Abstract
Therapy of cancer with radiolabeled monoclonal antibodies has produced impressive results in preclinical experiments and in clinical trials conducted in radiosensitive malignancies, particularly B cell lymphomas. Two “first generation”, directly radiolabeled anti-CD20 antibodies, 131Iodine-tositumomab and 90Yttrium-ibritumomab tiuxetan were FDA-approved more than a decade ago, but have been little utilized due to a variety of medical, financial, and logistic obstacles. Newer technologies employing multi-step “pretargeting” methods, particularly those utilizing bispecific antibodies, have greatly enhanced the therapeutic efficacy of radioimmunotherapy (RIT) and diminished its toxicities. The dramatically improved therapeutic index of bispecific antibody pretargeting appears to be sufficiently compelling to justify human clinical trials and reinvigorate enthusiasm for RIT in the treatment of malignancies, particularly lymphomas.
Keywords: Radioimmunotherapy, lymphoma, pretargeting
“To be, or not to be, that is the question: Whether ‘tis nobler in the mind to suffer the slings and
arrows of outrageous fortune, or to take arms against a sea of troubles,
And by opposing end them.”
Hamlet. William Shakespeare.
BACKGROUND
Impact of monoclonal antibodies on the field of clinical oncology
Antibody therapies have transformed the treatment of cancer in the last 20 years. This transformation has particularly impacted the treatment of B cell malignancies, where the addition of anti-CD20 antibodies (e.g. rituximab, obinutuzumab, ofatumomab) to conventional chemotherapy has improved overall response rates, complete response rates, progression-free survival, and overall survival of patients with chronic lymphocytic leukemia (CLL), follicular lymphoma and diffuse large B cell lymphomas in both front-line and relapsed settings. The dramatic impact of antibody therapy is not restricted to lymphomas. Trastuzumab has exhibited a potent and salutary impact on the outcome of patients treated for Her2/neu-expressing breast cancer, cetuximab and panitumomab (anti-EGFR antibodies) have improved outcomes for patients with cancer of the head and neck and metastatic colorectal cancer, bevacizumab is effective for metastatic colon cancer and advanced non-small cell lung cancer and daratumumab (anti-CD38) and elotuzumab (anti-SLAMF7) have demonstrated impressive efficacy in multiple myeloma(1–3). Most impressive are the recent results of immune “checkpoint inhibiting antibodies” such as ipilumimab (anti-CTLA4), nivolumab (anti-PD-1) and pembrolizumab (anti-PD-1) which are not directly cytotoxic for cancer cells, but “release the brakes” on the immune system, allowing cytotoxic T cells to be more effective at recognizing and killing cancer cells. Outstanding results have already been demonstrated with checkpoint inhibiting antibodies even in far advanced refractory solid tumors including melanoma, lung cancer, Hodgkin lymphoma, and are under study for a multitude of other malignancies(4–6).
Antibody-Drug Conjugates
Despite the impressive results obtained with unmodified monoclonal antibodies summarized above, single agent efficacy is generally limited and few cancer patients are permanently cured with antibody monotherapy. Consequently, investigators have explored the potential utility of augmenting the activity of antibodies by conjugating drugs, toxins, and radionuclides to them to produce more durable remissions. The first successful antibody-drug conjugate (ADC) was gemtuzumab ozogamicin (an anti-CD33 antibody conjugated to calicheamicin) which has significant efficacy in acute myeloid leukemias(7), particularly those with favorable cytogenetic profiles, including acute promyelocytic leukemia. More recently, brentuximab vedotin (anti-CD30-monomethyl auristatin E) has shown dramatic efficacy in relapsed and refractory Hodgkin lymphoma, with overall response rates (ORR) of >70% and complete response (CR) rates of 33%. Patients achieving CR enjoyed 3 year overall survival (OS) rates of 73% and 3 year progression-free survival (PFS) rates of 58%(8). Nor is ADC success restricted to hematologic malignancies. Dramatic results have been obtained with ado-trastuzumab-emtansine (an anti-Her2 antibody conjugated to the microtubule-inhibitory agent DM1) which provides superior PFS (9.6 vs 6.4 months, p<0.001) and OS (30.9 vs 25.1 months) compared to treatment with standard therapy (lapatinib plus capecitabine)(9). The ADC field is exploding, with many additional products expected to receive FDA-approval in the next few years.
Radiolabeled Antibodies
Combining monoclonal antibodies with radiation therapy was first studied in hematologic malignancies based on the rationale that these are the most radiosensitive tumors(10). Indeed, many clinicians believe that radiation therapy remains the single most effective agent for lymphomas. It is not surprising, therefore, that investigators began studies conjugating radionuclides to monoclonal antibodies shortly after the introduction of hybridoma technology in the late 1970s and early 1980s. To employ radioimmunotherapy (RIT) effectively, several important variables needed to be optimized, including selection of the best cell surface target antigen and targeting antibody. An ideal target antigen for RIT is expressed at a high, uniform density on the surface of all tumor cells, is not expressed on normal cells, is minimally internalized after antibody binding, and is not “shed” into the circulation. Equally important, the target’s cognate antibody should penetrate rapidly into tumor nodules, bind with high avidity to the target antigen, interact minimally with non-malignant tissues, and clear from the blood soon after maximal tumor binding is achieved. Although a “perfect” antigen-antibody pair does not exist, CD20, CD22, and HLA-DR have been effectively targeted on B cell lymphomas, CD33 and CD45 have shown promise in studies treating acute myeloid leukemia (AML) and early studies have suggested impressive efficacy targeting CD38 in multiple myeloma (MM)(11). Investigators have not reached a universal consensus on the best therapeutic radionuclide for RIT, but 131Iodine and 90Yttrium have been utilized in the vast majority of studies. These beta-particle emitting radionuclides are readily available, have favorable emission characteristics, and are stably retained on antibodies using simple radiolabeling methods(10). Recently, the increased availability of α–emitter radionuclides, in conjunction with advances in radiochemistry leading to radiolabeling platforms capable of providing critical stability to α–particle-labeled biomolecules,(12) has led to promising results using α–emitter RIT in leukemia(13,14), lymphoma (15) and MM (Green and Press unpublished observations). Based on their physical characteristics, α-emitters may be particularly efficacious in minimal residual disease (MRD) settings, where isolated cells and small tumor clusters prevail. MRD elimination prior to stem cell transplant has been associated with improved OS in patients with hematologic malignancies including AML(16) and MM (17). In light of these advances, our group and others are exploring a role for α-emitter RIT in a variety of settings.
First Generation Radiolabeled Antibodies
Early RIT studies attached radionuclides directly to the antibody protein, either using oxidizing agents such as chloramine T or IodoGen™ reactions for 131Iodine or derivatizing the antibody with a chelating agent such as the DOTA macrocycle for 111Indium and 90Yttrium. Most clinical trials to date have investigated the utility of either tostitumomab (anti-CD20) antibody followed by 131I-tositumomab (Bexxar™) or rituximab followed by 90Y-ibritumomab tiuxetan (Zevalin™) for treatment of B cell lymphomas. Dozens of published studies using these two products showed dramatic efficacy in patients with indolent B cell lymphomas with overall response rates (ORR) of 95% and CR rates of 75% in the front-line setting as a single agent (18) and ORR rates of 60–80% and CR rates of 20–40% in patients with relapsed or refractory indolent lymphomas(19–22). Many of these responses are durable with median remission durations exceeding 6 years after front-line therapy(18) and with 15–20% of relapsed/refractory patients enjoying remissions exceeding five years (19–23). Toxicities were mild in all single agent studies, with reversible myelosuppression being most common. Concern has frequently been expressed about radiation induced myelodysplasia (MDS) and AML, although the rates observed following RIT are comparable to the incidence of MDS/AML reported with many standard chemotherapeutic agents.(24) Non-hematologic adverse events following Bexxar™ and Zevalin™ consisted mainly of grade 1–2 fatigue, nausea, infusion reactions, and hypothyroidism (with Bexxar™). Efficacy appeared similar with either Bexxar™ or Zevalin™, however the two drugs have never been rigorously compared in a randomized setting.
At least seven Phase II studies and two Phase III studies have tested RIT in newly diagnosed patients receiving front-line therapy, either alone or as consolidation following chemotherapy(18,25–33). These studies have all demonstrated outstanding efficacy with ORR of 90–100% and CR rates of 60–100%. More importantly, the remissions induced have been very durable with the median remission durations exceeding 6 years in all studies with sufficient follow-up(25,33). The efficacy of this strategy was validated in a Phase III randomized trial of 90Y-ibritumomab tiuxetan consolidation after first remission in advanced-stage follicular lymphomas. In this study of 414 patients with newly diagnosed lymphoma who had achieved a complete or partial remission following first-line chemotherapy; the median PFS was 37 months for patients treated with 90Y-ibritumomab tiuxetan compared to only 13.5 months for those who did not receive consolidative RIT, (P ≤ 0.0001). These salutary findings led to the regulatory approval of 90Y-ibritumomab tiuxetan (Zevalin®) RIT as first-line consolidation in both Europe and the USA. A subsequent Phase III Intergroup Study compared front-line CHOP chemotherapy for 6 cycles followed by 131I-tositumomab consolidation with CHOP chemotherapy plus 6 doses of rituximab. The trial enrolled 554 patients with previously untreated, advanced stage follicular lymphoma(33–35). Although the initial report of this study showed no differences in either PFS or OS between the two treatment arms, follow-up has demonstrated a significant improvement in 10-year PFS for patients treated with CHOP + RIT (given without any rituximab) (57%) compared to patients treated on the CHOP-Rituximab arm (42%; p-value = 0.01) (34). There were no statistically significant differences in 10-year OS of patients treated on the two arms of this study, nor were there significant differences in the rates of MDS, secondary malignancies or grade 3–5 adverse events.
FDA Approval of Radiolabeled Antibodies
In February, 2002 Zevalin™ became the first radiolabeled antibody to receive FDA approval. Its initial indication was the treatment of relapsed or refractory low grade, follicular B-cell non-Hodgkin’s lymphoma (NHL), including patients with rituximab-refractory follicular NHL. The label was expanded in 2014 to include use of Zevalin™ for consolidation of patients following initial treatment with front-line chemotherapy. Bexxar™ was approved in 2003 for treatment of relapsed, refractory and transformed indolent lymphomas. However, to the surprise and disappointment of most RIT investigators, neither of the two approved RIT products found broad application in clinical practice. In February 2014, GlaxoSmithKline discontinued the manufacture and sale of Bexxar™ due to poor sales. Zevalin™ remains available in the United States, but sales are poor.
The reasons for the commercial failures of Bexxar™ and Zevalin™ remain controversial. One survey of physicians indicated a major impediment to the sales of RIT reagents was the fact that these reagents could not be administered in the offices or infusion rooms of practicing hematologists and oncologists, but required referral to Nuclear Medicine and Radiation Oncology consultants(36). These referrals often involved extensive “phone tag”, communication gaps, and frustration on the parts of the referring hematologist-oncologist, the treating Nuclear Medicine or Radiation Oncology physician and the patient. Reimbursement concerns and phobias concerning radiation therapy also probably played roles, particularly in the context of exaggerated concerns about the risks of MDS/AML. The simultaneous emergence of several competing therapies targeting B cell malignancies which could be easily administered by practicing oncologists (bendamustine) or could be conveniently dosed by oral administration (ibrutinib, idelalisib) undoubtedly also contributed significantly to limited integration of Bexxar™ and Zevalin™ into clinical practice. To overcome these formidable obstacles will require enhancements to RIT that produce outcomes far superior to those achievable by other options in the marketplace. “Pretargeted” RIT is an approach that might achieve this goal.
Pretargeted Radioimmunotherapy (PRIT)
PRIT employs multi-step delivery of reagents to dissociate the slow distribution phase of the large antibody molecule from the administration of the therapeutic radionuclide(37–40). Tumor-reactive antibodies are administered in a non-radioactive form, allowing them to accumulate in tumor sites without subjecting the body to non-specific irradiation from circulating radiolabeled antibodies(10). After a delay of 24–48 hours to allow maximal accretion of antibody in the tumor, a small molecular weight, radioactive reagent with high affinity for the tumor-reactive antibody is delivered. This second reagent is very small and rapidly penetrates tumors where the radioactive ligand is trapped by the pretargeted antibody. Unbound molecules of the radioactive reagent are rapidly cleared from the blood in the urine or bile, often facilitated by the use of a “clearing agent” injected immediately before the radiolabeled moiety. This intervention eliminates excess unbound antibody from the blood and avoids it from combining with the radiolabeled ligand outside the tumor environment(40). Several PRIT technologies have been developed including the use of antibody-streptavidin (SA) conjugates or fusion proteins used together with a dendrimeric N-acetylgalactosamine containing clearing agent, followed by 90Y-DOTA biotin(41). While this has been the most popular and widely employed PRIT approach, it has been criticized because of the immunogenicity of SA and the potential for “blocking” of SA binding sites by endogenous circulating biotin which might compete with binding to 90Y-DOTA-biotin.
To avoid the limitations imposed by SA-biotin PRIT, investigators have developed several alternative approaches. Foremost among these alternatives is the use of bispecific monoclonal antibody technologies. Goldenberg and colleagues developed bivalent haptens that permit cooperative binding, linking two bispecific antibodies together on the tumor surface using a bivalent histamine-succinyl-glycine hapten as a bridge(42). Another innovative approach utilizes molecularly engineered ‘dimerization and docking domains’ containing self-assembling protein kinase A motifs with engineered cysteine residues(43,44). A third approach(45), employs molecularly engineered bispecific antibodies incorporating complementary reactive groups in the antibody binding pocket, which bind covalently and irreversibly to radiolabeled electrophilic ligands(46,47). Wittrup perfected a versatile, modular (IgG-scFv) bispecific antibody format with an IgG portion specific for tumor and a high-affinity scFv specific for DOTA-yttrium generated by yeast surface display. Our own studies (48) have utilized a modification of the Wittrup’s approach employing two scFv domains rather than an IgG-scFv construct.(49) Finally, Hnatowich has developed a unique methodology employing complementary hybridization of phosphorodiamidate morpholino oligomers that rely on Watson-Crick pairing to capture radiolabeled ligands. (50) Although each of these “second generation” PRIT methods has been shown to be superior to “first generation” RIT with directly radiolabeled antibodies, until the publication of our comparative studies in Cancer Research(48), no head-to-head comparisons had been conducted to discern which of the PRIT approaches was most promising for clinical development.
Head to Head Comparison of SA-PRIT and Bispecific Antibody PRIT
To facilitate selection of the most promising PRIT construct for future clinical trials, we performed a comparative analysis of the biodistribution and therapeutic efficacy of the two most popular PRIT strategies, namely, SA-biotin and bispecific antibody PRIT. We engineered a bispecific fusion protein consisting of scFvs targeting human CD20 on one end and Yttrium-DOTA on the other end. The fusion protein traps the radiolabeled ligand (90Y-DOTA) using a very high affinity anti-Y-DOTA scFv (C825) generated by Dane Wittrup using yeast surface display(51). Head-to-head comparative biodistribution experiments comparing SA-biotin and bispecific Ab (2H7-Fc-C825 ) PRIT in mice bearing human lymphoma xenograft tumors demonstrated tumor targeting by the bispecific construct (2H7-Fc-C825) that was virtually identical to SA-biotin PRIT after 24 hours (8.37±1.21% ID/g vs 8.19±1.02% ID/g respectively). However, residual radioactivity in the blood and normal organs was consistently higher following SA-biotin PRIT than after bispecific Ab PRIT (2.09±0.46% ID/g vs 0.59±0.09% for the blood and 2.07±0.35% ID/g vs 0.55±0.07% for the kidneys, respectively). Consequently, tumor-to-normal ratios were superior for bispecific Ab PRIT with 2H7-Fc-C825. Therapy studies performed in mice bearing either Ramos or Granta subcutaneous lymphoma xenografts, demonstrated that bispecific 2H7-Fc-C825 PRIT was very effective and significantly less myelosuppressive than SA-biotin PRIT. All animals (100%) receiving optimal doses of bispecific 2H7-Fc-C825 fusion protein (2.8 nmol), followed by 90Y-DOTA-biotin (1000μCi) were alive and cured after 150 days, while tumor-bearing control groups demonstrated rapidly progressive tumor growth with 0% survival beyond 25 days (p<.001). In addition to reduced immunogenicity and the absence of endogenous biotin interference, these findings suggest that bispecific PRIT is preferred for future clinical trials because of a slightly superior biodistribution profile, less myelosuppression, and superior efficacy.
Implications and future directions
Despite impressive efficacy and safety profiles that led to FDA approval of two radioimmunoconjuages for the treatment of B cell lymphoma (131I-tositumomab and 90Y-ibritumumab tiuxitan), these agents have rarely been incorporated into clinical care. RIT targeting CD20 remains in the National Comprehensive Cancer Network Guidelines (NCCN) as a first-line therapy for elderly or infirm patients with follicular lymphoma and as a recommended approach to consolidation or second-line therapy for follicular NHL. Nonetheless, overall utilization remains low and RIT is administered disproportionately within the confines of academic centers(36). Limited use is likely a consequence of multiple factors which include the availability and ease of administration associated with other novel targeting agents and concerns about radiation toxicity, particularly to the bone marrow. Concerns regarding reimbursement to community oncologists cannot be trivialized, however the absolute cost of RIT for consolidation is lower than the cost of maintenance rituximab ($46,000; and $54,000 to $72,000 [12 – 16 courses]) respectively(52). RIT may also offer a quality of life advantage to patients because administration involves a single patient infusion visit as compared to frequent infusions during rituximab maintenance.
Innovations that improve targeting, diminish toxicity and highlight the unique favorable attributes associated with RIT may help to overcome a history of limited adoption. We have recently shown that parallel bispecific antibodies targeting CD38 on myeloma cells or CD45 on AML cells work as well as the CD20 antibodies published in Cancer Research(15) (and unpublished data, Green and Press). We are currently scaling up production of the bispecific antibodies for human clinical trials. Future studies will also investigate potential synergy of PRIT with small molecule inhibitors (e.g. ibrutinib, navitoclax, idelalisib), radiosensizing agents (Poly(ADP-ribose) polymerase inhibitors(53), bortezomib(54)) and a role for bispecific PRIT in stem cell transplantation. Ultimately, superior efficacy will provide the most compelling argument for the adoption of PRIT and well-designed clinical trials, convincingly recapitulating the impressive responses seen with bispecific antibody delivery systems in preclinical models, will be essential to reinvigorate interest in this field.
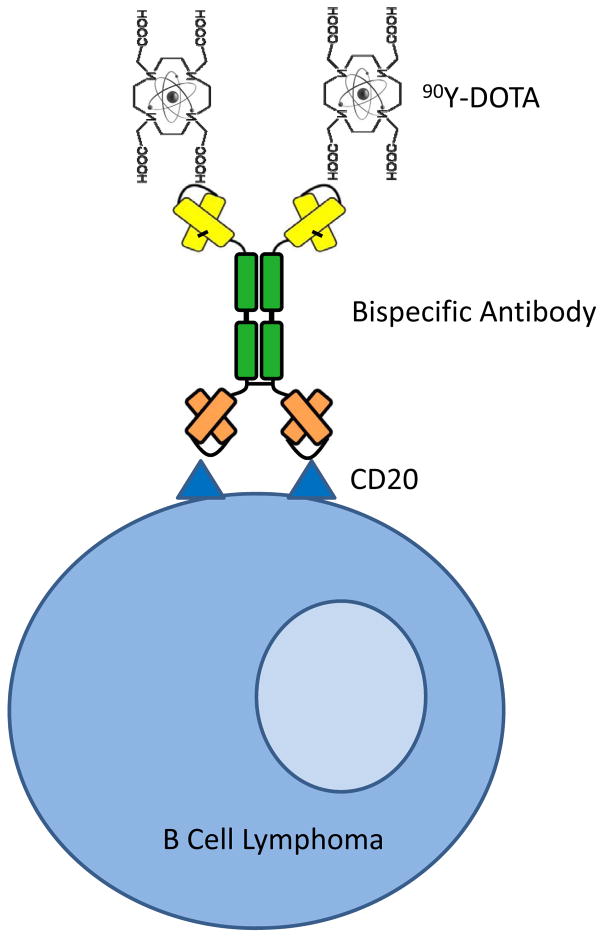
Figure 1. Bispecific Antibody Pretargeted Radioimmunotherapy.
A bispecific antibody construct was engineered that spontaneously dimerizes after expression by CHO-DG44 cells. One single chain antibody fragment binds with high affinity to the CD20 antigen on B cell malignancies, whereas the other single chain antibody fragment binds with exceptionally high affinity to an 90Yttrium-DOTA hapten.
Acknowledgments
Financial Support: This work was supported by grants from the US National Institutes of Health NCI K08 CA151682 (D.J.G.); NCI R01 CA205248 (D.J.G); NCI R01 CA076287 (O.W.P.), NCI R01 CA136639 (O.W.P.), NCI R01 CA154897 (O.W.P.), NCI P01 CA044991 (O.W.P.), NCI R21 CA155911 (D.J.G), NCI R01 CA109663 (O.W.P.), Multiple Myeloma Opportunities for Research and Education (MMORE), The Quest for Truth Foundation, The Brotherton Family Fund and by the David and Patricia Giuliani Family Foundation.
Footnotes
Disclosure of COI: The authors have no conflicts of interest to disclose.
Bibliography
- 1.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature reviews Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 2.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 3.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 4.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol. 2016;34:972–9. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 8.Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–43. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nature reviews Cancer. 2015;15:347–60. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DJ, Orgun NN, Jones JC, Hylarides MD, Pagel JM, Hamlin DK, et al. A Preclinical Model of CD38-Pretargeted Radioimmunotherapy for Plasma Cell Malignancies. Cancer research. 2014;74:1179–89. doi: 10.1158/0008-5472.CAN-13-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilbur DS, Chyan MK, Hamlin DK, Vessella RL, Wedge TJ, Hawthorne MF. Reagents for astatination of biomolecules. 2. Conjugation of anionic boron cage pendant groups to a protein provides a method for direct labeling that is stable to in vivo deastatination. Bioconjugate chemistry. 2007;18:1226–40. doi: 10.1021/bc060345s. [DOI] [PubMed] [Google Scholar]
- 13.Jurcic JG, Rosenblat TL, McDevitt MR, Pandit-Taskar N, Carrasquillo JA, Chanel SM, et al. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac-lintuzumab) (anti-CD33; HuM195) in acute myeloid leukemia (AML) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:6516. [Google Scholar]
- 14.Hagemann UB, Wickstroem K, Wang E, Shea AO, Sponheim K, Karlsson J, et al. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Molecular cancer therapeutics. 2016;15:2422–31. doi: 10.1158/1535-7163.MCT-16-0251. [DOI] [PubMed] [Google Scholar]
- 15.Green DJ, Shadman M, Jones JC, Frayo SL, Kenoyer AL, Hylarides MD, et al. Astatine-211 conjugated to an anti-CD20 monoclonal antibody eradicates disseminated B-cell lymphoma in a mouse model. Blood. 2015;125:2111–9. doi: 10.1182/blood-2014-11-612770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:329–36. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA oncology. 2017;3:28–35. doi: 10.1001/jamaoncol.2016.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. The New England journal of medicine. 2005;352:441–9. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 19.Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with Ibritumomab Tiuxetan Radioimmunotherapy in Patients With Rituximab-Refractory Follicular Non-Hodgkin’s Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:3262–9. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2453–63. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MS, Estes J, Zasadny KR, Francis IR, Ross CW, Tuck M, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96:1259–66. [PubMed] [Google Scholar]
- 22.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3918–28. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RI, Kaminski MS, Wahl RL, Knox SJ, Zelenetz AD, Vose JM, et al. Tositumomab and Iodine-131 Tositumomab Produces Durable Complete Remissions in a Subset of Heavily Pretreated Patients With Low-Grade and Transformed Non-Hodgkin’s Lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7565–73. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 24.Bennett JM, Kaminski MS, Leonard JP, Vose JM, Zelenetz AD, Knox SJ, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine 131I tositumomab. Blood. 2005;105:4576–82. doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 25.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I–131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4143–9. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 26.Leonard JP, Coleman M, Kostakoglu L, Chadburn A, Cesarman E, Furman RR, et al. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I 131 tositumomab for untreated follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5696–704. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs SA, Swerdlow SH, Kant J, Foon KA, Jankowitz R, Land SR, et al. Phase II Trial of Short-Course CHOP-R Followed by Y-90-ibritumomab Tiuxetan and Extended Rituximab in Previously Untreated Follicular Lymphoma. Clinical Cancer Research. 2008;14:7088–94. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 28.Zinzani PL, Tani M, Pulsoni A, Gobbi M, Perotti A, De Luca S, et al. Fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in previously untreated patients with follicular non-Hodgkin lymphoma trial: a phase II non-randomised trial (FLUMIZ) The Lancet Oncology. 2008;9:352–8. doi: 10.1016/S1470-2045(08)70039-1. [DOI] [PubMed] [Google Scholar]
- 29.Zinzani PL, Tani M, Fanti S, Stefoni V, Musuraca G, Castellucci P, et al. A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:769–73. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- 30.Zinzani PL, Rossi G, Franceschetti S, Botto B, Di Rocco A, Cabras MG, et al. Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3998–4004. doi: 10.1158/1078-0432.CCR-10-0162. [DOI] [PubMed] [Google Scholar]
- 31.Zinzani PL, Tani M, Fanti S, Stefoni V, Musuraca G, Vitolo U, et al. A phase 2 trial of fludarabine and mitoxantrone chemotherapy followed by yttrium-90 ibritumomab tiuxetan for patients with previously untreated, indolent, nonfollicular, non-Hodgkin lymphoma. Cancer. 2008;112:856–62. doi: 10.1002/cncr.23236. [DOI] [PubMed] [Google Scholar]
- 32.Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5156–64. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 33.Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:314–20. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shadman M, Li H, Rimsza L, LeBlanc M, Czuczman M, Kaminski M, et al. SWOG S0016: Long term follow-up of phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131) iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma. Blood. 2016 doi: 10.1200/JCO.2012.42.4101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, et al. A comparative analysis of prognostic factor models for follicular lymphoma based on a phase III trial of CHOP-rituximab versus CHOP + 131iodine--tositumomab. Clin Cancer Res. 2013;19:6624–32. doi: 10.1158/1078-0432.CCR-13-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaefer NG, Huang P, Buchanan JW, Wahl RL. Radioimmunotherapy in non-Hodgkin lymphoma: opinions of nuclear medicine physicians and radiation oncologists. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:830–8. doi: 10.2967/jnumed.110.085589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nature medicine. 2005;11:1250–5. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Yao Z, Garmestani K, Axworthy DB, Zhang Z, Mallett RW, et al. Pretargeting radioimmunotherapy of a murine model of adult T-cell leukemia with the alpha-emitting radionuclide, bismuth 213. Blood. 2002;100:208–16. doi: 10.1182/blood-2002-01-0107. [DOI] [PubMed] [Google Scholar]
- 39.Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1987;28:1294–302. [PubMed] [Google Scholar]
- 40.Axworthy DB, Reno JM, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1802–7. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axworthy DB, Fritzberg AR, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, et al. Preclinical evaluation of an anti-tumor monoclonal antibody/streptavidin conjugate for pretargeted Y-90 radioimmunotherapy in a mouse xenograft model. Journal of immunotherapy. 1994:16. [Google Scholar]
- 42.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:823–34. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 43.Chang CH, Rossi EA, Goldenberg DM. The dock and lock method: a novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13:5586s–91s. doi: 10.1158/1078-0432.CCR-07-1217. [DOI] [PubMed] [Google Scholar]
- 44.Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6841–6. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodwin DA, Meares CF. Advances in pretargeting biotechnology. Biotechnol Adv. 2001;19:435–50. doi: 10.1016/s0734-9750(01)00065-9. [DOI] [PubMed] [Google Scholar]
- 46.Chmura AJ, Orton MS, Meares CF. Antibodies with infinite affinity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8480–4. doi: 10.1073/pnas.151260298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butlin NG, Meares CF. Antibodies with infinite affinity: origins and applications. Acc Chem Res. 2006;39:780–7. doi: 10.1021/ar020275e. [DOI] [PubMed] [Google Scholar]
- 48.Green DJ, Frayo SL, Lin Y, Hamlin DK, Fisher DR, Frost SH, et al. Comparative Analysis of Bispecific Antibody and Streptavidin-Targeted Radioimmunotherapy for B-cell Cancers. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-16-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orcutt KD, Rhoden JJ, Ruiz-Yi B, Frangioni JV, Wittrup KD. Effect of small-molecule-binding affinity on tumor uptake in vivo: a systematic study using a pretargeted bispecific antibody. Molecular cancer therapeutics. 2012;11:1365–72. doi: 10.1158/1535-7163.MCT-11-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Dou S, Liu G, Liu X, Wang Y, Chen L, et al. Synthesis and in vitro characterization of a dendrimer-MORF conjugate for amplification pretargeting. Bioconjugate chemistry. 2008;19:1518–25. doi: 10.1021/bc8001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orcutt KD, Slusarczyk AL, Cieslewicz M, Ruiz-Yi B, Bhushan KR, Frangioni JV, et al. Engineering an antibody with picomolar affinity to DOTA chelates of multiple radionuclides for pretargeted radioimmunotherapy and imaging. Nuclear medicine and biology. 2011;38:223–33. doi: 10.1016/j.nucmedbio.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Q, Ayer T, Nastoupil LJ, Rose AC, Flowers CR. Comparing the cost-effectiveness of rituximab maintenance and radioimmunotherapy consolidation versus observation following first-line therapy in patients with follicular lymphoma. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18:189–97. doi: 10.1016/j.jval.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaefer NG, James E, Wahl RL. Poly(ADP-ribose) polymerase inhibitors combined with external beam and radioimmunotherapy to treat aggressive lymphoma. Nuclear medicine communications. 2011;32:1046–51. doi: 10.1097/MNM.0b013e32834a369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elstrom RL, Ruan J, Christos PJ, Martin P, Lebovic D, Osborne J, et al. Phase 1 study of radiosensitization using bortezomib in patients with relapsed non-Hodgkin lymphoma receiving radioimmunotherapy with 131I-tositumomab. Leukemia & lymphoma. 2015;56:342–6. doi: 10.3109/10428194.2014.914195. [DOI] [PMC free article] [PubMed] [Google Scholar]