationships between depression and SOL, TST, mesor (mean activity level), and amplitude (height of the rest-activity rhythm). SOL = Sleep Onset Latency, TST = Total Sleep Time. * indicates statistically significant contrast. Error bars represent the standard error of the mean. Women with CES-D ≤ 16 (n = 212), mean CES-D score = 6.2 (SD = 4.6); Women with CES-D > 16 (n = 40), mean CES-D = 24.2 (SD = 6.6); Men with CES-D ≤ 16 (n = 137), mean CES = 6.0 (SD = 4.7); Men with CES-D > 16 (n = 29), mean CES-D = 24.0 (SD = 7.4).
Discussion
Depressive symptoms were associated with disrupted sleep continuity and rest-activity rhythms in this population-based sample; however, the relationship with depressive symptoms differed by sex. Women with higher depression scores demonstrated more sleep disruption than less depressed women, particularly with a longer time to fall asleep and less total time asleep. This is consistent with previous research indicating worse self-reported sleep problems among depressed women (19). In contrast, rest-activity rhythms were less robust (i.e., lower mesor and amplitude) among men with higher depression scores, indicating more disruption in the full 24-hour cycle. Polysomnographic studies similarly suggest that sex moderates the relationship between depression and sleep; specifically, depressed women show increased slow wave activity while depressed men show no change or decreased slow wave activity (25, 26). It has been suggested that women may require more sleep than men (e.g., 17), which in turn could make sleep more vulnerable to disruption by psychological disorders like depression. Furthermore, since women report more frequent insomnia than men (15), it is possible that sleep disruption plays a more causal role in developing depression for women. In either case, our study provides additional support that sleep is an area of particular vulnerability for women with greater depressive symptoms.
Importantly, regardless of depression symptom severity, we found that men showed worse actigraphic sleep continuity than women on all measures, replicating previous actigraphic findings (16, 17). It is possible that this contributed to a floor effect, making it more difficult to detect a relationship between depressive symptoms and sleep disruption in men. Considering this finding in the context of rest-activity rhythms being less robust in men with greater depressive symptoms, men presenting with depressive symptoms would likely benefit from comprehensive assessment of the full 24-hour rest-activity pattern, including disruptions in sleep continuity and daytime symptoms such as behavioral withdrawal and decreased physical activity. Supplementing clinical interviews with sleep and daytime activity diaries or actigraphy monitoring may be particularly useful to provide information regarding both nighttime sleep and daytime activity and rest-activity rhythm disruption. In addition, depression interventions could take sex into account with greater focus on nighttime sleep disruption in women (e.g., cognitive-behavioral interventions for insomnia) and more attention to the 24-hour rhythm for men (e.g., adding behavioral activation and social rhythm strategies to insomnia treatment).
Across the full sample, as expected, depression was associated with greater sleep disruption. However, these findings were significant only for SOL and SE; because SE in part reflects SOL, it is likely that depressed individuals may struggle most with falling asleep. This could result from a more delayed rest-activity and sleep pattern that can be typical among depressed individuals, found in the present sample (i.e., a significant relationship between later acrophase and higher depressive symptoms) and elsewhere (4; 8; 9). Depressive symptoms were also related to a less robust overall rhythm (i.e., lower amplitude), consistent with previous studies (8, 9).
Unlike a study in older women (6), we did not find that depressive symptoms were related to impaired rest-activity rhythms among women. However, our sample did not include many women in the age range studied by Maglione and colleagues (i.e., over age 70) so it is possible that this relationship does not emerge until late adulthood. Of note, men and women did not differ in depression severity in this sample. Depression rates differ the least between sexes in older populations or children (27), so it is possible that the generally older age of this sample (mean 56.87 years) contributed to the similar depressive symptom severity between men and women.
Strengths of this study include the large, diverse, population-based sample and continuous recording of seven days of actigraphic data. Actigraphic indices included more traditional sleep-wake statistics that have the limitation of only being a proxy for sleep and wakefulness since actigraphy cannot distinguish sleep from quiet wakefulness. However, actigraphic indices also included cosinor indices categorizing the rest-activity pattern (i.e., mesor, amplitude, and acrophase), thus producing measures of the full 24-hour period for analysis.
Limitations include the inability to determine causal relationships from this cross-sectional study design. Previous research has observed that sleep and rest-activity rhythm disruption can predict development of depression, while current depression is associated with further disruption in these areas (2, 3). Furthermore, it is important to note that the effect sizes associated with the reported analyses are small to medium in size (see R2 reported in Tables 2 and 3). Thus, although depression and sex may be related to sleep continuity and rest-activity rhythms, it is likely that other variables not examined in this study also play an important role in determining these relationships. In addition, depressive symptom severity was assessed via self-report measure, which can be subject to over- or under-reporting; future replication using clinical interviews would be useful.
In summary, these findings emphasize the dynamic relationship between depression, sex, and sleep and rest-activity rhythms, pointing to the need for further research on the role of sex in improving assessment and treatment of sleep continuity and rest-activity dysregulation in depression.
Acknowledgments
Sources of Funding: All authors have received grant support from Merck; Dr. Benca has served as a consultant for Merck, Janssen, and Jazz; all unrelated to this work. The current analyses were support by the University of Wisconsin Center for Sleep Medicine and Research. The MIDUS I study (Midlife in the U.S.) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS II research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS I investigation. The research was further supported by the following grants: 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Abbreviations
- SOL
sleep onset latency
- WASO
wake time after sleep onset
- SE
sleep efficiency
- TST
total sleep time
- REM
rapid eye movement
- MIDUS
Midlife in the United States
- CES-D
Center for Epidemiologic Studies Depression Scale
- PSQI
Pittsburgh Sleep Quality Index
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Baglioni C, Riemann D. Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Current Psychiatry Reports. 2012;14:511–518. doi: 10.1007/s11920-012-0308-5. [DOI] [PubMed] [Google Scholar]
- 2.Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, Stone KL Group SoOFR. Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep. 2014;37:1179–1187. doi: 10.5665/sleep.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smagula SF, Ancoli-Israel S, Blackwell T, Boudreau R, Stefanick ML, Paudel ML, Stone KL, Cauley JA Group OFiMMR. Circadian Rest-Activity Rhythms Predict Future Increases in Depressive Symptoms Among Community-Dwelling Older Men. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crasson M, Kjiri S, Colin A, Kjiri K, L'Hermite-Baleriaux M, Ansseau M, Legros JJ. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology. 2004;29:1–12. doi: 10.1016/s0306-4530(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 5.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, Tranah GJ, Stone KL Research Group SoOF. Depressive symptoms and circadian activity rhythm disturbances in community-dwelling older women. Am J Geriatr Psychiatry. 2014;22:349–361. doi: 10.1016/j.jagp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30:1223–1230. doi: 10.3109/07420528.2013.813528. [DOI] [PubMed] [Google Scholar]
- 8.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18:105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 9.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Perlis ML, Giles DE, Buysse DJ, Thase ME, Tu X, Kupfer DJ. Which depressive symptoms are related to which sleep electroencephalographic variables? Biol Psychiatry. 1997;42:904–913. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 11.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- 12.Arfken CL, Joseph A, Sandhu GR, Roehrs T, Douglass AB, Boutros NN. The status of sleep abnormalities as a diagnostic test for major depressive disorder. J Affect Disord. 2014;156:36–45. doi: 10.1016/j.jad.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, Replication NCS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 14.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–1375. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 19.Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry. 2013;70:1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- 20.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Halberg F, Tong Y, Johnson E. Circadian system phase - an aspect of temporal morphology; procedures and illustrative examples. In: H vM, editor. The cellular aspects of biorhythms. New York: Springer-Verlag; 1968. pp. 20–48. [Google Scholar]
- 25.Plante DT, Goldstein MR, Landsness EC, Peterson MJ, Riedner BA, Ferrarelli F, Wanger T, Guokas JJ, Tononi G, Benca RM. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146:120–125. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armitage R, Hoffman R, Trivedi MH, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 27.Jorm AF. Sex and age differences in depression: a quantitative synthesis of published research. Aust N Z J Psychiatry. 1987;21:46–53. doi: 10.3109/00048678709160898. [DOI] [PubMed] [Google Scholar]