Abstract
Background
Hispanic women have lower breast cancer incidence rates than non-Hispanic white (NHW) women. To what extent genetic versus non-genetic factors account for this difference is unknown.
Methods
Using logistic regression, we evaluated the interactive influences of established risk factors and ethnicity (self-identified and identified by ancestral informative markers) on breast cancer risk among 2326 Hispanic and 1854 NHW postmenopausal women from the US and Mexico in the Breast Cancer Health Disparities Study.
Results
The inverse association between % Native American(NA) ancestry and breast cancer risk was only slightly attenuated after adjusting for known risk factors [lowest versus highest quartile: odds ratio(OR)=1.39, 95% confidence interval(CI)=1.00–1.92 among US Hispanics; OR=1.92 (1.29–2.86) among Mexican women]. The prevalence of several risk factors, as well as the associations with certain factors and breast cancer risk, differed according to genetic admixture. For example, higher BMI was associated with reduced risk among women with lower NA ancestry only [BMI <25 versus >30: OR=0.65 (0.44–0.98) among US Hispanics; OR=0.53 (0.29–0.97) among Mexicans]. The average number of risk factors among cases was inversely related to % NA ancestry.
Conclusions
The lower NA ancestry groups were more likely to have the established risk factors, with the exception of BMI. While the majority of factors were associated with risk in the expected directions among all women, BMI had an inverse association among Hispanics with lower NA ancestry.
Impact
These data suggest that the established risk factors are less relevant for breast cancer development among women with more NA ancestry.
Keywords: genetic ancestry, Latinas, breast cancer risk factors, gene-environment interaction, ethnic disparities
Introduction
In the United States (US), age-adjusted breast cancer incidence rates are about 25% lower among Hispanics/Latinas than among non-Hispanic white (NHW) women (1). This difference in breast cancer incidence is likely due to differences in genetic, reproductive, hormonal, lifestyle and environmental factors. To what extent genetic versus non-genetic factors, independently and interactively, account for this difference has not been well defined.
Hispanics are a genetically heterogeneous population (2), representing a mix of primarily European and Native American genotypes (3). Genetic admixture studies using ancestral informative markers (AIMs) of European and Native American ancestry have indicated that higher European ancestry is associated with increased breast cancer risk among both US Hispanic and Mexican women (2–5). The consistency of this observation is concordant with the discovery of a genetic variant near the estrogen receptor 1 gene (ESR1) that is only observed in women with at least some Native American ancestry and is associated with a reduced risk of breast cancer (6).
Our previous work within the 4-Corners Breast Cancer Study found several differences in the impact of known breast cancer risk factors according to self-reported ethnicity in a US population (7). We estimated that about two-thirds of breast cancers could be attributed to known risk factors among NHW women as compared with under one-third among Hispanic women (7). We now know that Hispanic women have genetic factors that lower the risk of breast cancer development. We hypothesize that there might be ancestry/ethnic-specific differences in the way that non-genetic factors affect breast cancer risk.
The diversity in genetic ancestry and lifestyle exposures among Hispanic and NHW women provides an opportunity to evaluate the influence of genetic versus non-genetic risk factors, thereby contributing to a more complete understanding of breast cancer etiology and possible causes for the observed disparities in incidence. We explored the interactive influences of breast cancer risk factors and both self-identified ethnicity and genetic ancestry estimates (AIMs) on breast cancer risk among postmenopausal women from the US and Mexico.
Materials and Methods
Study population
The Breast Cancer Health Disparities Study is comprised of participants from three population-based case-control studies: the 4-Corners Breast Cancer Study, the San Francisco Bay Area Breast Cancer Study, and the Mexico Breast Cancer Study. These three studies have been described in detail elsewhere (8–10). Briefly, participants from the 4-Corners Breast Cancer Study were NHW, Hispanic, or Native American women living in non-reservation areas in the states of Arizona, Colorado, New Mexico, or Utah at the time of diagnosis (or selection). Cases were ages 25 to 79 years with a histological confirmed diagnosis of in situ or invasive cancer between October 1999 and May 2004; controls were selected from the target and frequency matched on ethnicity and 5-year age distribution of cases. In Arizona and Colorado, controls under 65 years old were randomly selected from a commercial mailing list. In New Mexico and Utah, they were randomly selected from driver’s license lists. In all states, women 65 years and older were randomly selected from the Center for Medicare Services lists. Participants from the San Francisco Bay Area Breast Cancer Study were women ages 35 to 79 years from the San Francisco Bay Area. Cases were women diagnosed with a first primary histologically confirmed invasive breast cancer between April 1997 and April 2002, and controls were identified through random-digit dialing, frequency-matched by ethnicity and age distribution. Participants from the Mexico Breast Cancer Study were women ages 28 to 74 years, living in the states of Monterrey or Veracruz or in Mexico City. Cases were women diagnosed with either a new histologically confirmed in situ or invasive breast cancer between January 2004 and December 2007, whereas controls were women randomly selected from the catchment area of the cases using a probabilistic multi-stage design.
Prior studies have observed a stronger association between genetic admixture and breast cancer risk among postmenopausal versus premenopausal women (4, 5). Given this evidence and the limited power to adequately evaluate three-way interactions by menopausal status, this analysis was restricted to postmenopausal women who had both genetic and risk factor data available. This analysis included 875 NHW cases and 979 NHW controls, 614 US Hispanic cases and 785 US Hispanic controls, and 426 Mexican cases and 501 Mexican controls. Due to relatively small number of Native American women (55 cases and 73 controls) in the 4-Corners Breast Cancer Study, they were included with Hispanic women. Ethical approval of this study was obtained by the Institutional Review Boards at the University of Colorado, the University of Utah, the University of Arizona, the University of New Mexico, the Cancer Prevention Institute of California, the Instituto Nacional de Salud Publica (INSP), and the Instituto Mexicano de Seguridad Social (IMSS). All participants signed informed written consent prior to participation.
Risk factor data
Data were harmonized across all study centers and questionnaires (5). This process involved transforming of variables that used the same or the closest information possible and assessing the distribution of variables across studies for comparability. Women who reported still having periods during the referent year (defined as the calendar year before diagnosis for cases or before selection into the study for controls) without taking menopausal hormone therapy (HT) were classified as pre-menopausal. Women were classified as post-menopausal if they met any of the following criteria: 1) reported a natural menopause, or 2) were still having periods while taking HT and were at or above the 95th percentile of age for those who reported having a natural menopause (i.e., ≥12 months since their last period). Age at menopause was site and ethnic-specific: 58 years for NHW and 56 for Hispanic women from the 4-Corners Breast Cancer Study; 54 for the Mexico Breast Cancer Study; and 55 for NHW and 56 for Hispanic women from the San Francisco Bay Area Breast Cancer Study.
The following variables were included in this analysis: age at menopause (<50, ≥50 years), HT use (never, former, current use), age at menarche (≤11, 12–13, ≥14 years), family history of breast cancer among first-degree relatives (yes, no), parity and age at first full-term live or still birth (nulliparous, 1–2 births at age <25 years, 1–2 births at age ≥25 years, ≥3 births at age <25 years, ≥3 births at age ≥25 years), breast-feeding (never, ≤12, >12 months), body mass index (BMI) (<25, 25–29.9, ≥30 kg/m2), and alcohol intake (none, <10 g/day, ≥10 g/day). With the exception of BMI, all variables were self-reported. BMI (kg/m2) was calculated based on self-reported weight (kg) in the reference year and height (m) measured at interview. Self-reported height was utilized for those who were missing measurement data. Alcohol intake was based on lifetime average intake (4-Corners and Mexico subjects), or intake during the reference year (SFBCS). All multivariable models were also adjusted for age (continuous) and study center (4-Corners or San Francisco Bay Area for US-based analyses only). A total of 104 AIMs were genotyped in order to provide an estimate of European and Native American ancestry (11).
Genetic data
DNA was extracted from either whole blood (7286 subjects) or mouthwash samples (637 subjects) provided by study participants. Whole Genome Amplification (WGA) was applied to the mouthwash derived DNA samples prior to genotyping. Genotyping was conducted as part of a larger study of 1,466 single nucleotide polymorphisms (SNPs) in 205 candidate genes in several inflammation-related pathways hypothesized to be involved in breast carcinogenesis (5). A total of 104 AIMs were included to estimate European and Native American ancestry (11). As previously described, genotyping was done using a multiplexed bead array assay format based on GoldenGate chemistry (Illumina, San Diego, California). A genotyping call rate of 99.93% was attained (99.65% for WGA samples). For quality control, 132 internal replicates were included, representing 1.6% of the sample set. The duplicate concordance rate was 99.996% as determined by 193,297 matching genotypes among sample pairs.
Statistical analyses
All statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, NC). Bivariate analyses were utilized to compare averages (or proportions) of risk factors between controls and/or cases within and across the different ethnic or admixture groups. Statistical significance was based on two-sided tests at a significance level of less than 0.05. Logistic regression models were utilized to compute odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the ethnic-specific relationship between genetic admixture (quartiles) and postmenopausal breast cancer risk among women in the US and Mexico, as well as the relationships between risk factors and breast cancer risk among postmenopausal women according to genetic admixture (below or above the population-specific median values) among Hispanic women in the US and Mexico. Data were stratified by ethnic and/or genetic admixture groups to obtain ethnic/admixture-specific risk estimates. Ethnic/admixture-specific risk estimates were also evaluated based on ER status for US subjects only.
Separate analyses were performed for the US and Mexico studies because of the considerable differences in the distributions of the genetic and non-genetic risk factors, as well as the potential for confounding by unmeasured risk factors (2). The relationship between % NA ancestry and postmenopausal breast cancer risk was evaluated using quartiles within each ethnic/regional group. Among ordinal variables, a Wald P value for linear trend was obtained when converting the variable from categorical to numerical data and including it in the multivariable regression model. For all risk factors, a P value for interaction was obtained from the Wald P value when creating an interaction term between each risk factor and the ethnic/admixture group within each region.
Comparative density plots based on the distribution of total number of aforementioned risk factors among cases and controls were created using SAS. Two sample t-tests and one-way ANOVAs (Bonferroni correction for pairwise comparisons) were used to obtain P values for comparisons with the average number of risk factors across the ethnic/admixture groups.
Results
Among US controls, NHWs were significantly more likely to have many of the established risk factors compared to Hispanics (P < 0.05) (Table 1). The only risk factor that US Hispanics were more likely to report was a higher BMI. Interestingly, this pattern was similar when comparing US Hispanics to Mexican controls. US Hispanics were significantly more likely to have all of the same risk factors when compared to Mexican women, whereas Mexican women had a higher BMI. As expected, there were large differences in the % NA ancestry, with NHW controls having the lowest (4%), followed by US Hispanics (41%) and Mexicans (71%). Among US cases, Hispanic women had a higher percentage of ER negative tumors compared with NHW women (23.3% versus 18.1%).
Table 1.
Breast cancer risk factors among postmenopausal women according to region and self-reported ethnicity.
| Characteristics | US Studies | Mexico Study | ||||
|---|---|---|---|---|---|---|
| Non-Hispanic White | Hispanic | |||||
|
|
|
|
||||
| Cases (n = 875) | Controls (n =979) | Cases (n =614) | Controls (n =785) | Cases (n = 426) | Controls (n = 501) | |
| US study site, No. (%) | ||||||
| 4-Corners | 740 (84.6) | 838 (85.6) | 309 (50.3) | 392 (49.9) | N/A | N/A |
| San Francisco Bay Area | 135 (15.4) | 141 (14.4) | 305 (49.7) | 393 (50.1) | ||
| Reference agea,b, y, mean (SD) | 60.7 ( 9.2) | 62.0 ( 9.7) | 59.2 ( 9.1) | 59.2 (9.1) | 57.6 (7.4) | 56.3 (6.9) |
| Family history*,#, No. (%) | ||||||
| Yes | 201 (23.0) | 162 (16.6) | 121 (19.7) | 102 (13.0) | 29 (6.8) | 12 (2.4) |
| No | 674 (77.0) | 817 (83.4) | 493 (80.3) | 683 (87.0) | 397 (93.2) | 489 (97.6) |
| No. of birthsb,c, mean (SD) | 2.4 (1.6) | 2.6 (1.7) | 3.1 (2.1) | 3.7 (2.5) | 3.3 (2.4) | 4.3 (2.7) |
| Age at first birtha,b,c, y, mean (SD) | 23.5 (4.3) | 23.5 (4.5) | 23.0 (5.2) | 22.5 (4.9) | 23.0 (5.7) | 21.4 (4.9) |
| Breast-feedingb,d, No. (%) | ||||||
| Any | 459 (52.5) | 570 (58.2) | 320 (52.1) | 492 (62.7) | 326 (76.5) | 429 (85.6) |
| None | 416 (47.5) | 409 (41.8) | 294 (47.9) | 293 (37.3) | 100 (23.5) | 72 (14.4) |
| Body mass index in referent yeara, kg/m2, mean (SD) | 27.6 (5.8) | 27.5 (6.0) | 29.6(6.1) | 30.2 (6.2) | 30.2(5.9) | 31.0 (5.6) |
| Age at menarche, y, mean (SD) | 12.6 (1.6) | 12.8 (1.6) | 12.6 (1.7) | 12.8 (1.8) | 12.8 (1.7) | 13.0 (1.6) |
| Age at menopauseb, y, mean (SD) | 46.2 (8.2) | 45.2 (8.2) | 45.7 (7.7) | 44.7 (7.6) | 45.7 (6.2) | 45.9 (6.4) |
| Hormone therapy usea,b, No. (%) | ||||||
| Current | 469 (53.6) | 478 (48.8) | 243 (39.6) | 207 (26.4) | 18 (4.2) | 10 (2.0) |
| Former | 189 (21.6) | 270 (27.6) | 121 (19.7) | 246 (31.3) | 84 (19.7) | 64 (12.8) |
| Never | 217 (24.8) | 231 (23.6) | 250 (40.7) | 332 (42.3) | 324 (76.1) | 427 (85.2) |
| Alcohol consumption,b,e, No. (%) | ||||||
| Any | 462 (52.8) | 469 (47.9) | 194 (31.6) | 234 (29.8) | 70 (16.4) | 33 (6.6) |
| None | 413 (47.2) | 510 (52.1) | 420 (68.4) | 551 (70.2) | 356 (83.6) | 468 (93.4) |
| Percent Native American ancestrya,b, mean (SD) | ||||||
| ER statusa | 0.04 (0.06) | 0.04 (0.04) | 0.41 (0.17) | 0.44 (0.18) | 0.66 (0.20) | 0.71 (0.19) |
| ER+ | 493 (82.9) | 348 (76.7) | Not available | |||
| ER− | 102 (18.1) | 106(23.3) | ||||
P < 0.05 when comparing NHW controls to US Hispanic controls or NHW cases to US Hispanic cases (ER status only).
P < 0.05 when comparing US Hispanic controls to Mexico controls.
Among parous (live or still birth) women only (749 cases and 854 controls for NHW; US Hispanic= 559 cases and 737 controls for US Hispanic; 375 cases and 479 controls for Mexico).
Includes both parous and non-parous women.
Alcohol consumption was based on lifetime average intake (4-Corners and Mexico subjects), or intake during the reference year (SFBCS).
Overall, a wide range of % NA ancestry was observed among Hispanic women (IQR = 32 to 54 % for US; IQR = 56 to 84% for Mexico). Despite considerable differences in the distribution of % NA ancestry between US Hispanic and Mexican women, having less NA ancestry was associated with higher breast cancer risk in both populations (Table 2). This association was only slightly diminished after adjusting for known risk factors, and remained statistically significant or marginally significant (bottom versus top quartile: OR = 1.39; 95% CI = 1.00 to 1.92, Ptrend = 0.04 among US Hispanic women; OR = 1.92; 95% CI = 1.29 to 2.86, Ptrend = 0.004 among Mexican women). When stratified by ER status, the association appeared to be stronger among ER negative cases (Supplemental Table 1). However, the percentage of ER positive tumors did not differ based on quartile of % NA ancestry within ethnic group. No association with NA ancestry was observed among NHW women, who had a very limited range of % NA ancestry (interquartile range (IQR) = 1.8 to 4.7%), irrespective of ER status (Table 2 and Supplemental Tables 1 and 2).
Table 2.
Relationship between percent Native American ancestry and breast cancer among postmenopausal women.
| Percent Native American ancestry, quartiles, No. (%) | Cases | Controls | Minimally Adjusted OR (95% CI)a | Multivariate Adjusted OR (95% CI)b |
|---|---|---|---|---|
| Non-Hispanic White | n = 875 | n = 979 | ||
| 0, 1.80% | 267 (30.5) | 254 (25.9) | 1.12 (0.87, 1.44) | 1.17 (0.91, 1.51) |
| 1.81, 2.80% | 186 (21.3) | 237 (24.2) | 0.84 (0.65, 1.10) | 0.86 (0.66, 1.13) |
| 2.81, 4.70% | 199 (22.7) | 251 (25.6) | 0.84 (0.64, 1.09) | 0.87 (0.66, 1.13) |
| >4.70% | 223 (25.5) | 237 (24.2) | Referent | Referent |
| Ptrend = 0.33 | Ptrend = 0.21 | |||
| US Hispanic | n = 614 | n =785 | ||
| 0, 32% | 165 (26.9) | 188 (24.0) | 1.51 (1.11, 2.05) | 1.39 (1.00, 1.92) |
| 32.1, 43.0% | 167 (27.2) | 181 (23.1) | 1.59 (1.17, 2.17) | 1.43 (1.04, 1.98) |
| 43.1, 54.0% | 156 (25.4) | 201 (25.6) | 1.33 (0.98, 1.81) | 1.30 (0.95, 1.79) |
| >54.0% | 126 (20.5) | 215 (27.4) | Referent | Referent |
| Ptrend = 0.005 | Ptrend = 0.04 | |||
| Mexico | n = 426 | N = 501 | ||
| 0, 56.0% | 131 (30.8) | 108 (21.6) | 2.19 (1.50, 3.19) | 1.92 (1.29, 2.86) |
| 56.1, 69.0% | 105 (24.7) | 127 (25.4) | 1.53 (1.05, 2.24) | 1.45 (0.97, 2.17) |
| 69.1, 84.0% | 112 (26.3) | 123 (24.6) | 1.67 (1.14, 2.43) | 1.62 (1.09, 2.41) |
| >84.0% | 78 (18.3) | 143 (28.5) | Referent | Referent |
| Ptrend = 0.0002 | Ptrend = 0.004 |
OR, odds ratio; CI, confidence interval.
Adjusted for referent age and study site
Adjusted for referent age, study site, family history of breast cancer, parity, age at first birth, duration of breast feeding, body mass index in referent year, age at menarche, age at menopause, menopausal hormone therapy use, and alcohol consumption.
To explore the influence of NA ancestry on the relationship between the described risk factors and postmenopausal breast cancer risk, the population was stratified according to region, self-reported ethnicity and % NA ancestry (below or above the median) (Table 3). Among NHW women, the majority of factors evaluated were associated with breast cancer risk in the expected direction. Specifically, positive family history, younger age at menarche, and older age at menopause were significantly associated with increased risk. Associations with low parity/older age at first birth, alcohol consumption and no prior history of breast-feeding were also in the expected direction, although they were weak and not statistically significant.
Table 3.
Relationship between risk factors and breast cancer risk among postmenopausal non-Hispanic Whites and US Hispanics according to percent Native American ancestry
| Risk Factors | Non-Hispanic White | US Hispanic | Pinteraction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–43% Native American Ancestry | 43.1%–100% Native American Ancestry | |||||||||
|
|
|
|
||||||||
| Cases (n = 875) | Controls (n=979) | OR (95% CI)a | Cases (n =332) | Controls (n = 369) | OR (95% CI)a | Cases (n = 282) | Controls (n = 416) | OR (95% CI)a | ||
| Ageb, y | 0.98 (0.97, 0.99) | 1.01 (0.99, 1.02) | 1.00 (0.98, 1.02) | 0.20 | ||||||
| Family history of breast cancer, No. (%) | 0.93 | |||||||||
| Yes | 201 (23.0) | 162 (16.6) | 1.57 (1.24, 1.99) | 76 (22.9) | 53 (14.4) | 1.82 (1.22, 2.73) | 45 (16.0) | 49 (11.8) | 1.61 (1.01, 2.55) | |
| No | 674 (77.0) | 817 (83.4) | Referent | 256 (77.1) | 316 (85.6) | Referent | 237 (84.0) | 367 (88.2) | Referent | |
| No. births and age at first birthc, No. (%) | 0.76 | |||||||||
| Nulliparous | 126 (14.4) | 125 (12.8) | 1.00 (0.72, 1.40) | 25 (7.5) | 28 (7.6) | 0.89 (0.47, 1.68) | 30 (10.6) | 20 (4.8) | 2.10 (1.07, 4.12) | |
| 1–2, <25 y | 186 (21.3) | 192 (19.6) | 1.13 (0.68, 1.48) | 62 (18.7) | 56 (15.2) | 1.30 (0.82, 2.07) | 48 (17.0) | 49 (11.8) | 1.60 (0.98, 2.62) | |
| 1–2, ≥25 y | 172 (19.7) | 171 (17.5) | 1.14 (0.87, 1.50) | 61 (18.4) | 58 (15.7) | 1.24 (0.79, 1.95) | 36 (12.8) | 47 (11.3) | 1.15 (0.69, 1.93) | |
| ≥3, <25 y | 305 (34.9) | 378 (38.6) | Referent | 143 (43.1) | 184 (49.9) | Referent | 143 (50.7) | 237 (57.0) | Referent | |
| ≥3, ≥25 y | 86 (9.8) | 113 (11.5) | 0.99 (0.71, 1.37) | 41 (12.4) | 43 (11.7) | 1.14 (0.69, 1.89) | 25 (8.9) | 63 (15.1) | 0.67 (0.40, 1.14) | |
| Breastfeedingd, mos, No. (%) | 0.31 | |||||||||
| None | 416 (47.5) | 409 (41.8) | 1.11 (0.84, 1.46) | 159 (47.9) | 147 (39.8) | 1.42 (0.95, 2.12) | 135 (47.9) | 146 (35.1) | 1.43 (0.94, 2.17) | |
| ≤12 | 272 (31.1) | 356 (36.1) | 0.86 (0.66, 1.12) | 96 (28.9) | 117 (32.3) | 0.95 (0.62, 1.46) | 73 (25.9) | 124 (29.8) | 1.03 (0.67, 1.58) | |
| >12 | 187 (21.4) | 214 (21.9) | Referent | 77 (23.2) | 103 (27.9) | Referent | 74 (26.2) | 146 (35.1) | Referent | |
| Ptrend = 0.31 | Ptrend = 0.06 | Ptrend = 0.09 | ||||||||
| Body mass indexb, kg/m2, No. (%) | 0.76 | |||||||||
| <25.0 | 347 (39.7) | 397 (40.6) | Referent | 96 (28.9) | 85 (23.0) | Referent | 48 (17.0) | 63 (15.1) | Referent | |
| 25.0–29.9 | 279 (31.9) | 304 (31.1) | 1.09 (0.87, 1.37) | 110 | 126 (34.2) | 0.76 (0.51, 1.14) | 102 (36.2) | 151 (36.3) | 1.08 (0.66, 1.75) | |
| ≥30 | 249 (28.4) | 278 (28.4) | 1.03 (0.81, 1.30) | (33.1) | 158 (42.8) | 0.65 (0.44, 0.98) | 132 (46.8) | 202 (48.6) | 1.07 (0.67, 1.71) | |
| Ptrend = 0.78 | 126 (38.0) | Ptrend = 0.04 | Ptrend = 0.82 | |||||||
| Age at menarche, y, No. (%) | 0.80 | |||||||||
| ≤11 | 197 (22.5) | 183 (18.7) | 1.38 (1.05, 1.82) | 93 (28.0) | 81 (22.0) | 1.44 (0.93, 2.21) | 66 (23.4) | 84 (20.2) | 1.04 (0.66, 1.63) | |
| 12–13 | 469 (53.6) | 525 (53.6) | 1.13 (0.90, 1.41) | 148 (44.6) | 175 (47.4) | 1.08 (0.75, 1.56) | 126 (44.7) | 203 (48.8) | 0.81 (0.55, 1.17) | |
| ≥14 | 209 (23.9) | 271 (27.7) | Referent | 91 (27.4) | 113 (30.6) | Referent | 90 (31.9) | 129 (31.0) | Referent | |
| Ptrend = 0.02 | Ptrend = 0.11 | Ptrend = 0.99 | ||||||||
| Age at menopause, y, No. (%) | 0.30 | |||||||||
| ≤50 | 493 (56.3) | 593 (61.1) | Referent | 205 (61.8) | 232 (62.9) | Referent | 176 (62.4) | 294 (70.7) | Referent | |
| >50 | 382 (43.7) | 377 (38.9) | 1.35 (1.11, 1.65) | 129 (38.2) | 137 (37.1) | 1.05 (0.75, 1.46) | 106 (37.6) | 122 (29.3) | 1.60 (1.13, 2.28) | |
| Hormone therapy useb, No. (%) | 0.07 | |||||||||
| Current | 469 (53.6) | 478 (48.8) | 1.04 (0.83, 1.31) | 139 (41.9) | 111 (30.1) | 1.49 (1.03, 2.15) | 104 (36.9) | 96 (23.1) | 1.63 (1.11, 2.41) | |
| Former | 189 (21.6 ) | 270 (27.6) | 0.75 (0.57, 0.98) | 70 (21.1) | 112 (30.4) | 0.72 (0.48, 1.09) | 51 (18.1) | 134 (32.2) | 0.52 (0.35, 0.79) | |
| Never | 217 (24.8) | 231 (23.6) | Referent | 123 (37.0) | 146 (39.5) | Referent | 127 (45.0) | 186 (44.7) | Referent | |
| Ptrend = 0.39 | Ptrend = 0.04 | Ptrend = 0.06 | ||||||||
| Alcohol consumptione, g/day, No. (%) | 0.58 | |||||||||
| None | 413 (47.2) | 510 (52.1) | Referent | 219 (66.0) | 235 (63.7) | Referent | 201 (71.3) | 316 (76.0) | Referent | |
| <10 | 328 (37.5) | 336 (34.3) | 1.19 (0.94, 1.47) | 85 (25.6) | 106 (28.7) | 0.79 (0.55, 1.14) | 66 (23.4) | 79 (19.0) | 1.10 (0.73, 1.65) | |
| ≥10 | 134 (15.3) | 133 (13.6) | 1.26 (0.94, 1.67) | 27 (8.4) | 28 (7.6) | 0.99 (0.56, 1.77) | 15 (5.3) | 21 (5.0) | 0.92 (0.45, 1.90) | |
| Ptrend = 0.06 | Ptrend = 0.49 | Ptrend = 0.89 | ||||||||
OR, odds ratio; CI, confidence interval. Results in bold are statistically significant, results in italics are marginally significant.
Adjusted for study site (San Francisco Bay Area Breast Cancer Study versus 4-Corners) and all the listed factors.
In referent year.
Includes live or still birth
Includes both parous and non-parous women.
Alcohol consumption was based on lifetime average intake (4-Corners and Mexico subjects), or intake during the reference year (SFBCS).
Among Hispanic women with lower % NA ancestry, factors significantly associated in the expected direction included positive family history and current HT use. Associations with age at menarche, breast-feeding, and parity/age at first birth were in the expected direction, though not statistically significant. In contrast, BMI was significantly inversely associated with risk. Among US Hispanic women with higher % NA ancestry, positive family history, later age at menopause, and current HT use were significantly associated with increased breast cancer risk. No history of breast-feeding was associated with a marginally significant increased risk. When comparing across all US cases, BMI was the only risk factor that was associated in the unexpected direction among Hispanic women with lower % NA ancestry only. Higher BMI was associated with reduced breast cancer risk among US Hispanic women with lower NA ancestry only (BMI <25 versus > 30 kg/m2: OR = 0.65, 95% CI = 0.44 to 0.98), although there was no statistically significant interaction when comparing these groups (Table 3).
Among Mexican women with lower % NA ancestry (Table 4), positive family history, low parity/older age at first birth, HT use, alcohol consumption and no history of breast-feeding (borderline, P = 0.06) were associated with increased breast cancer risk. A non-significant positive association was observed with younger age at menarche. Among Mexican women with higher % NA ancestry, most associations were in the expected direction, but only family history was significant (wide CIs). Non-significant elevated ORs were found for low parity/older age at first birth, younger age at menarche and alcohol consumption. The only risk factor with an unexpected association was BMI. Similar to the US Hispanics, higher BMI was associated with reduced breast cancer risk among women with lower NA ancestry only (BMI <25 versus > 30kg/m2: OR=0.53; 95% CI = 0.29 to 0.97) among Mexican women), although this interaction was not statistically significant. There was a suggestive interaction for no history of breastfeeding (Pinteraction = 0.06), but only among Mexican women with lower % NA ancestry.
Table 4.
Relationship between risk factors and breast cancer risk according to percent Native American ancestry among postmenopausal Mexican women
| Risk Factors | 0–68% Native American Ancestry | 69–100% Native American Ancestry | P interaction | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Cases (n= 236) | Controls (n =232) | OR (95% CI)a | Cases (n = 190) | Controls (n = 269) | OR (95% CI)a | ||
| Ageb, y | 1.06 (1.02, 1.09) | 1.02 (1.00, 1.05) | 0.27 | ||||
| Family history of breast cancer, No. (%) | 0.36 | ||||||
| Yes | 21 (8.9) | 10 (4.2) | 2.53 (1.10, 5.85) | 8 (4.2) | 2 (0.7) | 5.57 (1.15, 27.01) | |
| No | 215 (91.1) | 222 (95.7) | Referent | 182 (95.8) | 267 (99.3) | Referent | |
| No. births and age at first birthc, No. (%) | 0.14 | ||||||
| Nulliparous | 31 (13.1) | 7 (3.0) | 3.60 (1.22, 10.61) | 20 (10.5) | 15 (5.6) | 2.48 (0.94, 6.60) | |
| 1–2, < 25 y | 21 (8.9) | 21 (9.1) | 1.36 (0.66, 2.84) | 23 (12.1) | 31 (11.5) | 1.16 (0.62, 2.18) | |
| 1–2, ≥25 y | 44 (18.6) | 28 (12.1) | 1.68 (0.91, 3.12) | 29 (15.3) | 30 (11.2) | 1.54 (0.83, 2.87) | |
| ≥3, <25 y | 101 (42.8) | 154 (66.4) | Referent | 100 (52.6) | 162 (60.2) | Referent | |
| ≥3, ≥25 y | 39 (16.5) | 22 (9.5) | 2.21 (1.17, 4.15) | 18 (9.5) | 31 (11.5) | 0.93 (0.48, 1.81) | |
| Breastfeeding, mos, No. (%)d | 0.06 | ||||||
| None | 63 (26.7) | 29 (12.5) | 2.19 (0.99, 4.86) | 37 (19.5) | 43 (16.0) | 1.06 (0.47, 2.39) | |
| ≤12 | 140 (59.3) | 140 (60.3) | 1.35 (0.77, 2.37) | 120 (63.2) | 160 (59.5) | 1.42 (0.84, 2.42) | |
| >12 | 33 (14.0) | 63 (27.2) | Referent | 33 (17.4) | 66 (24.5) | Referent | |
| Ptrend = 0.06 | Ptrend = 0.61 | ||||||
| Body mass indexb, kg/m2, No. (%) | 0.43 | ||||||
| <25.0 | 43 (18.2) | 27 (11.7) | Referent | 25 (13.2) | 31 (11.5) | Referent | |
| 25.0, 29.9 | 90 (38.1) | 75 (32.3) | 0.77 (0.41, 1.44) | 77 (40.5) | 106 (39.4) | 0.89 (0.48, 1.66) | |
| ≥30 | 103 (43.6) | 130 (56.0) | 0.53 (0.29, 0.97) | 88 (46.3) | 132 (49.1) | 0.82 (0.44, 1.51) | |
| Ptrend = 0.02 | Ptrend = 0.51 | ||||||
| Age at menarche, y, No. (%) | 0.75 | ||||||
| ≤11 | 46 (19.5) | 37 (16.0) | 1.62 (0.89, 2.94) | 38 (20.0) | 54 (20.1) | 1.33 (0.75, 2.33) | |
| 12, 13 | 115 (48.7) | 101 (43.5) | 1.37 (0.87, 2.15) | 100 (52.6) | 118 (43.9) | 1.52 (0.97, 2.38) | |
| ≥14 | 75 (31.8) | 94 (40.5) | Referent | 52 (27.4) | 97 (36.0) | Referent | |
| Ptrend = 0.09 | Ptrend = 0.23 | ||||||
| Age at menopause, y, No. (%) | 0.46 | ||||||
| ≤50 | 158 (67.0) | 152 (65.5) | Referent | 131 (69.0) | 189 (70.3) | Referent | |
| >50 | 78 (33.0) | 80 (34.5) | 0.70 (0.44, 1.11) | 59 (32.0) | 80 (29.7) | 1.01 (0.66, 1.56) | |
| Hormone therapy useb No. (%) | 0.13 | ||||||
| Ever | 66 (28.0) | 33 (14.2) | 1.94 (1.16, 3.26) | 36 (18.9) | 39 (15.2) | 1.17 (0.70, 1.96) | |
| Never | 170 (72.0) | 199 (85.8) | Referent | 154 (81.1) | 228 (84.8) | Referent | |
| Alcohol consumptionb,e | 0.11 | ||||||
| No. (%) | 189 (80.1) | 219 (94.4) | Referent | 167 (87.9) | 249 (92.6) | Referent | |
| None Any | 47 (19.9) | 13 (5.6) | 3.61 (1.77, 7.36) | 23 (12.1) | 20 (7.4) | 1.62 (0.84, 3.12) | |
OR, odds ratio; CI, confidence interval. Results in bold are significant; results in italics are marginally significant.
Adjusted for all listed factors as well as study site.
In referent year.
Includes live or still birth.
Includes both parous and non-parous women.
Alcohol consumptionwas based on lifetime average intake (4-Corners and Mexico subjects), or intake during the reference year (SFBCS).
When stratified by ER status, there were no notable differences in any of the observed relationships (Supplemental Tables 3 and 4). However, power was limited, and data were not available for Mexican cases. The inverse relationship between BMI and breast cancer risk observed among US Hispanic women with lower NA ancestry appeared to be more relevant for risk of ER negative breast cancer (BMI <25 versus BMI > 30: OR=0.30; 95% CI = 0.14 to 0.65 for ER negative BC; OR=0.86; 95% CI = 0.52 to 1.43 for ER positive BC); however, there was limited power to assess these relationships by tumor subtype.
Among US postmenopausal cases, the prevalence of risk factors was highest among NHWs, intermediate among Hispanics with lower % NA ancestry, and lowest among Hispanics with higher % NA ancestry (Table 5). There were a few exceptions: 1) history of breast-feeding was comparable across the groups, 2) Hispanics with lower % NA had a younger age at menarche, and 3) Hispanics were more likely to be overweight. When comparing Hispanic cases from the US and Mexico, the prevalence of many of the risk factors was much lower for Mexican cases, including positive family history, no history of breast-feeding, HT use and alcohol consumption. Similarly, Mexican cases were more likely to have a higher BMI compared with US Hispanic women.
Table 5.
Comparative summary of prevalence and direction of association for risk factors among the different subpopulations.
| Risk Factorsa | Prevalence of Risk Factors Among Cases | Direction of Association With Breast Cancer Risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| US | Mexico | US | Mexico | |||||||
|
|
|
|
|
|||||||
| NHW | Hispanic 0–43% | Hispanic 43.1–100% | 0–68% | 68.1–100% | NHW | Hispanic 0–43% | Hispanic 43.1–100% | 0–68% | 68.1–100% | |
| Family history of breast cancerc,d | 23.1% | 23.2% | 16.0% | 8.9% | 4.2% | Increase | Increase | Increase | Increase | Increase |
| Few births/Later age at first birth (<3 births and/or >25 y)b,d | 65.1% | 56.9% | 49.3% | 57.2% | 47.4% | Increasef | Increasef | Increase | Increase | Increasef |
| No Breast-feedinge | 47.4% | 47.4% | 47.9% | 26.7% | 19.5% | Increasef | Increasef | Increasef | Increase | |
| Higher body mass index (≥25kg/m2)b,c,e | 60.1% | 71.0% | 83.0% | 81.8% | 86.8% | Decrease | Decrease | |||
| Earlier age at menarche (≤11 y)b,e | 22.6% | 28.4% | 23.4% | 19.5% | 20.0% | Increase | Increasef | Increasef | Increasef | |
| Later age at menopause (>50 y) | 43.9% | 37.9% | 37.6% | 33.1% | 31.1% | Increase | Increase | |||
| Hormone therapy use (Yes)b,c,d,e | 75.4% | 63.3% | 55.0% | 28.0% | 19.0% | Increasef | Increase | Increase | Increase | |
| Alcohol consumption (Any)b,d,e | 53.0% | 33.6% | 28.7% | 19.9% | 12.1% | Increasef | Increase | |||
Risk factors were dichotomized (e.g. presence versus absence) as presented.
P < 0.05 comparing prevalence among NHW versus US Hispanic (0–43%).
P < 0.05 comparing prevalence among US Hispanic (0–43%) versus US Hispanic (43.1–100%).
P < 0.05 comparing prevalence among Mexican (0–68%) versus Mexican(68.1–100%).
P < 0.05 comparing US Hispanic (0–43%) versus Mexican (0–68%).
Associations that were suggestive but not statistically significant.
When comparing the direction of the association with risk factors (Table 5), family history was the only risk factor that was significantly associated with breast cancer risk among all subgroups. In general, associations with low parity/older age at first birth were in the expected direction across all subgroups, although weak and not significant for some subgroups. With the exception of BMI, all other risk factors were associated in the expected direction among at least two of the subgroups. There were no obvious patterns or trends with respect to associations with risk factors when comparing regional, ethnic and genetic admixture groups. Overall, differences in the prevalence of risk factors were more pronounced than differences in the magnitude of associations when comparing ORs across the different groups.
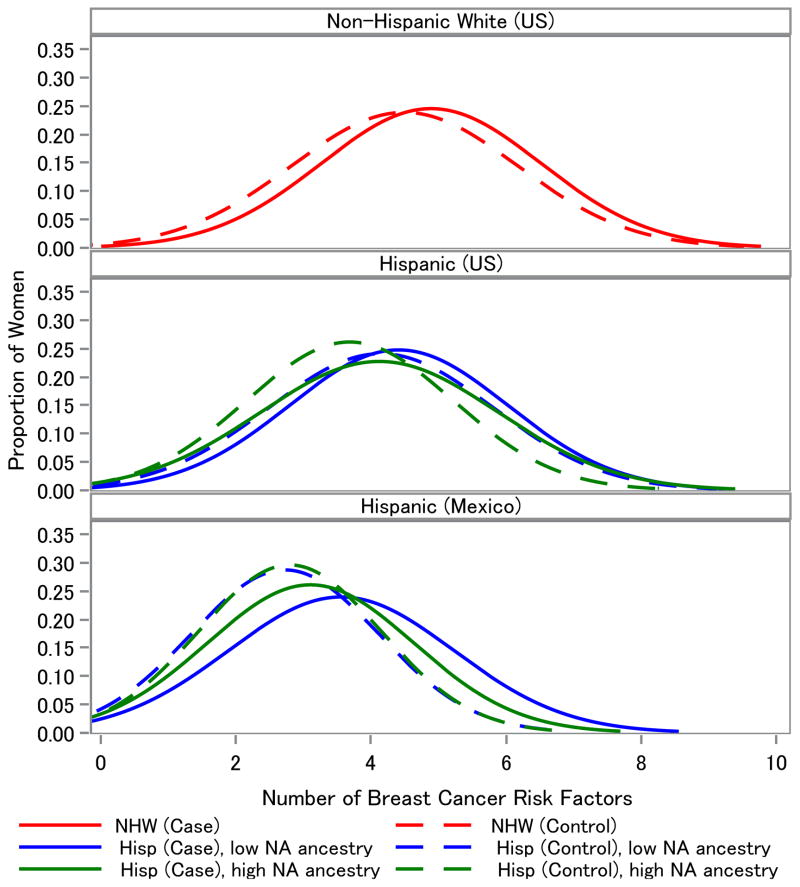
We hypothesized that women with breast cancer, on average, should have a higher number of risk factors when compared to controls, irrespective of region, ethnicity or extent of NA ancestry. Furthermore, if these factors behaved similarly across ethnic populations such that the observed increase in incidence rate among Hispanics who immigrate to the US is solely due to acquiring more risk factors, then we would expect to see US Hispanic cases possessing a relatively similar number of risk factors as NHW cases. To explore this, we compared the distribution of the total number risk factors that cases and controls had according to region, ethnicity and % NA group (Figure 1). Cases were more likely to have a higher average number of the evaluated risk factors compared to controls for all subgroups (4.24 versus 3.91, P < 0.0001 for NHW; 3.88 versus 3.60, P = 0.02 for US Hispanic, low NA; 3.64 versus 3.20, P = 0.0002 for US Hispanic, high NA; 3.07 versus 2.24, P < 0.0001 for Mexico, low NA; 2.66 versus 2.34, P = 0.01 for Mexico, high NA). Additionally, the average number of risk factors differed according to ethnicity and region (data not shown), with NHW cases having the highest, followed by US Hispanics, and Mexican cases (P < 0.05). Differences were also observed according to admixture group within a region (P = 0.056 when comparing low and high admixture groups among US Hispanic cases and P = 0.005 among Mexican cases).
Figure 1. The average number of risk factors among breast cancer cases is inversely related to extent of Native American (NA) ancestry.
Breast cancer cases were more likely to have a higher average number of the evaluated breast cancer risk factors compared to controls, irrespective of region, self-reported ethnicity and % NA ancestry. These comparative density plots were based on the distribution of total number of risk factors among cases and controls, as categorized in Table 5.
Discussion
As previously reported (2–5), higher NA ancestry is associated with lower risk of postmenopausal breast cancer among Hispanic women residing in either the US or Mexico. The risk estimates were only slightly attenuated when adjusting for known breast cancer risk factors. When evaluating the relationship between these risk factors and postmenopausal breast cancer risk according to level of NA ancestry, we observed considerable differences in risk factor prevalence. Overall, risk factor prevalence and extent of NA ancestry were inversely related: the proportion of women with a given risk factor was highest among NHW women, intermediate among US Hispanics, and lowest among women living in Mexico. Likewise, within each region, the lower NA ancestry groups were more likely to have risk factors than those with higher NA ancestry. BMI was the one exception where women with more NA ancestry were more likely to have a higher BMI.
There were no striking differences or trends when comparing the magnitude and direction of the associations between reported risk factors and breast cancer among regional and ethnic subgroups. The majority of factors we evaluated were associated with risk in the expected directions among women in each group, although not all reached statistical significance. Overall, the majority of the associations did not appear to be dependent upon ER status. However, we did not have data on ER status for Mexico, and we had limited power among US Hispanics. A suggestive inverse association was observed with BMI and breast cancer risk among US Hispanic and Mexican women with lower NA ancestry. An inverse relationship between BMI and risk of ER negative breast cancer has been observed among postmenopausal African-American women (12). Interestingly, our data also supported a stronger inverse relationship for risk of ER negative breast cancer. This could be partially attributed to Hispanic women with lower % NA having more African influence, since Hispanic populations have been shown to have between 0–8% African ancestry. However, these results should be interpreted cautiously due to small sample size. Since the intent of this analysis is to assess risk factors collectively, our interpretations of this finding are speculative. A more in-depth analysis with BMI on this study population has been conducted (13, 14). We did not have sufficient power in this analysis to evaluate BMI according to hormone therapy use, which has been shown to mask the effect of BMI.
In the US, Hispanic women have a higher prevalence of obesity compared to NHW women, yet lower rates of postmenopausal breast cancer (1, 15). The few studies that have evaluated the relationship between obesity and breast cancer among Hispanic women are conflicting (16–21). BMI is a large determinant of endogenous sex steroid hormone levels among postmenopausal women, a speculated mechanism for the associated increase in breast cancer risk (22). Independent of ethnic differences in BMI, some studies have found differences between Hispanics and NHWs with respect to endogenous steroid hormone levels, while others have not (23, 24). Obesity and body size may also affect risk through other mechanisms, such as influencing inflammatory and insulin-related pathways (25–27). It is unknown whether the hypothesized biological mechanisms behave similarly among the different populations, and additional research is warranted.
Breast cancer risk is higher in US-born Hispanics than foreign-born, and risk increases with longer duration of residency, which is at least partially attributed to changes in risk factor profiles from acculturation (9, 28). Our previous work found that known risk factors account for fewer breast cancers among Hispanic women, suggesting that there are other unidentified factors involved (7). The findings from this study provide additional evidence for the contribution of both known and yet to be identified risk factors among Hispanic women. We do observe an increase in the prevalence of many risk factors among US Hispanics when compared with Mexican women, and the average total number of risk factors among cases is significantly higher than among controls, irrespective of region, ethnicity and NA ancestry. However, the average number of risk factors among breast cancer cases is inversely related to extent of NA ancestry, suggesting that breast cancer development among Hispanic women is not just the consequence of acquiring more risk factors such that their profile simply resembles that of NHW women. Additionally, a shift in risk factor profile cannot explain the observed ethnic differences in tumor characteristics. For example, US Hispanic women are more likely to have tumor characteristics associated with poorer prognosis, such as estrogen receptor-negative tumors, and these differences are not solely attributed to socioeconomic factors (29–31). These data suggest that certain genetic and/or non-genetic factors that contribute to breast cancer among Hispanic women differ from those among NHW women.
Irrespective of ethnicity, region or extent of NA ancestry, having a positive family history was associated with an increase in breast cancer risk. Interestingly, the proportion of women with a family history declined with increasing % NA ancestry, reflecting both the differences in overall breast cancer incidence and possible genetic differences in breast cancer susceptibility. Given that this is a case-control study, there is also the potential for culturally driven recall differences that may contribute to the observed differences in ancestry. Little is known about the effects of genetic and/or non-genetic factors within the context of genetic background on breast cancer risk. We previously found that some of the associations with SNPs identified by previous GWAS exploration were stronger among women with intermediate to high levels of NA ancestry and similar between the Hispanic women with low NA ancestry and NHWs (32). In addition, a GWAS conducted in a sample of Hispanic/Latina women reported the finding of a protective variant of Native American origin (6). In future studies it would be important to combine information on non-genetic risk factors with information on genetic risk factors to evaluate what proportion of the genetic ancestry-breast cancer risk association can be explained by these known factors.
This study has both strengths and weaknesses. This study represents one of the largest comparative breast cancer studies among Hispanic and NHW women. The availability of genetic admixture data provides the unique opportunity to explore gene-environment interactions and provides insight into ethnic differences in breast cancer risk. Our study did not include estimates of African ancestry, a minor component representing between 0–8% of the ancestral background of Hispanic women from Mexico and Central America. However, when we compared the Indigenous American ancestry estimates obtained with a two-way admixture model to those obtained with a supervised three-way admixture model in a subset of these Hispanic women, the use of a two-way admixture model was shown to be adequate (2). In spite of our large sample size, power was limited to assess differences in risk according to tumor characteristics partly due to the lack of this information for the Mexico cases.
This study was prompted by the accumulating evidence suggesting that either genetic and/or non-genetic factors may modify susceptibility to the development of breast cancer among Hispanic women. As previously shown, NA ancestry is associated with lower breast cancer risk even after accounting for differences in established breast cancer risk factors. In addition, our data suggest that these breast cancer risk factors, as a whole, contribute less to breast cancer development among women with more NA ancestry, and further research is needed to gain a better understanding of how genetic and non-genetic risk factors act independently and collectively to affect risk.
Supplementary Material
Acknowledgments
Financial Support: The Breast Cancer Health Disparities Study was funded by grant CA14002 (to M.L. Slattery) from the National Cancer Institute to Dr. Slattery. The San Francisco Bay Area Breast Cancer Study was supported by grants CA63446 and CA77305 (to E.M. John) from the National Cancer Institute, grant DAMD17-96-1-6071 (to E.M. John) from the U.S. Department of Defense, and grant 7PB-0068 (to E.M. John) from the California Breast Cancer Research Program. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000036C awarded to the Cancer Prevention Institute of California; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The 4-Corners Breast Cancer Study was funded by grants CA078682 (to M.L. Slattery), CA078762 (K.B. Baumgartner), CA078552 (to T. Byers), and CA078802 (to A.R. Giuliano) from the National Cancer Institute. The research also was supported by the Utah Cancer Registry, which is funded by contract N01-PC-67000 from the National Cancer Institute, with additional support from the State of Utah Department of Health, the New Mexico Tumor Registry, and the Arizona and Colorado cancer registries, funded by the Centers for Disease Control and Prevention National Program of Cancer Registries and additional state support. The Mexico Breast Cancer Study was funded by Consejo Nacional de Ciencia y Tecnología (CONACyT) (SALUD-2002-C01-7462) (to G. Torres-Mejia). Work for this study was also supported by the National Cancer Institute (CA 160607 to L. Fejerman).
We would also like to acknowledge the contributions of the following individuals to the study: Sandra Edwards for data harmonization oversight; Erica Wolff and Michael Hoffman for laboratory support; Carolina Ortega for her assistance with data management for the Mexico Breast Cancer Study, Jocelyn Koo for data management for the San Francisco Bay Area Breast Cancer Study, and Dr. Josh Galanter for assistance in selection of AIMS markers for the study.
Footnotes
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute or endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Disclosure of Potential Conflicts of Interests: The authors declare that they have no competing interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Fejerman L, Romieu I, John EM, Lazcano-Ponce E, Huntsman S, Beckman KB, et al. European ancestry is positively associated with breast cancer risk in Mexican women. Cancer Epidemiol Biomarkers Prev. 2010;19:1074–82. doi: 10.1158/1055-9965.EPI-09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziv E, John EM, Choudhry S, Kho J, Lorizio W, Perez-Stable EJ, et al. Genetic ancestry and risk factors for breast cancer among Latinas in the San Francisco Bay Area. Cancer Epidemiol Biomarkers Prev. 2006;15:1878–85. doi: 10.1158/1055-9965.EPI-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, et al. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008;68:9723–8. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slattery ML, John EM, Torres-Mejia G, Lundgreen A, Herrick JS, Baumgartner KB, et al. Genetic variation in genes involved in hormones, inflammation and energetic factors and breast cancer risk in an admixed population. Carcinogenesis. 2012;33:1512–21. doi: 10.1093/carcin/bgs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, et al. Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun. 2014;5:5260. doi: 10.1038/ncomms6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hines LM, Risendal B, Slattery ML, Baumgartner KB, Giuliano AR, Sweeney C, et al. Comparative analysis of breast cancer risk factors among Hispanic and non-Hispanic white women. Cancer. 2010;116:3215–23. doi: 10.1002/cncr.25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slattery ML, Edwards S, Murtaugh MA, Sweeney C, Herrick J, Byers T, et al. Physical activity and breast cancer risk among women in the southwestern United States. Ann Epidemiol. 2007;17:342–53. doi: 10.1016/j.annepidem.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14:2905–13. doi: 10.1158/1055-9965.EPI-05-0483. [DOI] [PubMed] [Google Scholar]
- 10.Angeles-Llerenas A, Ortega-Olvera C, Perez-Rodriguez E, Esparza-Cano JP, Lazcano-Ponce E, Romieu I, et al. Moderate physical activity and breast cancer risk: the effect of menopausal status. Cancer Causes Control. 2010;21:577–86. doi: 10.1007/s10552-009-9487-8. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HJ, Choudhry S, Naqvi M, Rodriguez-Cintron W, Burchard EG, Ziv E. Comparison of three methods to estimate genetic ancestry and control for stratification in genetic association studies among admixed populations. Hum Genet. 2005;118:424–33. doi: 10.1007/s00439-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 12.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–66. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, et al. Overall and abdominal adiposity and premenopausal breast cancer risk among hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomarkers Prev. 2015;24:138–47. doi: 10.1158/1055-9965.EPI-13-1007-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, et al. Body size throughout adult life influences postmenopausal breast cancer risk among hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomarkers Prev. 2015;24:128–37. doi: 10.1158/1055-9965.EPI-14-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 16.Sexton KR, Franzini L, Day RS, Brewster A, Vernon SW, Bondy ML. A review of body size and breast cancer risk in Hispanic and African American women. Cancer. 2011;117:5271–81. doi: 10.1002/cncr.26217. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland FD, Hunt WC, Baumgartner KB, Crumley D, Nicholson CS, Fetherolf J, et al. Reproductive risk factors for breast cancer in Hispanic and non-Hispanic white women: the New Mexico Women’s Health Study. Am J Epidemiol. 1998;148:683–92. doi: 10.1093/aje/148.7.683. [DOI] [PubMed] [Google Scholar]
- 18.Wenten M, Gilliland FD, Baumgartner K, Samet JM. Associations of weight, weight change, and body mass with breast cancer risk in Hispanic and non-Hispanic white women. Ann Epidemiol. 2002;12:435–4. doi: 10.1016/s1047-2797(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 19.Slattery ML, Sweeney C, Edwards S, Herrick J, Baumgartner K, Wolff R, et al. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2007;102:85–101. doi: 10.1007/s10549-006-9292-y. [DOI] [PubMed] [Google Scholar]
- 20.John EM, Phipps AI, Sangaramoorthy M. Body size, modifying factors, and postmenopausal breast cancer risk in a multiethnic population: the San Francisco Bay Area Breast Cancer Study. SpringerPlus. 2013;2:239. doi: 10.1186/2193-1801-2-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr. 2015;6:803–19. doi: 10.3945/an.115.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–33. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 23.Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, Henderson BE. Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1849–55. doi: 10.1158/1055-9965.EPI-06-0307. [DOI] [PubMed] [Google Scholar]
- 24.Wacker M, Risendal B, Westerlind K, Lezotte D, Byers T. Ethnicity, body size, and estrogen levels in postmenopausal Hispanic and non-Hispanic white women. J Womens Health. 2009;18:487–91. doi: 10.1089/jwh.2008.0835. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res. 2012;5:515–21. doi: 10.1158/1940-6207.CAPR-12-0091. [DOI] [PubMed] [Google Scholar]
- 26.Dizdar O, Alyamac E. Obesity: an endocrine tumor? Med Hypotheses. 2004;63:790–2. doi: 10.1016/j.mehy.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 27.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 28.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. JNCI. 2005;97:439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 29.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 31.Watlington AT, Byers T, Mouchawar J, Sauaia A, Ellis J. Does having insurance affect differences in clinical presentation between Hispanic and non-Hispanic white women with breast cancer? Cancer. 2007;109:2093–9. doi: 10.1002/cncr.22640. [DOI] [PubMed] [Google Scholar]
- 32.Fejerman L, Stern MC, Ziv E, John EM, Torres-Mejia G, Hines LM, et al. Genetic ancestry modifies the association between genetic risk variants and breast cancer risk among Hispanic and non-Hispanic white women. Carcinogenesis. 2013;34:1787–93. doi: 10.1093/carcin/bgt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.