Abstract
Introduction/Background
Locoregional recurrence after resection of non-small cell lung cancer (NSCLC) is common. We examined outcomes after definitive radiotherapy (RT) to identify prognostic factors for survival and further recurrence.
Patients and Methods
We reviewed 152 patients receiving RT for LR-NSCLC, analyzing subsequent overall survival (OS), locoregional failure (LRF), distant metastasis (DM) and any progression (LRF + DM).
Results
Two and 5-year OS were 49% and 28% respectively. Two and 5-year LRF, DM, and any-progression rates were 40%/45%, 33%/37%, and 53%/57%. Performance status and IMRT were independently associated with OS, as was RT dose ≥60Gy. Stage, chemotherapy at recurrence, and surgery-to-recurrence interval were not independently associated with outcome. Chemotherapy at initial presentation, adenocarcinoma histology, and male gender were independently associated with higher DM.
Conclusion
This is the largest reported series of LR-NSCLC treated with definitive RT. Survival appears comparable to or greater than that of primary NSCLC. Subsequent locoregional failure is more common than distant failure. Established prognostic factors for primary NSCLC, such as chemotherapy and stage, were not clearly prognostic in this analysis. IMRT and higher RT doses were associated with improved survival, though IMRT patients were also treated more recently. This data supports definitive-intent RT with optimal dose and technique in such patients.
Keywords: non-small cell lung cancer, local recurrence, radiotherapy
INTRODUCTION
Surgery is typically the treatment of choice for resectable non-small-cell lung cancer. Though distant metastasis is the primary determinant of subsequent survival, isolated intrathoracic recurrence is a common pattern of failure after surgery, with reported rates ranging from 5% to 38%.[1, 2] In the absence of distant metastases, a second attempt at curative local therapy is often warranted. However, many such patients are no longer fit to undergo further surgery due to diminished pulmonary reserve, technical unresectability of recurrent disease, or both. Definitive radiation therapy (RT) is then the local treatment modality of choice.
Although locally recurrent NSCLC after surgery is common, there is little data to guide treatment for these patients or predict their outcome. In the absence of conclusive data, many clinicians prescribe RT in the same way as primary NSCLC of comparable disease extent. Small series suggest that definitive RT can achieve outcomes similar to those of primary NSCLC, but this has not been evaluated in a large cohort of patients.[1, 3–6] In addition, it is unclear whether the same prognostic factors and treatment approaches that have proven valid in primary NSCLC, such as the use of sequential or concurrent chemotherapy with RT, are also applicable to the locally recurrent setting.
We therefore sought to review a large cohort of patients who were treated with definitive RT for localized recurrence of NSCLC after surgical resection. In addition to characterizing the outcomes of this group as whole, we also evaluated whether patient and disease factors at initial presentation and at recurrence were predictive of disease control. In particular, we examined whether extent of disease at initial presentation and recurrence, interval between surgery and recurrence, use of chemotherapy, and choice of RT dose and technique had an impact on outcome.
MATERIAL AND METHODS
Patient Selection
Institutional review and privacy boards approved this study, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. Institutional databases were queried to identify patients receiving curative-intent (“salvage”) RT for locoregionally recurrent NSCLC between 1994 and 2012 at our institution. All patients had to have received curative-intent surgery that was at least macroscopically complete, and then developed recurrent NSCLC within five years of surgery. Disease was only considered recurrent if it was limited to the ipsilateral lung, ipsilateral thoracic nodes and/or bilateral mediastinal and supraclavicular nodes, and was of the same histology as the initial tumor. In cases where histologic confirmation of recurrent tumor was not obtained, we only included cases if the treating physician considered the patient to have locally recurrent disease based on the clinical scenario.
Treatment
Patients receiving radiation with palliative intent (<40Gy in conventional fractionation) were excluded. Patients were immobilized in a customized foam cradle with arms above their heads, and radiation dose was prescribed to the isodose line encompassing the PTV. Treatment fields for conventionally fractionated RT were generally limited to the recurrent tumor, involved nodes, and the ipsilateral hilum. Typical margins for conventional RT were 1.2–1.5cm from gross tumor volume (GTV) to planning tumor volume (PTV), or 2cm to block edge.
Patients treated with stereotactic body radiotherapy (SBRT) received ≥600cGy per fraction (median of four fractions), with on-board CT guidance at each fraction. Patients were offered SBRT according to the judgment of the treating physician, using criteria analogous for those used to select SBRT for primary lung cancer at our institution. In general, SBRT was considered for patients with node-negative tumors measuring ≤5cm. Four-dimensional CT simulation was employed to define the respiratory excursion of the GTV, and the PTV consisted of an additional 7–8mm expansion. Dose was then prescribed to the 100% isodose line encompassing the PTV using intensity-modulated beams.
Data Collection
All patient charts were retrospectively reviewed to obtain patient, disease, and treatment factors at the time of initial presentation of NSCLC, as well as recurrence. Patient factors included age, gender, weight loss and performance status. Disease factors included AJCC stage (based on 7th edition criteria), histology, margin status after surgery (R0 vs. R1), interval between resection and recurrence, and maximum standardized uptake value (SUVmax) in patients who underwent PET imaging at the time of recurrence. All patients were assigned a recurrent “stage,” applying the same AJCC staging criteria as used for primary NSCLC. We also distinguished patients who recurred in lymph nodes only from those who had recurrences involving lung parenchyma. Treatment factors included use of chemotherapy at time of surgery, chemotherapy at time of salvage RT (either sequential or concurrent), RT technique (non-IMRT vs. IMRT vs. SBRT), and RT dose (≥60Gy or <60Gy, in the subset of patients not receiving SBRT). Subsequent locoregional failure (after salvage RT) was defined as clinical evidence of disease progression within the ipsilateral hemithorax.
Statistical Analysis
Overall survival was estimated by Kaplan-Meier methods. The cumulative incidence of locoregional failure, distant metastasis, and any progression were estimated, and death without disease progression was treated as a competing event. All followup was calculated from the date of RT completion. Cox regression analysis was performed to examine clinical and treatment factors related to overall survival, while competing risk regression was used to analyze association with time to locoregional failure, distant metastasis and any progression. Overall p-values were derived from likelihood ratio tests comparing a model with the variable to a null model.
Multivariable analysis was performed where all variables with p<0.1 on univariate analysis were included into a model for each endpoint. (The cutoff was p<0.05 for the endpoint of distant metastasis, due to many associated variables and the small number of events.) Additional variables of significant clinical interest were also examined in the multivariable model. Specifically, recurrent stage was added to the model for overall survival, and surgery-to- recurrence interval was added to the model for distant metastasis. Overall p-values were derived from likelihood ratio tests comparing a model with and without the variable. Because radiation doses for patients treated with stereotactic body radiation therapy (SBRT) are not comparable to doses with non-SBRT techniques, a separate multivariable model was fitted on the subset of non-SBRT patients to examine the importance of RT dose on overall survival.
RESULTS
Patient Characteristics
We identified 152 patients who satisfied the study criteria and are included in the present study. Patients underwent RT between the years 1994 and 2012. The median age at time of salvage RT was 70 years (range 48–89 years). Median interval between surgery and diagnosis of locoregional recurrence was 17 months (range 2–60 months). For non-SBRT patients, the median RT dose was 66Gy (range 45–90Gy) and all patients were treated with conventional fractionation (1.8–2.0Gy per fraction). The median SBRT dose was 45Gy (range 30–60Gy). Patient characteristics are listed in Table 1. The median followup for the cohort was 23 months (25 months among surviving patients).
Table 1.
Patient characteristics
| Characteristic | N | % |
|---|---|---|
| Gender | ||
| Male | 63 | 41% |
| Female | 89 | 59% |
| KPS at recurrence | ||
| <80% | 31 | 20% |
| ≥80% | 121 | 80% |
| Surgical Margin Status | ||
| Negative | 141 | 93% |
| Positive or unknown | 11 | 7% |
| Histology | ||
| Adenocarcinoma | 99 | 65% |
| Squamous cell | 44 | 29% |
| Other | 9 | 6% |
| Initial Stage | ||
| I | 84 | 55% |
| II | 29 | 19% |
| III | 39 | 26% |
| Recurrent Stage | ||
| I | 34 | 22% |
| II | 31 | 20% |
| III | 87 | 57% |
| Location of Recurrence | ||
| Lung only | 58 | 38% |
| Nodal only | 52 | 34% |
| Lung and nodal | 42 | 28% |
| Chemotherapy At Surgery | ||
| Yes | 43 | 28% |
| No | 109 | 72% |
| Chemotherapy For Recurrence | ||
| Yes (concurrent=32, sequential=28) | 60 | 39% |
| No | 92 | 61% |
| RT Technique | ||
| 3D/Conventional | 73 | 48% |
| IMRT | 60 | 39% |
| SBRT | 19 | 13% |
| RT Technique by Recurrent Stage | ||
| Recurrent Stage I | ||
| 3D/Conventional | 12 | 35% |
| IMRT | 6 | 18% |
| SBRT | 16 | 47% |
| Recurrent Stage II | ||
| 3D/Conventional | 13 | 42% |
| IMRT | 16 | 52% |
| SBRT | 2 | 6% |
| Recurrent Stage III | ||
| 3D/Conventional | 48 | 55% |
| IMRT | 38 | 44% |
| SBRT | 1 | 1% |
| RT Dose (non-SBRT patients) | ||
| ≤60Gy | 117 | 77% |
| <60Gy | 16 | 11% |
Overall Survival
Median survival for the entire cohort was 23 months (95% confidence interval: 18–34 months), and the 2- and 5-year estimate of OS was 49% (40–57%) and 28% (20–37%), respectively (See Figure 1). On univariate analysis, the following factors were associated with improved OS (p<0.1): Lower stage at initial presentation, KPS≥80% and use of IMRT. Improved survival was also observed for RT dose ≥60Gy in the non-SBRT subset (p=0.02). Recurrent stage was marginally associated with survival (p=0.13) and was also chosen for multivariable modeling.
Figure 1.
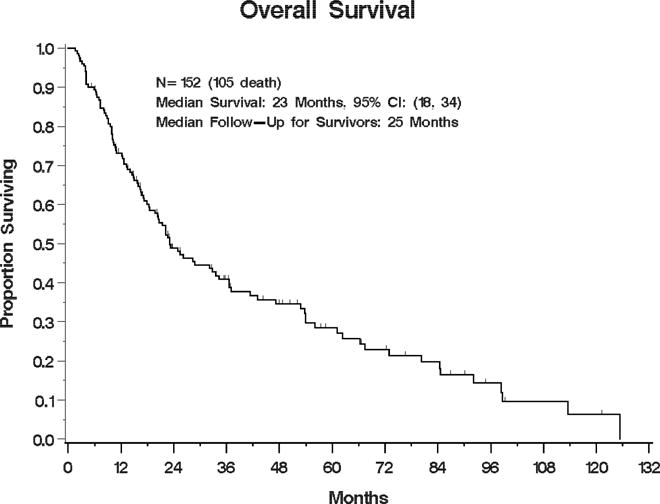
Kaplan-Meier estimate of overall survival for the entire cohort
In a multivariable model, higher KPS (HR 0.63, 95%CI (0.39, 0.99), p=0.05) remained independently associated with improved survival and initial stage II vs. stage I was borderline significant (p=0.06) (see Table 2). In a separate multivariable model excluding the SBRT patients, RT dose ≥60Gy remained significantly associated with improved survival (HR 0.5, 95%CI (0.28, 0.88) p=0.02), as did use of IMRT (HR 0.61, 95%CI (0.39, 0.94), p=0.03).
Table 2.
Multivariate analysis of overall survival
| Characteristic | HR (95%CI) | p-value |
|---|---|---|
| Initial stage | 0.06 | |
| I | Reference | |
| II | 0.57 (0.32, 1.03) | |
| III | 1.22 (0.76, 1.97) | |
| Recurrent stage | 0.25 | |
| I | Reference | |
| II | 1.53 (0.77, 3.07) | |
| III | 1.61 (0.91, 2.84) | |
| KPS | 0.05 | |
| <80 | Reference | |
| ≥80 | 0.63 (0.39, 1) | |
| RT technique | 0.07 | |
| 3D/Unknown | Reference | |
| IMRT | 0.62 (0.4, 0.96) | |
| SBRT | 0.56 (0.21, 1.51) |
Locoregional Failure
Sixty-four patients (42%) experienced subsequent locoregional failure. The 2- and 5-year estimate of LRF was 40% (32–48%) and 45% (37–54%), respectively (see Figure 2). On univariate analysis, higher recurrent stage and use of chemotherapy at time of initial surgery were significantly associated with increased LRF (p<0.05). Higher initial stage and shorter interval between surgery and recurrence were borderline significant (p<0.1) and also included in the multivariable analysis. However, none of the candidate factors was independently associated with LRF, including RT dose in the non-SBRT patients. IMRT and SBRT were associated with a numerically lower risk of LRF (HR 0.81 and 0.56 respectively), but the association of RT technique with LRF was not statistically significant (p=0.38).
Figure 2.
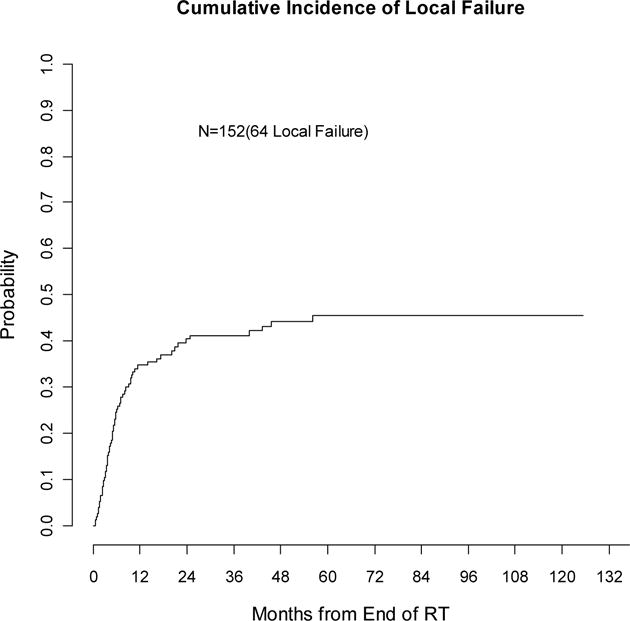
Cumulative incidence of local failure (competing-risks analysis)
Distant Metastasis
Fifty-three patients (35%) experienced distant metastasis. The 2- and 5-year estimate of distant metastasis was 33% (25–40%) and 37% (28–46%) respectively (see Figure 3). On univariate analysis, male gender, younger age, adenocarcinoma histology, and use of chemotherapy with initial surgery were significantly correlated with higher risk of distant metastasis (p≤0.05) and included in the multivariate analysis. Higher initial stage, recurrent stage, and use of chemotherapy with salvage RT showed borderline association with distant metastasis but were not evaluated by multivariable analysis due to the limited number of distant metastasis events. By multivariable modeling, male gender (HR 1.82, 95%CI (1.04, 3.18), p=0.04), use of chemotherapy with initial surgery (HR 1.93, 95%CI (1.1, 3.37), p=0.02), and adenocarcinoma vs. squamous cell histology (HR 2.1, 95%CI (1.01, 4.38), p=0.05) were independently associated with greater risk of distant metastasis, while younger age was borderline significant (p=0.06).
Figure 3.
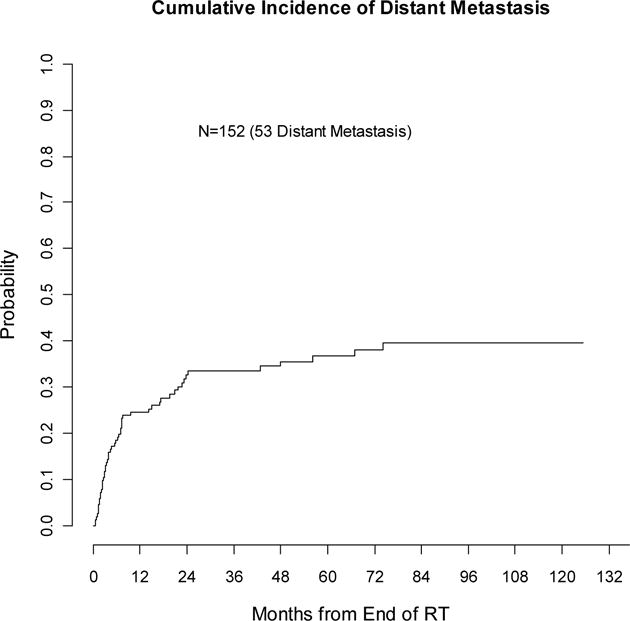
Cumulative incidence of distant metastasis (competing-risks analysis)
Any Progression
The 2- and 5-year rate of any progression (locoregional or distant) was 53% (44–61%) and 57% (49–64%) respectively (see Figure 4). On univariate, shorter surgery to recurrence interval, use of chemotherapy with surgery, and younger age correlated with increased risk of progression (p≤0.05). Initial stage (p=0.08) and recurrent stage (p=0.11) were also included in the multivariable analysis. None of the factors were independently associated with progression.
Figure 4.
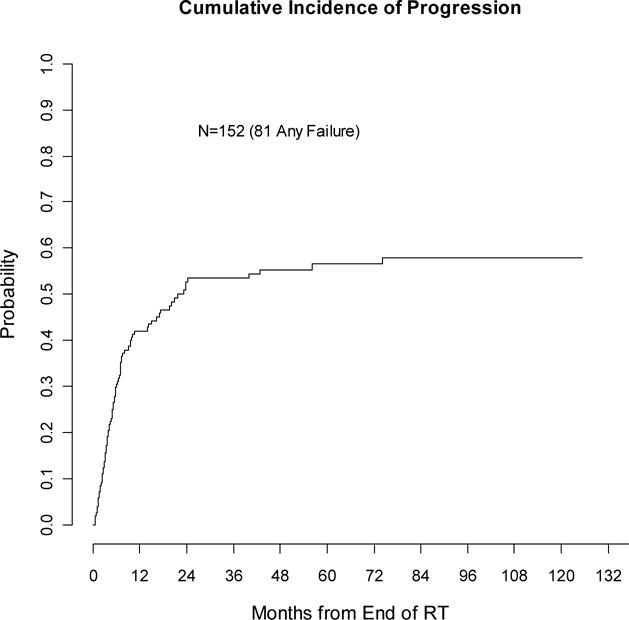
Cumulative incidence of any recurrence (competing-risks analysis)
DISCUSSION
This is the largest series yet reported of locoregionally recurrent NSCLC after surgical resection treated with definitive RT. A large cohort of post-resection recurrent NSCLC was recently described by investigators in Taiwan, but a relatively small proportion received radiation therapy.[7] Our purpose was to describe the outcomes of patients deemed eligible for definitive treatment with radiation therapy.
We found overall survival broadly comparable to that of primary locally-advanced NSCLC, with median survival of 23 months and 5-year survival of 28%. This compares favorably with reported median survivals ranging from 15–27 months in primary Stage III NSCLC patients treated with chemotherapy and radiation.[8, 9] We hypothesized that tumor stage, particularly stage at the time of recurrence, would correlate with outcome. Although patients with recurrent stage II and III disease had worse survival than recurrent Stage I, recurrent stage was not a significant prognostic factor on univariate or multivariable analysis.
Previously published series have also indicated that median survival in these patients is comparable to or better than primary NSCLC. An early report from Fox Chase Cancer Center compared 37 recurrent patients with 759 primary NSCLC patients and found no significant difference in survival.[5] A more recent report from the University of Michigan compared 54 recurrent patients with 607 primary NSCLC patients and reported median survival of 20 months in the recurrent patients, which was significantly better than the primary NSCLC patients.[4]
Other groups have evaluated the impact of combining chemotherapy with RT in this setting. A small Canadian series focused exclusively on patients treated with concurrent chemoradiation and reported a relatively favorable median survival of 27 months.[10] The most recent and heretofore largest series treated with definitive RT, from Korea, suggested that concurrent chemoradiation led to significantly better outcomes compared to radiotherapy alone.[11] However, we did not see any clear benefit of chemotherapy on outcomes in our larger cohort, which may be related in part to the observation that locoregional failure was a higher risk for these patients than distant metastasis.
Though we did not identify a clear correlation of chemotherapy with outcomes, we did for radiation technique and dose. For patients with primary NSCLC, the optimal RT dose is considered to be 60Gy or greater.[12] RT dose ≥60Gy also was independently associated with improved OS among patients receiving conventionally fractionated RT in our cohort, even after adjusting for other factors likely to affect prescribed dose, such as recurrent stage (since bulkier disease is less likely to be amenable to high-dose RT). On the other hand, RT dose ≥60Gy was not significantly associated with the risk of local recurrence or distant metastasis, which raises the question of how higher radiation dose contributes to increased survival.
We also found that IMRT was associated with improved survival compared to conventional radiation techniques. Though the benefit of IMRT has not been conclusively established in NSCLC, this association has also been observed in primary NSCLC, and may be due to the lower toxicity that can be achieved with this technique.[13] Reducing RT-related toxicity may be especially relevant for these patients, who have reduced lung capacity and other post-surgical changes from prior resection. However, we cannot rule out the possibility that the observed benefit of higher doses and IMRT is due to other confounding factors not accounted for in our multivariable analysis. In particular, IMRT was much more likely to be used in the more recently treated patients (10% of patients treated prior to 2006, compared to 69% of patients treated after 2006), and therefore the IMRT patients may have benefitted from other advances in treatment over this time. It must be acknowledged that the long timeframe of this study, and the significant heterogeneity in the patient population, makes it inherently difficult to isolate the effect of radiation technique and dose on outcome, and these associations must be considered only hypothesis-generating.
This is also the first series of locoregionally recurrent NSCLC to include patients treated with stereotactic body radiotherapy, which has demonstrated great promise in primary NSCLC and therefore is an attractive option for patients whose local recurrences are technically amenable to this technique.[14] Use of SBRT was not an independent prognostic factor for outcome in our series, but the limited number of SBRT patients limits our ability to draw firm conclusions about the comparative effectiveness of SBRT in this setting. Overall, however, this data supports the use of high-dose RT and modern radiation techniques for locally recurrent NSCLC, as is the case for primary NSCLC.
Our study confirmed that a number of factors known to impact outcome for primary NSCLC are also applicable in the post-resection recurrent setting. In particular, poor performance status is an adverse prognostic factor, and adenocarcinoma histology is associated with a higher risk of distant metastasis. Some previous series suggested that longer interval between surgery and recurrence was favorable.[6, 11] On univariate analysis, we observed correlation with local failure and with any progression, but no significant association was seen after multivariable analysis.
A major strength of our analysis is the large number of patients, which provided the opportunity to assess the impact of multiple clinical and treatment factors on disease control and survival outcomes. Only a handful of studies of post-resection recurrent NSCLC have analyzed multiple prognostic factors and disease control endpoints in addition to survival, and none have analyzed the impact of IMRT or SBRT.[11, 15] Our data supports an aggressive approach to definitive RT, utilizing high-dose IMRT or SBRT. On the other hand, this data does not provide clear evidence for integrating chemotherapy into the salvage treatment, which is otherwise a well-established strategy for improving outcomes in primary NSCLC.
Another distinguishing feature of this series is our analysis of patterns of failure. Most published series have only reported survival. We recorded whether patients experienced disease progression in locoregional or distant sites after definitive RT, and found that locoregional failure is a somewhat more likely event than distant metastasis. This observation may reflect the underlying biology of locally recurrent NSCLC: since these tumors did not widely metastasize after surgery, they may not be as prone to distant metastasis after salvage RT either. This suggests that attempts to optimize local control, such as through RT technique and dose, may be particularly beneficial for this population.
The major limitation of this data is its retrospective nature, and the heterogeneity of the patients included with respect to stage and treatment of both initial and recurrent disease. Though we made efforts to adjust for clinical and treatment factors that may confound the analysis of prognostic factors, it is impossible to account for every potential factor, especially when the individual patient scenarios and treatments are so diverse. However, no prospective data exists for this subset of NSCLC patients, nor is any likely to be produced in the near future.
CONCLUSION
This large cohort and detailed analysis of prognostic factors and disease outcomes may be of interest and help to clinicians facing the scenario of locoregionally recurrent NSCLC after surgical resection. We conclude that favorable outcomes can be achieved with definitive RT, and that the use of IMRT and higher RT doses is encouraged.
MICROABSTRACT.
The optimal management and prognosis of locally recurrent NSCLC after surgery is not well described. This is the largest reported series of such patients treated with definitive radiation. We found generally favorable survival with this approach (median 23 months), and patients treated with higher radiation doses and intensity-modulated radiation did particularly well.
CLINICAL PRACTICE POINTS.
Localized recurrence of NSCLC after surgery is relatively common. Radiation therapy can be utilized in this situation with curative intent, but relatively little is known about outcomes or prognostic factors with this approach. Most published series have not analyzed the impact of factors such as interval between surgery and recurrence, use of chemotherapy, and dose and type of radiation therapy, because patient numbers are small. This is the largest known series of such patients (n=152), and we undertook a detailed analysis of potential prognostic factors and analyzed local and distant failure rates, as well as survival. We found a relatively favorable 5-year survival rate of 28%. Interval between surgery and recurrence, and use of chemotherapy were not clearly prognostic, but higher radiation doses and the use of intensity-modulated radiation therapy (IMRT) were independently associated with improved outcomes. This data supports the use of curative-intent radiation therapy for locally recurrent NSCLC, using optimal doses and techniques.
Acknowledgments
none
Sources of Support: The research of Meier Hsu and Zhigang Zhang were partly supported by an NIH Core Grant P30 CA008748.
This work was supported by the National Institutes of Health (Core Grant P30 CA008748)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This data was presented, in preliminary and abstract form, at the ASTRO Annual Meeting in Boston, Massachusetts in October 2012.
References
- 1.Jeremic B, Bamberg M. External beam radiation therapy for bronchial stump recurrence of non-small-cell lung cancer after complete resection. Radiother Oncol. 2002;64:251–257. doi: 10.1016/s0167-8140(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 2.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–81. doi: 10.1016/j.jtcvs.2012.09.030. discussion 81–72. [DOI] [PubMed] [Google Scholar]
- 3.Bar J, Ng D, Moretto P, Goss GD, Sun A, Macrae R, Laurie SA, Leighl N, Nicholas G. Chemoradiotherapy for locoregional recurrence of non-small-cell lung cancer after surgical resection: a retrospective analysis. Clin Lung Cancer. 2013;14:200–204. doi: 10.1016/j.cllc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Cai XW, Xu LY, Wang L, Hayman JA, Chang AC, Pickens A, Cease KB, Orringer MB, Kong FM. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1100–1105. doi: 10.1016/j.ijrobp.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ, Jr, Herbert SH, Stafford PM, Sandler HM, Rosenthal SA, McKenna WG, Hughes E, Dougherty MJ, Keller S. Should patients with post-resection locoregional recurrence of lung cancer receive aggressive therapy? Int J Radiat Oncol Biol Phys. 1992;24:25–30. doi: 10.1016/0360-3016(92)91016-g. [DOI] [PubMed] [Google Scholar]
- 6.Kelsey CR, Clough RW, Marks LB. Local recurrence following initial resection of NSCLC: salvage is possible with radiation therapy. Cancer J. 2006;12:283–288. doi: 10.1097/00130404-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hung JJ, Yeh YC, Jeng WJ, Chien HC, Wu YC, Chou TY, Hsu WH. Prognostic Factors of Survival after Recurrence in Patients with Resected Lung Adenocarcinoma. J Thorac Oncol. 2015;10:1328–1336. doi: 10.1097/JTO.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncology. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor MD, Nagji AS, Bhamidipati CM, Theodosakis N, Kozower BD, Lau CL, Jones DR. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93:1813–1820. doi: 10.1016/j.athoracsur.2012.03.031. discussion 1820–1811. [DOI] [PubMed] [Google Scholar]
- 11.Bae SH, Ahn YC, Nam H, Park HC, Pyo HR, Shim YM, Kim J, Kim K, Ahn JS, Ahn MJ, Park K. High dose involved field radiation therapy as salvage for loco-regional recurrence of non-small cell lung cancer. Yonsei Med J. 2012;53:1120–1127. doi: 10.3349/ymj.2012.53.6.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez CA, Stanley K, Grundy G, Hanson W, Rubin P, Kramer S, Brady LW, Marks JE, Perez-Tamayo R, Brown GS, Concannon JP, Rotman M. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer. 1982;50:1091–1099. doi: 10.1002/1097-0142(19820915)50:6<1091::aid-cncr2820500612>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Liao ZX, Komaki RR, Thames HD, Jr, Liu HH, Tucker SL, Mohan R, Martel MK, Wei X, Yang K, Kim ES, Blumenschein G, Hong WK, Cox JD. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeremic B, Shibamoto Y, Milicic B, Milisavljevic S, Nikolic N, Dagovic A, Aleksandrovic J, Radosavljevic-Asic G. External beam radiation therapy alone for loco-regional recurrence of non-small-cell lung cancer after complete resection. Lung Cancer. 1999;23:135–142. doi: 10.1016/s0169-5002(99)00007-0. [DOI] [PubMed] [Google Scholar]


