Abstract
Background
Prostate cancer has a propensity to invade and grow along nerves, a phenomenon called perineural invasion (PNI). Recent studies suggest that presence of PNI in prostate cancer has been associated with cancer aggressiveness.
Methods
We investigated the association between PNI and lethal prostate cancer in untreated and treated prostate cancer cohorts: the Swedish Watchful Waiting Cohort of 615 men who underwent watchful waiting, and the U.S. Health Professionals Follow-Up Study of 849 men treated with radical prostatectomy. One pathologist performed a standardized histopathologic review assessing PNI and Gleason grade. Patients were followed from diagnosis until metastasis or death.
Results
The prevalence of PNI was 7% and 44% in the untreated and treated cohorts, respectively. PNI was more common in high Gleason grade tumors in both cohorts. PNI was associated with enhanced tumor angiogenesis, but not tumor proliferation or apoptosis. In the Swedish study, PNI was associated with lethal prostate cancer (odds ratio, OR, 7.4, 95% confidence interval, CI, 3.6, 16.6; p<0.001). A positive, though not statistically significant, association persisted after adjustment for age, Gleason grade, and tumor volume (OR 1.9, 95% CI 0.8, 5.1; p=0.17). In the U.S. study, PNI predicted lethal prostate cancer independent of clinical factors (HR 1.8, 95% CI 1.0, 3.3; p=0.04).
Conclusion
These data support the hypothesis that perineural invasion creates a microenvironment that promotes cancer aggressiveness.
Impact
Our findings suggest that PNI should be a standardized component of histopathologic review, and highlights a mechanism underlying prostate cancer metastasis.
INTRODUCTION
An important determinant of tumor behavior is the ability to breach basement membranes and spread outside the confines of the organ of origin. The classic paradigm of tumor metastasis is that of tumor spread via blood vessels and lymphatic channels. However, prostate cancer has long been recognized to show a propensity to invade and grow along prostatic nerves, which lead out of the prostate to the pelvic plexus (1). While this phenomenon, known as perineural invasion (PNI), has classically been thought to occur because nerves provide cancer cells a low resistance path out of the prostate, recent studies suggest a more dynamic interaction (2–4). Prostate cancer cells adjacent to nerves show increased proliferation compared to those located further away (5, 6). Capsular nerve count as assessed by image analysis has also been shown to be significantly higher in areas adjacent to prostate tumor than normal tissue, suggesting the induction of nerve growth (7). Thus, the perineural space may be a microenvironment that promotes both cancer spread and growth.
Despite the biologic plausibility of PNI being a potential determinant of prostate cancer behavior, an association between PNI and prostate cancer progression continues to be a subject of debate, and PNI is not routinely reported on prostate cancer pathology reports. While some studies have failed to find an association between PNI and prostate cancer outcomes (8–26), many others have demonstrated the clinical significance of PNI detection in both biopsy (27–37) and prostatectomy (38–43) samples. In Tables 1 and 2 we summarize previously published studies assessing the association between PNI in prostate biopsy (Table 1) and radical prostatectomy (Table 2) specimens and risk of recurrence and cancer-specific mortality.
Table 1.
Summary of selected studies evaluating the prognostic value of PNI in prostate biopsy specimens
| Study Size |
Treatment | PNI Prevalence no. (%) |
Follow-Up (yr) |
Presence of PNI in biopsy specimen predicts: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Pathologic Features |
Recurrence/Progression |
Survival |
|||||||
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| Moreira et al, 2015 (27) | 302 | AS | 11 (3.6) | NS | NA | Yes | Yes | NR | NR |
| Cohn et al, 2014 (28) | 165 | AS | 14 (8.5) | 0.5 | NA | Yes | Yes | NR | NR |
| Tollefson et al, 2014 (29) | 451 | RP | 102 (22.7) | 12.9 | NR | Yes | Yes | Yes | Yes |
| DeLancey et al, 2013 (30) | 3226 | RP | 634 (19.7) | NS | EPE, SVI, PSM | Yes | Yes | Yes | Yes |
| Katz et al, 2013 (31) | 599 | RP | 105 (17.5) | 3.9 | GS, PS | Yes | NR | NR | NR |
| Feng et al, 2011 (32) | 651 | EBRT | 220 (33.8) | 5.2 | NA | Yes | Yes | Yes | Yes |
| Loeb et al, 2010 (8) | 1256 | RP | 188 (15.0) | 2.8 | EPE, SVI | Yes | No | NR | NR |
| Vora et al, 2007 (33) | 416 | EBRT | 41 (9.9) | 5.0 | NA | Yes | Yes | NR | NR |
| Yu et al, 2007 (34) | 586 | EBRT | 112 (19.1) | 5.7 | NA | Yes | Yes | NR | NR |
| Beard et al, 2006 (35) | 517 | EBRT | 84 (16.2) | 4.5 | NA | NR | NR | Yes | NR |
| Merrick et al, 2005 (9) | 512 | BT | 133 (26.0) | 5.3 | NA | No | No | NR | NR |
| Beard et al, 2004 (10) | 381 | EBRT | 86 (22.5) | 2.8 | NA | Yes | No | NR | NR |
| Pollack et al, 2004 (11) | 839 | EBRT | 98 (11.7) | 5.3 | NA | NR | No | NR | NR |
| Nelson et al, 2003 (12) | 1414 | RP | 199 (16.1) | 3.1 | NR | NR | No | NR | NR |
| Quinn et al, 2003 (13) | 696 | RP | 72 (10.4) | 4.6 | GS, EPE, PSM | Yes | No | NR | NR |
| D’Amico et al, 2001 (36) | 750 | RP | 53 (7.1) | 4.0 | EPE | NR | Yes | NR | NR |
| de la Taille et al, 1999 (37) | 319 | RP | 77 (24.1) | 2.1 | NR | Yes | Yes | NR | NR |
PNI: perineural invasion; AS: active surveillance; RP: radical prostatectomy; EBRT: external-beam radiation therapy; BT: brachytherapy; NA: not applicable; NR: not recorded; PS: pathologic stage; EPE: extraprostatic extension; SVI: seminal vesicle invasion; PSM: positive surgical margin; GS: Gleason score
Table 2.
Summary of selected studies evaluating the prognostic value of PNI in radical prostatectomy specimens
| Study Size |
Treatment | PNI Prevalence no. (%) |
Follow-Up (yr) |
Presence of PNI in RP specimen predicts: |
||||
|---|---|---|---|---|---|---|---|---|
| Recurrence/Progression |
Survival |
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Hashimoto et al, 2015 (14) | 784 | RP | 557 (71.0) | 1.5 | Yes | No | NR | NR |
| Kozal et al, 2015 (15) | 742 | RP | 21 (2.8) | 2.6 | Yes | No | NR | NR |
| Reeves et al, 2015 (16) | 1497 | RP | 1173 (78.4) | 1.2 | Yes | No | NR | NR |
| Tanimoto et al, 2015 (17) | 439 | RP | NR | 1.3 | Yes | No | NR | NR |
| Andersen et al, 2014 (38) | 535 | RP | 134 (25.0) | 7.4 | Yes | Yes | Yes | Yes |
| Ost et al, 2011 (39) | 278 | RP+RT±ADT | 134 (84.2) | 3.6–5.0 | Yes | Yes | NR | NR |
| Psutka et al, 2011 (40) | 300 | RP | NR | 12.0 | Yes | Yes | NR | NR |
| Tanaka et al, 2011 (41) | 468 | RP±RT/ADT | 226 (50.7) | 4.4 | Yes | Yes | NR | NR |
| Lee et al, 2010 (18) | 361 | RP | 188 (52.1) | 3.5 | Yes | No | No | No |
| Menon et al, 2010 (19) | 1384 | RP | 832 (60.1) | 5.0 | Yes | No | NR | NR |
| Jeon et al, 2009 (42) | 237 | RP | 41 (17.3) | 3.5 | Yes | Yes | NR | NR |
| Kumano et al, 2009 (20) | 267 | RP | 165 (61.8) | NR | Yes | No | NR | NR |
| Mizuno et al, 2009 (21) | 164 | RP | 117 (71.3) | NR | Yes | No | NR | NR |
| Miyake et al, 2005 (22) | 202 | RP | NR | NR | Yes | No | NR | NR |
| Ng et al, 2004 (23) | 364 | RP | 287 (78.8) | 1.0 | No | NR | NR | NR |
| Mian et al, 2002 (24) | 188 | RP | NR | 5.0 | No | No | NR | NR |
| Maru et al, 2001 (43) | 640 | RP | 477 (74.5) | 4.0 | Yes | Yesa | NR | NR |
| Quinn et al, 2001 (25) | 732 | RP±RT | 375 (51.3) | 3.3 | Yes | No | NR | NR |
| van den Ouden et al, 1997 (26) | 273 | RP | 207 (75.8) | 4.1 | Yes | No | No | No |
PNI: perineural invasion; AS: active surveillance; RP: radical prostatectomy; EBRT: external-beam radiation therapy; ADT: androgen-deprivation therapy; NA: not applicable; NR: not recorded;
PNI diameter
Most prior studies looking at PNI as a prognostic factor in radical prostatectomy specimens have focused on biochemical recurrence as the outcome of interest. Only three studies (18, 26, 38) have examined PNI as a predictor of prostate cancer-specific mortality in men treated with surgery, with only one of them (38) showing PNI to predict mortality on multivariate analysis. This is critical as most men who develop a biochemical recurrence are not destined to develop lethal prostate cancer (44–46). Our work focuses on exploring the relationship between PNI and prostate cancer-specific deaths in a novel fashion: no prior study has explored this association among initially untreated men. Using two large population-based prostate cancer cohorts, we set out to determine whether PNI is an independent predictor of lethal prostate cancer in men treated with watchful waiting or radical prostatectomy, as well as to evaluate the association between PNI and tumor markers of disease aggressiveness.
MATERIALS AND METHODS
Study Population
The study was nested within two prostate cancer cohorts: the Swedish Watchful Waiting Cohort and the U.S. Health Professionals Follow-Up Study (HPFS) prostate tumor cohort. The Swedish cohort included men from the southeast region of Sweden diagnosed with clinically localized prostate cancer between 1977 and 1999 after undergoing transurethral resection of the prostate (TURP) for benign prostatic hyperplasia (BPH) (47, 48). As per the standard of care in Sweden at the time, these men were followed expectantly as part of a watchful waiting protocol. Men who went on to develop symptomatic locally advanced or metastatic disease were treated with androgen deprivation therapy. We retrieved archival TURP tissue specimens for 661 of the men who form the study cohort, as described in more detail elsewhere (49), with 615 of these specimens ultimately being evaluated for PNI.
In 1986, the HPFS enrolled 51,529 male health professionals aged 40 to 75 years, all of who were cancer-free at baseline. The men are followed every two years with detailed questionnaires to collect information on lifestyle factors, medications, and health-related information. For this project, we included men diagnosed with incident prostate cancer between 1986 and 2004 for whom archival prostatectomy (95%) or TURP (5%) tissue specimens were available. Our sample of 849 cases represents 49% of the prostate cancer cases in the cohort that were treated with radical prostatectomy. Details of the HPFS prostate tumor cohort are described elsewhere (50, 51). Importantly, men whose tumors were evaluated for PNI did not differ from those men who were not sampled in terms of their clinical characteristics.
Approval for the study was obtained from the Institutional Review Board of the Harvard School of Public Health, Partners Health Care and the Uppsala-Örebro and Linköping Ethical Review Board.
Data Collection
Data on age, clinical stage, and PSA level at diagnosis (for HPFS cases only) were abstracted from medical records and pathology reports. The vital status of all patients in the Swedish cohort was assessed using the Swedish Death Register and was up-to-date as of March 1st, 2006. In the HPFS, deaths were ascertained by periodic review of the U.S. National Death Index and through report from next of kin (last reviewed in December 2012). Cause of death was determined through review of medical records and autopsy reports by endpoint committees of physicians. Data on biochemical recurrence and distant metastases were tracked through patient questionnaires and review of medical records from treating physicians.
Histopathologic and Tumor Biomarker Evaluation
One dedicated genitourinary pathologist (MF) undertook a systematic histopathologic review of all available H&E slides for each case, blinded to patient outcome, for the presence or absence of PNI, Gleason grade, pathologic tumor stage (1997 American Joint Committee on Cancer TNM Classification), high-grade prostatic intraepithelial neoplasia (hgPIN), acute and chronic inflammation, and patterns of atrophy (simple atrophy, simple atrophy cyst formation, partial atrophy, and post-atrophic hyperplasia). TURP specimens were also assessed for tumor volume (as measured by the percentage of the specimen involved with cancer). The presence or absence of PNI was assessed in each available tumor slide. PNI was defined as the presence of complete circumferential encirclement of nerve structures by malignant glands. Cases with non-circumferential PNI or a single focus of PNI among multiple tumor slides were deemed PNI-negative.
Tumor proliferation, apoptosis and angiogenesis were also examined in a subset of the patients in the HPFS. Ki67 immunostaining (N=399 cases) was performed on tumor tissue microarrays using a polyclonal antibody (Vector Labs), diluted 1:2,000 after citrate-based antigen retrieval, and scored by quantitative image analysis. A TUNEL assay (N=335) on tissue microarrays was used to assess apoptosis. For a subset of patients (N=339), whole section specimens were analyzed for morphologic markers of tumor angiogenesis (vessel density, area, diameter, and irregularity) by staining for the CD34 marker and using image analysis as described previously (52).
Statistical Analysis
Associations between PNI status and clinical and pathologic characteristics were assessed using the chi-square test for categorical variables and generalized linear models or the Wilcoxon rank sum test for continuous variables. Ki67 and TUNEL scores were categorized into quartiles to account for their non-normal distributions, while angiogenesis parameters were log-transformed.
Multivariable regression analyses were performed to determine whether PNI is an independent predictor of lethal prostate cancer. In the Swedish cohort, a nested case-control design was devised to maximize efficiency in the pathologic review and included men who died of prostate cancer at any point during follow-up as cases (N=228), and men who survived for 10 years or more as controls (N=387). Unconditional logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI).
The HPFS was analyzed using Cox proportional hazards regression to calculate hazard ratios (HR) and 95% CI. The event was defined as the development of bone or visceral metastases or death due to prostate cancer. Person-time was calculated from the date of diagnosis to development of metastases, death due to prostate cancer (whichever occurred earlier), death from other causes, or the end of follow-up (December 2011). Competing risks regression was used to assess the impact of death from other causes on the effect estimates.
Models were adjusted for age at diagnosis (continuous) and Gleason grade (ordinal; ≤6, 3+4, 4+3, 8 and ≥9). Additionally, models for the Swedish cohort were adjusted for percentage of tumor involved with cancer (continuous), while the HPFS models were adjusted for pathologic TNM stage (≤pT2, pT3, pT4/N+). Patients in the HPFS with TURP specimens were excluded from models that adjusted for pathologic TNM stage. We tested whether the relationship between PNI and lethal prostate cancer varied by Gleason grade and tumor volume/stage using interaction terms and nested log likelihood models.
Statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1 (Cary, NC) and R version 3.2.2.
RESULTS
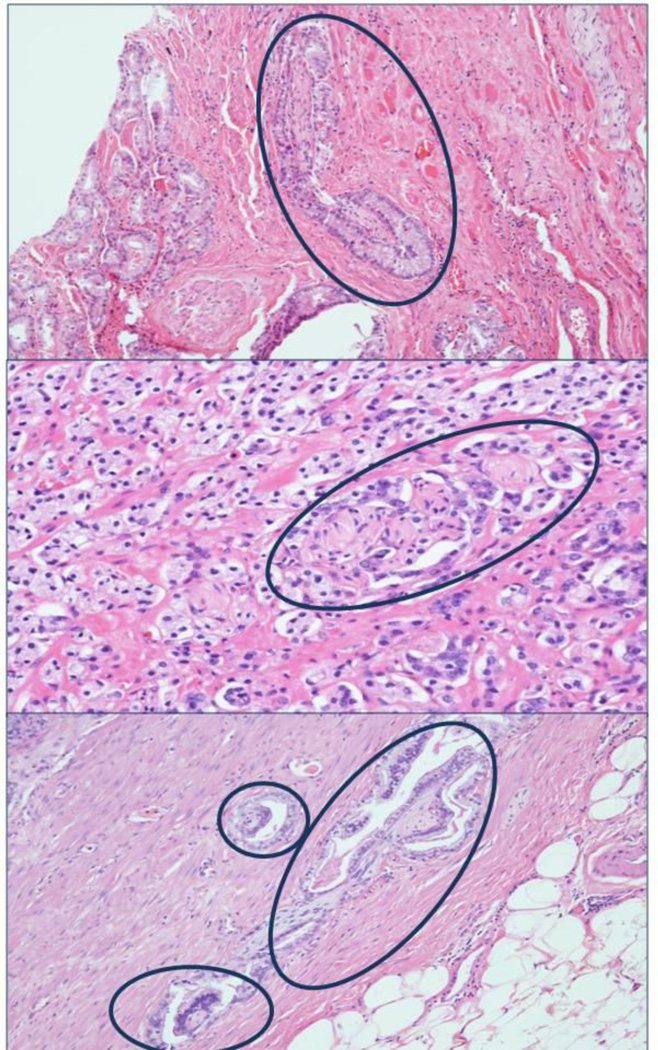
Men in the Swedish cohort were, on average, older at the time of diagnosis than those in the HPFS (mean age 73.0 years vs. 66.0 years, respectively). Tumors in the Swedish cohort tended to be of lower grade: although the proportion of men with high grade (Gleason ≥8) tumors was similar (19% vs. 25% in the Swedish cohort and HPFS, respectively), the proportion of men with Gleason 7 tumors was significantly higher in the HPFS (58% vs. 34%). Most tumors (59%) in the Swedish cohort were staged as T1b (defined as >5% of resected tissue involved with cancer or Gleason grade ≥7). In the HPFS, 72% of men had localized tumors at prostatectomy (≤pT2). Figure 1 shows H&E slides with PNI adjacent to prostate cancer.
Figure 1.
Hematoxylin & eosin-stained tissue slides showing presence of PNI adjacent to prostate cancer, Health Professionals Follow-Up Study.
Swedish Watchful Waiting Cohort
PNI was present in 43 of 615 (7%) prostate cancer cases in the Swedish cohort. Patients with tumors harboring PNI tended to be slightly older (mean age 74.8 years vs. 72.9 years, Table 3). PNI was strongly associated with high Gleason grade; two-thirds of tumors with PNI were Gleason grade ≥8 tumors compared to only 16% of tumors without PNI (ptrend<0.001). PNI was also positively associated with tumor volume and presence of hgPIN, a precursor lesion of prostate cancer (p=0.01). There was no association between the presence of PNI and chronic inflammation or any of the atrophy lesions.
Table 3.
Clinical and pathologic characteristics of prostate cancer cases in the Swedish Watchful Waiting Cohort and Health Professionals Follow-Up Study by presence of PNI
| Swedish Cohort | HPFS | |||
|---|---|---|---|---|
| No PNI | PNI | No PNI | PNI | |
| Overall, n (%) | 572 (93) | 43 (7) | 479 (56) | 370 (44) |
| Lethal prostate cancer, n (%) | 194 (85) | 34 (15) | 29 (33) | 58 (67) |
| Death from other causes, n (%) | – | 149 (60) | 98 (40) | |
| Diagnosis prior to 1995, n (%) | 449 (93) | 34 (7) | 154 (56) | 120 (44) |
| Age at diagnosis (years), mean (SD) | 72.9 (6.6) | 74.8 (6.8) | 65.9 (6.1) | 66.1 (5.6) |
| PSA (ng/dL), median (IQR) | – | 6.1 (4.7, 9.4) |
6.6 (4.8, 10.5) |
|
| Gleason score, n (%) | ||||
| ≤6 | 285 (99) | 2 (1) | 132 (28) | 18 (5) |
| 3+4 | 117 (97) | 4 (3) | 192 (40) | 99 (27) |
| 4+3 | 79 (90) | 9 (10) | 89 (19) | 110 (30) |
| 8 | 33 (89) | 4 (11) | 36 (52) | 33 (48) |
| ≥9 | 58 (71) | 24 (29) | 30 (21) | 110 (79) |
| Pathologic TNM stage, n (%) | ||||
| pT2 | – | 373 (64) | 207 (36) | |
| pT3 | 64 (31) | 141 (69) | ||
| pT4/N+ | 5 (23) | 17 (77) | ||
| Percent specimen with cancer, median (IQR) |
5 (2, 20) | 40 (10, 60) | – | |
| hg PIN, n (%) | 69 (86) | 11 (14) | 226 (54) | 194 (46) |
| Acute inflammation, n (%) | 83 (99) | 1 (1) | 135 (60) | 91 (40) |
| Chronic inflammation, n (%) | ||||
| None | 146 (90) | 17 (10) | 59 (50) | 60 (50) |
| Mild | 277 (95) | 16 (5) | 244 (58) | 176 (42) |
| Moderate | 132 (93) | 10 (7) | 134 (58) | 96 (42) |
| Severe | 17 (100) | 0 (0) | 35 (53) | 31 (47) |
| Simple atrophy, n. (%) | 344 (94) | 22 (6) | 357 (58) | 263 (42) |
| Simple atrophy cyst formation, n (%) | 36 (95) | 2 (5) | 75 (57) | 56 (43) |
| Partial atrophy, n (%) | 10 (91) | 1 (9) | 15 (71) | 6 (29) |
| Post-atrophic hyperplasia, n (%) | 117 (94) | 8 (6) | 118 (65) | 63 (35) |
HPFS, Health Professionals Follow-Up Study; PNI, perineural invasion; SD, standard deviation; PSA, prostate-specific antigen; IQR, interquartile range; hgPIN, high-grade prostatic intraepithelial neoplasia; reported percentages correspond to the percentage of patients within each group with and without PNI
PNI was strongly associated with risk of lethal prostate cancer (see Table 4). Men with evidence of PNI in tumor tissue were seven times more likely to die of cancer than men without PNI (crude OR 7.4, 95% CI 3.6, 16.6; p<0.001). After adjustment for clinical factors, there remained a non-statistically significant higher risk of prostate cancer-related death in men with PNI (OR 1.9, 95% CI 0.8, 5.1; p=0.17). This association was similar for men with high and low Gleason grade tumors as well as large and small volume tumors (data not shown).
Table 4.
Association between PNI and lethal prostate cancer in the Swedish Watchful Waiting Cohort and US Health Professionals Follow-Up Study
| Lethal Prostate Cancer |
Crude OR/HRa (95% CI) |
Multivariate |
||
|---|---|---|---|---|
| Model 1b | Model 2c | |||
| Swedish Cohort | ||||
| No PNI | 194/572 (34%) | Ref. | Ref. | Ref. |
| PNI | 34/43 (79%) | 7.4 (3.6, 16.6) | 2.0 (0.8, 4.9) | 1.9 (0.8, 5.1) |
| HPFS | ||||
| No PNI | 29/479 (6%) | Ref. | Ref. | Ref. |
| PNI | 58/370 (16%) | 3.1 (2.0, 4.9) | 1.8 (1.1, 3.0) | 1.8 (1.0, 3.3) |
Odds ratios (OR) and hazard ratios (HR) used as measures of association for the Swedish Cohort and HPFS, respectively;
adjusted for age and Gleason grade;
additionally adjusted for tumor volume (Swedish cohort), and pathologic tumor stage (HPFS); CI confidence interval
Health Professionals Follow-Up Study
In the HPFS, which predominantly includes radical prostatectomy specimens (95%), PNI was present in 370 of 849 cases (44%), a higher proportion than in the Swedish cohort (Table 3). Men with tumors exhibiting PNI did not differ from those without PNI in age at diagnosis (mean 66.1 vs. 65.9 years, p=0.68) nor preoperative PSA levels (median 6.6 vs. 6.1 ng/dL, p=0.12). Similar to the Swedish cohort, there was a strong association between the presence of PNI and higher Gleason grade (ptrend<0.001). Moreover, PNI was significantly more common with higher pathologic TNM stage (ptrend<0.001). Tumors harboring PNI had a lower prevalence of post-atrophic hyperplasia (18% vs. 25%, p=0.01). No associations between PNI and hgPIN, acute or chronic inflammation, or other patterns of atrophy were found.
During a median follow-up of 10.7 years, 87 men developed distant metastases or died of prostate cancer. In the crude analysis, there was a strong positive association between PNI and risk of lethal prostate cancer (HR 3.1, 95% CI 2.0, 4.9; p<0.001). This association was attenuated but remained significant after adjusting for age at diagnosis and Gleason score (HR 1.8, 95% CI 1.1, 3.0; p=0.01), as well as after further adjustment for pathologic TNM stage (HR 1.8, 95% CI 1.0, 3.3; p=0.04). Estimates from competing risks models were similar to those from the Cox models. The association between PNI and lethal disease was similar among high and low Gleason grade tumors as well as by pathologic TNM stage (analyses not shown). However, many subgroups had a low number of events such that our analysis was likely underpowered to detect significant effect modification by Gleason grade and stage.
There was no association between presence of PNI and extent of tumor proliferation or apoptosis (Table 5). Tumors harboring PNI did, however, demonstrate increased tumor angiogenesis, as manifested by higher microvessel density (p=0.008) and smaller vessel diameter and area (p<0.001). The differences in angiogenesis parameters were consistent across Gleason grade (analyses not shown).
Table 5.
Prostate tumor biomarkers by PNI status in the Health Professionals Follow-Up Study. Measures expressed as medians (interquartile range).
| PNI | No PNI | p-value | |
|---|---|---|---|
| TUNEL | 0.5 (0, 2.0) | 0.5 (0, 2.0) | 0.45 |
| Ki67 | 0.2% (0%, 0.6%) | 0.1% (0, 0.5%) | 0.61 |
| Microvessel densitya | 75 (58, 100) | 64 (48, 95) | 0.008 |
| Vessel diameterb (µm) | 22.9 (20.1, 26.1) | 25.6 (22.5, 29.2) | <0.001 |
| Vessel areab (µm2) | 414 (302, 574) | 520 (399, 716) | <0.001 |
| Vessel lumen regularitya | 4.1 (3.3, 4.8) | 3.9 (3.1, 4.7) | 0.17 |
Higher values indicate increased tumor angiogenesis;
lower values indicate increased tumor angiogenesis
DISCUSSION
In two independent prostate cancer cohorts, we found PNI to be rarely present in low Gleason grade tumors but much more common in high Gleason grade disease. Moreover, in both a watchful waiting and prostatectomy cohort, PNI conferred a twofold higher risk of lethal prostate cancer even after adjustment for other pathologic factors. The association between PNI and lethal prostate cancer was apparent even among men with high grade cancers, and may indicate an independent mechanism by which cancers cells can leave the prostate.
The prevalence of PNI among RP specimens in our study (44%) is lower than the prevalence reported by most prior studies, which was typically in the range of 50–80% (see Table 2). This is likely explained by the fact that we used a strict definition of PNI, that of circumferential encirclement of nerves by malignant glands. In contrast, prior studies reporting a higher PNI prevalence either did not describe how PNI was defined (14, 19, 20, 25, 39) or used vague descriptions such as presence of tumor cells “in the perineural space” (16, 18) or “along the perineural sheath” (21). Because the perineural space can often be difficult to identify using H&E stains alone, abutment of the perineural space by both malignant and benign glands can, in the absence of circumferential involvement, be misinterpreted as PNI (53, 54), resulting in misclassification. Use of a strict definition of PNI is further supported by studies showing that the extent of infiltration of the perineural space with malignant glands as measured by PNI diameter is associated with clinical recurrence, tumor proliferation as measured by Ki67, and increased expression of the anti-apoptotic biomarker NFΚB (43, 55). The lower prevalence of PNI in our study may alternately be explained by the fact that we did not have access to all H&E slides for some of the HPFS cases.
Although the associations between PNI and lethal prostate cancer were similar, there was a profound difference in the prevalence of PNI by cohort (7% in the Swedish cohort and 44% in the HPFS). This difference can likely explained by differences in the zonal location of the tumors. The HPFS prostatectomy cases were primarily peripheral zone tumors whereas tumors from the Swedish cases arose primarily in the transition zone. Indeed, among 40 HPFS TURP specimens, the PNI prevalence of 12% was on par with the Swedish TURP cohort. Although this suggests that there may be unique differences in the prostate microenvironment within these two zones, it is also possible that transition zone tumors may be smaller and therefore less likely to be diagnosed by a limited transurethral resection.
The novelty of our study is that it is one of the few studies, and the largest to date, to assess the association between PNI in radical prostatectomy (as opposed to biopsy) specimens and prostate cancer-specific mortality (as opposed to biochemical recurrence). Of the more than 30 studies that have examined the association between PNI in radical prostatectomy specimens and prostate cancer outcomes (see Table 2), only three (18, 26, 38) have assessed whether PNI predicts distant metastases or death, with the remainder focusing on biochemical recurrence as the outcome of interest. This is important given that most men who develop a biochemical recurrence are not destined to die of their disease (44–46). Two of these studies (18, 26) did not find PNI to be an independent predictor of cancer-specific mortality, although both were significantly smaller (361 and 273 patients) and had shorter follow-up (median 3.5 and 4.1 years) than our study (18, 26). The third study, of 535 Norwegian men who were followed for a median of 7.4 years after radical prostatectomy, showed the presence of PNI in the prostatectomy specimen to be associated with a worse cancer-specific survival even after adjustment for preoperative PSA, prostatectomy Gleason score, pathologic stage, and surgical margin status (38). In contrast, multiple prior studies have shown PNI discovered on needle core biopsy to predict adverse clinical outcomes, including mortality (see Table 1). A recent study of 451 men treated with radical prostatectomy at the Mayo Clinic and followed for a median of 13 years thereafter found PNI in the biopsy specimen to predict cancer-specific mortality independent of Gleason grade (29). The prognostic value of PNI in biopsy specimens may be related to its correlation with extraprostatic extension in prostatectomy specimens (56).
PNI, defined as the presence of cancer cells within any of the three connective tissue layers enveloping a nerve, was first described in the mid-1800’s in head and neck cancers (3). PNI was initially thought to be a variant of lymphatic spread, until it was shown that the perineural space is devoid of lymphatics (57). Among 78 prostatectomy specimens with extraprostatic extension, Villers et al showed spread to occur exclusively along the prostatic nerves in half (2). This and similar studies were the basis for the prevalent belief that the interface between prostatic nerves and prostate tissue is a low-resistance plane that provides a convenient route for cancer spread out of the prostate.
In contrast, recent research increasingly suggests that PNI is a manifestation of a complex interaction between nerve and cancer cells (3). Ayala et al developed an in vitro model of PNI consisting of co-cultured mouse dorsal root ganglion (DRG) cells and DU-145 prostate cancer cells. This model shows that DRG neurite outgrowth is significantly increased in the presence of prostate cancer cells, with cancer cells subsequently migrating retrograde along the neurites towards the DRG cell bodies (5). Prostate cancer-induced neuronogenesis has since been confirmed in vivo (58). More importantly, nerves have been shown to have a reciprocal effect on prostate cancer growth. On tissue microarrays, prostate cancer cells located near nerves demonstrate a higher proliferative index and lower apoptotic index than cancer cells located further away (6). A phase I clinical trial is currently underway to determine whether periprostatic injection of botulinum toxin A can impair prostate cancer growth (59).
Two candidate mechanisms have been identified to account for the pro-survival effect of PNI. NFΚB and its targets PIM-2 and DAD-1, which together constitute part of an anti-apoptotic pathway, have been shown to be overexpressed in prostate cancer cells associated with nerves compared to those located further away (6). The upstream activator of NFΚB, NCAM, is upregulated in the setting of nerve injury (60). Caveolin-1, a scaffolding protein with anti-apoptotic properties that is expressed by perineural stromal cells during wound repair, has likewise been found to be overexpressed in PNI (61). It is therefore possible that prostate cancer invasion of nerves and subsequent nerve injury results in upregulation of these anti-apoptotic pathways. We did identify a potential novel biological mechanism by which PNI could influence prostate cancer aggressiveness. Our finding of an association between the presence of PNI and increased angiogenesis has not been previously reported in prostate cancer, nor in any other tumors with a propensity for perineural spread, and presents a promising avenue for future study.
The strength of our study lies in its large sample size, prospective data collection, additional tissue biomarker data, and complete follow-up. Unlike most previous studies, all specimens in both cohorts were reviewed systematically for the presence or absence of PNI by a single dedicated genitourinary pathologist. This likely increased the validity of information for our analysis as PNI is not considered a required component of the histopathologic evaluation of prostate cancer specimens by the College of American Pathologists, and is therefore likely underreported in clinical practice (62, 63). The difference in prevalence of PNI in the TURP tumors, which arise primarily in the transitional zone, and prostatectomy samples, which are primarily peripheral zone cancers, provides further evidence of these cancers representing distinct biological entities. Despite the large sample size and standardized pathologic review, the low prevalence of PNI in the Swedish cohort likely significantly decreased the precision of our effect estimates, and possibly accounts for why PNI did not reach statistical significance as an independent predictor in this cohort. Importantly, men in the HPFS cohort who underwent RP had predominantly low to intermediate-grade, organ-confined tumors. Given the increasing use of active surveillance for low-risk tumors and the expected reverse stage migration precipitated by declining rates of PSA screening, it is expected that an increasing number of patients undergoing RP in the contemporary era will have high-risk disease. Given the power limitations of our subgroup analyses, it is unclear whether our findings are therefore generalizable to a more contemporary population of men undergoing RP.
In summary, we present evidence that PNI is a strong predictor of lethal prostate cancer among men undergoing radical prostatectomy and describe for the first time an association between PNI and prostate cancer-related mortality in a cohort without definitive treatment. Our findings, in concert with those from previously published studies, suggest that PNI should be a standardized component of the pathologic review of radical prostatectomy specimens and that this finding may identify another group of high-risk patients who may benefit from adjuvant therapy post-RP. Further research into PNI may shed light on key mechanisms underlying the metastatic potential of prostate cancer.
Acknowledgments
We would like to thank the participants and staff of the Swedish Watchful Waiting Cohort and the Health Professionals Follow-up Study for their valuable contributions. We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial support: This study was supported by funding for L.A. Mucci from the National Institutes of Health (grant Nos. PO1 CA055075, CA141298, CA13389, and UM1 CA167552), the US Army Prostate Cancer Research Program Idea Development Award (PC060389), DF/HCC Prostate SPORE Career Development Award (NIH/NCI P50 CA90381) and the Swedish Cancer Society (CAN 2006/1341). J.R. Rider and L.A. Mucci have received Prostate Cancer Foundation Young Investigator Awards. A. Pettersson was supported by the Swedish Research Council (Reg. No. 2009-7309) and the Royal Physiographic Society in Lund. The funding bodies had no influence on the design or conduct of the study, analysis and interpretation of the data, or preparation of the article.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Pennington JW, Prentiss RJ, Howe G. Radical prostatectomy for cancer: significance of perineural lymphatic invasion. J Urol. 1967;97:1075–1077. doi: 10.1016/S0022-5347(17)63180-X. [DOI] [PubMed] [Google Scholar]
- 2.Villers A, McNeal JE, Redwine EA, Freiha FS, Stamey TA. The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J Urol. 1989;142:763–768. doi: 10.1016/s0022-5347(17)38881-x. [DOI] [PubMed] [Google Scholar]
- 3.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 4.Fromont G, Godet J, Pires C, Yacoub M, Dore B, Irani J. Biological significance of perineural invasion (PNI) in prostate cancer. Prostate. 2012;72:542–548. doi: 10.1002/pros.21456. [DOI] [PubMed] [Google Scholar]
- 5.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–223. doi: 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- 6.Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–6090. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 7.Brundl J, Schneider S, Weber F, Zeman F, Wieland WF, Ganzer R. Computerized quantification and planimetry of prostatic capsular nerves in relation to adjacent prostate cancer foci. Eur Urol. 2014;65:802–808. doi: 10.1016/j.eururo.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Epstein JI, Humphreys EB, Walsh PC. Does perineural invasion on prostate biopsy predict adverse prostatectomy outcomes? BJU Int. 2010;105:1510–1513. doi: 10.1111/j.1464-410X.2009.08845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Adamovich E. Prognostic significance of perineural invasion on biochemical progression-free survival after prostate brachytherapy. Urology. 2005;66:1048–1053. doi: 10.1016/j.urology.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Beard CJ, Chen MH, Cote K, Loffredo M, Renshaw AA, Hurwitz M, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 2004;58:19–24. doi: 10.1016/s0360-3016(03)01433-0. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the Fox Chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 12.Nelson CP, Dunn RL, Wei JT, Rubin MA, Montie JE, Sanda MG. Contemporary preoperative parameters predict cancer-free survival after radical prostatectomy: a tool to facilitate treatment decisions. Urol Oncol. 2003;21:213–218. doi: 10.1016/s1078-1439(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 13.Quinn DI, Henshall SM, Brenner PC, Kooner R, Golovsky D, O’Neill GF, et al. Prognostic significance of preoperative factors in localized prostate carcinoma treated with radical prostatectomy: importance of percentage of biopsies that contain tumor and the presence of biopsy perineural invasion. Cancer. 2003;97:1884–1893. doi: 10.1002/cncr.11263. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto T, Yoshioka K, Nagao G, Nakagami Y, Ohno Y, Horiguchi Y, et al. Prediction of biochemical recurrence after robot-assisted radical prostatectomy: analysis of 784 Japanese patients. Int J Urol. 2015;22:188–193. doi: 10.1111/iju.12624. [DOI] [PubMed] [Google Scholar]
- 15.Kozal S, Peyronnet B, Cattarino S, Seisen T, Comperat E, Vaessen C, et al. Influence of pathological factors on oncological outcomes after robot-assisted radical prostatectomy for localized prostate cancer: results of a prospective study. Urol Oncol. 2015;33:330.e1–330.e1. doi: 10.1016/j.urolonc.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Reeves F, Hovens CM, Harewood L, Battye S, Peters JS, Costello AJ, et al. Does perineural invasion in a radical prostatectomy specimen predict biochemical recurrence in men with prostate cancer? Can Urol Assoc J. 2015;9:e252–e255. doi: 10.5489/cuaj.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanimoto R, Fashola Y, Scotland KB, Calvaresi AE, Gomella LG, Trabulsi EJ, et al. Risk factors for biochemical recurrence after robotic assisted radical prostatectomy: a single surgeon experience. BMC Urol. 2015;15:27. doi: 10.1186/s12894-015-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JT, Lee S, Yun CJ, Jeon BJ, Kim JM, Ha HK, et al. Prediction of perineural invasion and its prognostic value in patients with prostate cancer. Korean J Urol. 2010;51:745–751. doi: 10.4111/kju.2010.51.11.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010;58:838–846. doi: 10.1016/j.eururo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Kumano M, Miyake H, Muramaki M, Kurahashi T, Takenaka A, Fujisawa M. Adverse prognostic impact of capsular incision at radical prostatectomy for Japanese men with clinically localized prostate cancer. Int Urol Nephrol. 2009;41:581–586. doi: 10.1007/s11255-008-9467-z. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno R, Nakashima J, Mukai M, Okita H, Kosugi M, Kikuchi E, et al. Tumour length of the largest focus predicts prostate-specific antigen-based recurrence after radical prostatectomy in clinically localized prostate cancer. BJU Int. 2009;104:1215–1218. doi: 10.1111/j.1464-410X.2009.08548.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyake H, Sakai I, Harada K, Eto H, Hara I. Limited value of perineural invasion in radical prostatectomy specimens as a predictor of biochemical recurrence in Japanese men with clinically localized prostate cancer. Hinyokika Kiyo. 2005;51:241–246. [PubMed] [Google Scholar]
- 23.Ng JC, Koch MO, Daggy JK, Cheng L. Perineural invasion in radical prostatectomy specimens: lack of prognostic significance. J Urol. 2004;172:2249–2251. doi: 10.1097/01.ju.0000143973.22897.f8. [DOI] [PubMed] [Google Scholar]
- 24.Mian BM, Troncoso P, Okihara K, Bhadkamkar V, Johnston D, Reyes AO, et al. Outcome of patients with Gleason score 8 or higher prostate cancer following radical prostatectomy alone. J Urol. 2002;167:1675–1680. [PubMed] [Google Scholar]
- 25.Quinn DI, Henshall SM, Haynes AM, Brenner PC, Kooner R, Golovsky D, et al. Prognostic significance of pathologic features in localized prostate cancer treated with radical prostatectomy: implications for staging systems and predictive models. J Clin Oncol. 2001;19:3692–3705. doi: 10.1200/JCO.2001.19.16.3692. [DOI] [PubMed] [Google Scholar]
- 26.van den Ouden D, Hop WC, Kranse R, Schroder FH. Tumour control according to pathological variables in patients treated by radical prostatectomy for clinically localized carcinoma of the prostate. Br J Urol. 1997;79:203–211. doi: 10.1046/j.1464-410x.1997.33011.x. [DOI] [PubMed] [Google Scholar]
- 27.Moreira DM, Fleshner NE, Freedland SJ. Baseline perineural invasion is associated with shorter time to progression in men with prostate cancer undergoing active surveillance: results from the REDEEM study. J Urol. 2015;194:1258–1263. doi: 10.1016/j.juro.2015.04.113. [DOI] [PubMed] [Google Scholar]
- 28.Cohn JA, Dangle PP, Wang CE, Brendler CB, Novakovic KR, McGuire MS, et al. The prognostic significance of perineural invasion and race in men considering active surveillance. BJU Int. 2014;114:75–80. doi: 10.1111/bju.12463. [DOI] [PubMed] [Google Scholar]
- 29.Tollefson MK, Karnes RJ, Kwon ED, Lohse CM, Rangel LJ, Mynderse LA, et al. Prostate cancer Ki-67 (MIB-1) expression, perineural invasion, and Gleason score as biopsy-based predictors of prostate cancer mortality: the Mayo model. Mayo Clin Proc. 2014;89:308–318. doi: 10.1016/j.mayocp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 30.DeLancey JO, Wood DP, Jr, He C, Montgomery JS, Weizer AZ, Miller DC, et al. Evidence of perineural invasion on prostate biopsy specimen and survival after radical prostatectomy. Urology. 2013;81:354–357. doi: 10.1016/j.urology.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Katz B, Srougi M, Dall’Oglio M, Nesrallah AJ, Sant’anna AC, Pontes J, Jr, et al. Perineural invasion detection in prostate biopsy is related to recurrence-free survival in patients submitted to radical prostatectomy. Urol Oncol. 2013;31:175–179. doi: 10.1016/j.urolonc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Feng FY, Qian Y, Stenmark MH, Halverson S, Blas K, Vance S, et al. Perineural invasion predicts increased recurrence, metastasis, and death from prostate cancer following treatment with dose-escalated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:e361–e367. doi: 10.1016/j.ijrobp.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 33.Vora SA, Wong WW, Schild SE, Ezzell GA, Halyard MY. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 34.Yu HH, Song DY, Tsai YY, Thompson T, Frassica DA, DeWeese TL. Perineural invasion affects biochemical recurrence-free survival in patients with prostate cancer treated with definitive external beam radiotherapy. Urology. 2007;70:111–116. doi: 10.1016/j.urology.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Beard C, Schultz D, Loffredo M, Cote K, Renshaw AA, Hurwitz MD, et al. Perineural invasion associated with increased cancer-specific mortality after external beam radiation therapy for men with low- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:403–407. doi: 10.1016/j.ijrobp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 36.D’Amico AV, Wu Y, Chen MH, Nash M, Renshaw AA, Richie JP. Perineural invasion as a predictor of biochemical outcome following radical prostatectomy for select men with clinically localized prostate cancer. J Urol. 2001;165:126–129. doi: 10.1097/00005392-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 37.de la Taille A, Rubin MA, Bagiella E, Olsson CA, Buttyan R, Burchardt T, et al. Can perineural invasion on prostate needle biopsy predict prostate specific antigen recurrence after radical prostatectomy? J Urol. 1999;162:103–106. doi: 10.1097/00005392-199907000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Andersen S, Richardsen E, Nordby Y, Ness N, Storkersen O, Al-Shibli K, et al. Disease-specific outcomes of radical prostatectomies in Northern Norway: a case for the impact of perineural infiltration and postoperative PSA-doubling time. BMC Urol. 2014;14:49. doi: 10.1186/1471-2490-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316–1322. doi: 10.1016/j.ijrobp.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 40.Psutka SP, Feldman AS, Rodin D, Olumi AF, Wu CL, McDougal WS. Men with organ-confined prostate cancer and positive surgical margins develop biochemical failure at a similar rate to men with extracapsular extension. Urology. 2011;78:121–125. doi: 10.1016/j.urology.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Fujimoto K, Hirayama A, Torimoto K, Okajima E, Tanaka M, et al. Risk-stratified survival rates and predictors of biochemical recurrence after radical prostatectomy in a Nara, Japan, cohort study. Int J Clin Oncol. 2011;16:553–559. doi: 10.1007/s10147-011-0226-2. [DOI] [PubMed] [Google Scholar]
- 42.Jeon HG, Bae J, Yi JS, Hwang IS, Lee SE, Lee E. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int J Urol. 2009;16:682–686. doi: 10.1111/j.1442-2042.2009.02331.x. [DOI] [PubMed] [Google Scholar]
- 43.Maru N, Ohori M, Kattan MW, Scardino PT, Wheeler TM. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32:828–833. doi: 10.1053/hupa.2001.26456. [DOI] [PubMed] [Google Scholar]
- 44.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54:884–890. doi: 10.1016/s0090-4295(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 45.Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:629–633. doi: 10.1016/s0360-3016(00)00717-3. [DOI] [PubMed] [Google Scholar]
- 46.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 47.Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–471. [PubMed] [Google Scholar]
- 48.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 49.Davidsson S, Fiorentino M, Andren O, Fang F, Mucci LA, Varenhorst E, et al. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2280–2287. doi: 10.1158/1055-9965.EPI-11-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt430. djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, et al. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst. 2013;105:1881–1890. doi: 10.1093/jnci/djt332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mucci LA, Powolny A, Giovannucci E, Liao Z, Kenfield SA, Shen R, et al. Prospective study of prostate tumor angiogenesis and cancer-specific mortality in the Health Professionals Follow-up Study. J Clin Oncol. 2009;27:5627–5633. doi: 10.1200/JCO.2008.20.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali TZ, Epstein JI. Perineural involvement by benign prostatic glands on needle biopsy. Am J Surg Pathol. 2005;29:1159–1163. doi: 10.1097/01.pas.0000160980.62586.05. [DOI] [PubMed] [Google Scholar]
- 54.Tsuzuki T, Ujihira N, Ando T. Usefulness of epithelial membrane antigen (EMA) to discriminate between perineural invasion and perineural indentation in prostatic carcinoma. Histopathology. 2005;47:159–165. doi: 10.1111/j.1365-2559.2005.02177.x. [DOI] [PubMed] [Google Scholar]
- 55.Olar A, He D, Florentin D, Ding Y, Wheeler T, Ayala G. Biological correlates of prostate cancer perineural invasion diameter. Hum Pathol. 2014;45:1365–1369. doi: 10.1016/j.humpath.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cozzi G, Rocco BM, Grasso A, Rosso M, Abed El Rahman D, Oliva I, et al. Perineural invasion as a predictor of extraprostatic extension of prostate cancer: a systematic review and meta-analysis. Scand J Urol. 2013;47:443–448. doi: 10.3109/21681805.2013.776106. [DOI] [PubMed] [Google Scholar]
- 57.Hassan MO, Maksem J. The prostatic perineural space and its relation to tumor spread: an ultrastructural study. Am J Surg Pathol. 1980;4:143–148. doi: 10.1097/00000478-198004000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 59.Ayala G, Florentin D, Au JK, Ding Y, He D, Creighton CJ, et al. Targeting the neural microenvironment in prostate cancer: a neoadjuvant Botox clinical trial. J Urol. 2012;187:e660. [Google Scholar]
- 60.Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986;103:929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayala GE, Dai H, Tahir SA, Li R, Timme T, Ittmann M, et al. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 62.Srigley JR, Humphrey PA, Amin MB, Chang SS, Egevad L, Epstein JI, et al. Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Northfield, Ill: College of American Pathologists; 2011. [DOI] [PubMed] [Google Scholar]
- 63.Harnden P, Shelley MD, Clements H, Coles B, Tyndale-Biscoe RS, Naylor B, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]