Abstract
Osteoblastoma is a benign bone tumor that can often be difficult to distinguish from malignant osteosarcoma. Because misdiagnosis can result in unfavorable clinical outcomes, we have investigated microRNAs as potential diagnostic biomarkers for distinguishing between these two tumor types. Next generation RNA sequencing was used as an expression screen to evaluate >2,000 microRNAs present in tissue derived from rare formalin fixed paraffin embedded (FFPE) archival tumor specimens. MicroRNAs displaying the greatest ability to discriminate between these two tumors were validated on an independent tumor set, using qPCR assays. Initial screening by RNA-seq identified four microRNA biomarker candidates. Expression of three miRNAs (miR-451a, miR-144-3p, miR-486-5p) was higher in osteoblastoma, while the miR-210 was elevated in osteosarcoma. Validation of these microRNAs on an independent data set of 22 tumor specimens by qPCR revealed that miR-210 is the most discriminating marker. This microRNA displays low levels of expression across all of the osteoblastoma specimens and robust expression in the majority of the osteosarcoma specimens. Application of these biomarkers to a clinical test case showed that these microRNA biomarkers permit re-classification of a misdiagnosed FFPE tumor sample from osteoblastoma to osteosarcoma. Our findings establish that the hypoxia-related miR-210 is a discriminatory marker that distinguishes between osteoblastoma and osteosarcoma. This discovery provides a complementary molecular approach to support pathological classification of two diagnostically challenging musculoskeletal tumors. Because miR-210 is linked to the cellular hypoxia response, its detection may be linked to well-established pro-angiogenic and metastatic roles of hypoxia in osteosarcomas and other tumor cell types.
Keywords: osteosarcoma, osteoblastoma, microRNA, biomarker, FFPE
Osteoblastoma is a locally aggressive bone tumor that can be treated with local curettage without the need for radiation or chemotherapy. In contrast, osteosarcoma is a life threatening malignancy requiring wide surgical resection and chemotherapy. Although these tumors exhibit very different clinical behaviors, they can appear histologically and radiographically similar, making them difficult to differentiate clinically. Osteosarcoma is the most common primary malignant bone tumor in children and adolescents.1 It typically involves the metaphyseal region of long bones, and most frequently arises within the femur, tibia, or humerus.2 Osteoblastoma is a rare primary bone tumor, accounting for approximately 1% of all bone tumors.3 It is most likely to present in the 3rd to 4th decade of life, where it commonly affects the posterior elements of the spine.4,5 Although osteosarcoma and osteoblastoma have a predilection for different anatomic sites, each of these tumors can occur within any bone and can affect patients at any age. When misdiagnosis does occur, the results can be catastrophic resulting in unnecessary surgical resections with high morbidity (i.e., amputation), or even death when osteosarcomas are inadequately treated.
Because of the histological similarities and potential for misdiagnosis between osteoblastoma and high-grade osteosarcoma, there is a compelling need to improve current multi-disciplinary diagnostic methods (including clinical, histological, and radiographic findings) by developing precise molecular biomarkers. Recurrent chromosome 22 loss in osteoblastoma, which encompasses loci for Wnt signaling inhibitors, has been investigated as a biomarker to differentiate between osteosarcoma and osteoblastoma. This chromosomal alteration is associated with high canonical Wnt signaling and alterations in beta-catenin in osteoblastoma that may facilitate tumor diagnosis.6–8 Despite their utility, these biomarkers are not definitive and have not yet gained widespread acceptance for routine clinical use. Key impediments to biomarker development are the rarity of osteoblastomas compared to osteosarcomas, and the resulting limited availability of patient biopsies. Consequently, studies are restricted to archived formalin-fixed paraffin embedded biopsies and analysis of selected candidate genes and proteins. Additionally, these tumors frequently need to be de-calcified using harsh reagents prior to paraffin embedding that can limit the utility of conventional staining techniques. Yet, microRNAs, which are small noncoding RNAs (20–24 nucleotides in length) that act as post-transcriptional regulators of gene expression, are sufficiently stable for detection in de-calcified archival specimens.
MicroRNAs display aberrant expression patterns in a wide array of tumors including osteosarcoma.9 Their small size makes these molecules highly resistant to degradation and potentially versatile clinical biomarkers for tumor diagnosis.10 Studies have shown that micro-RNAs can be reliably extracted from FFPE tumor specimens that have been stored for decades, and that expression profiles correlate well with fresh frozen specimens.11 Several studies have examined microRNA expression in osteosarcoma and directly correlated changes in microRNA levels with clinical outcomes and response to chemotherapy.12–15 These studies suggest that microRNAs may be effective biomarkers for orthopedic tumors, although it is not clear yet whether miRNAs can be used to differentiate between osteosarcoma and osteoblastoma. Because of the clinical importance of accurately diagnosing osteosarcoma and osteoblastoma, we performed a high-resolution expression screen using RNA sequencing (RNA-seq) to examine which of the more than 2,000 currently known miRNAs in the human genome can differentiate between these two clinically challenging tumors. The main finding of this study is the definition of a novel bone tumor signature that permits clinical separation and correct classification of osteoblastoma versus osteosarcoma.
METHODS
Tumor Collection and RNA Isolation
A total of 30 FFPE tumor specimens (16 osteosarcomas and 14 osteoblastomas) were collected for research use from either Mayo Clinic or Leiden University Medical Center (LUMC). The osteosarcomas included, one osteoclast-rich, two chondroblastic, and 13 osteoblastic subtypes. The histology and x-ray radiographs for each tumor specimen was evaluated by a trained musculoskeletal pathologist (JT or JB), to ensure the correct tumor diagnosis, and that representative areas of the tumor were sampled. The 12 tumor specimens obtained from LUMC were also stained for beta-catenin using previously described methods.7,8
For RNA isolation a total of three, 10 micron sections were cut from each paraffin block. Tumor cells were macro-dissected from each section and subsequently used for micro-RNA isolation. None of the tumors evaluated in this investigation had been treated with pre-operative chemotherapy or radiation. FFPE sections were deparaffinized using xylene, and microRNAs were extracted using the Qiagen miRNeasy FFPE kit (Qiagen, Hilden, Germany). Total RNA was quantified using the NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific, Wilmington, Delaware). RNA samples were subsequently examined by RNA-seq analysis and/or real-time quantitative PCR. All human tumor specimens used in this study were collected in compliance with ethical standards for research and were in compliance with the rules and institutional guidelines governing human subjects research (IRB #11-008574, “Differentiating musculo-skeletal tumors using microRNA expression profiling”).
RNA Sequencing and Bioinformatics Analysis
High-throughput next generation microRNA sequencing was performed using five osteoblastoma, and four osteosarcoma specimens (three osteoblastic subtypes and one chondroblastic subtype). MicroRNAs were sequenced using the NEBNext Small RNA library prep kit on an Illumina HiSeq 2000. Short reads were trimmed of adapters with Cutadapt.16 Trimmed microRNA sequences greater than 17 nucleotides in length were then aligned to the reference genome and miRBase reference sequences using Bowtie.17 Known micro-RNA expression and novel microRNA prediction and quantification were performed using miRDeep2.18 All secondary data analyses were carried out using robustly expressed microRNAs, with an average expression of at least 10 normalized reads per million in either the osteoblastomas or osteosarcomas. Unsupervised hierarchical clustering was performed using the Pearson correlation method. MicroRNA target prediction was performed using ComiR, a combinatorial microRNA analysis program that takes into account microRNA expression levels was used for computational target prediction19,20 for microRNAs that showed a greater than threefold difference in expression between osteosarcoma and osteoblastoma. Functional gene annotation was performed for genes differentially expressed between osteoblastoma and osteosarcoma using the Database for Annotation and Visualization and Integrated Discovery v6.7 (DAVID 6.7).21,22
PCR Validation of MicroRNA Biomarkers
Selected microRNAs that were differentially expressed based on RNA-seq data were validated using TaqMan® microRNA assays (Life Technologies, Carlsbad, California). Real-time qPCR reactions were performed using the CFX384 Real-Time qPCR System (BioRad). Ribosomal RNA U6 and miR-103a-3p were included as reference genes. All microRNAs were normalized to miR-103a-3p, because this miRNA showed the lowest variability based on our RNA-seq analysis. MicroRNA expression levels were quantified using the 2ΔCt method. TaqMan® microRNA assays were used for U6 snRNA, hsa-miR-103, hsa-miR-320c, hsa-miR-210, hsa-miR-451, hsa-miR-144-3p, hsa-miR-486-5p, and hsa-miR-144-5p (Life Technologies, Carlsbad, California). Samples with poor amplification (Ct values greater than 35 for miR-103a-3p) were excluded, in total two samples were omitted.
RESULTS
Evaluation of MicroRNA Sequencing Data from FFPE Tumors Specimens
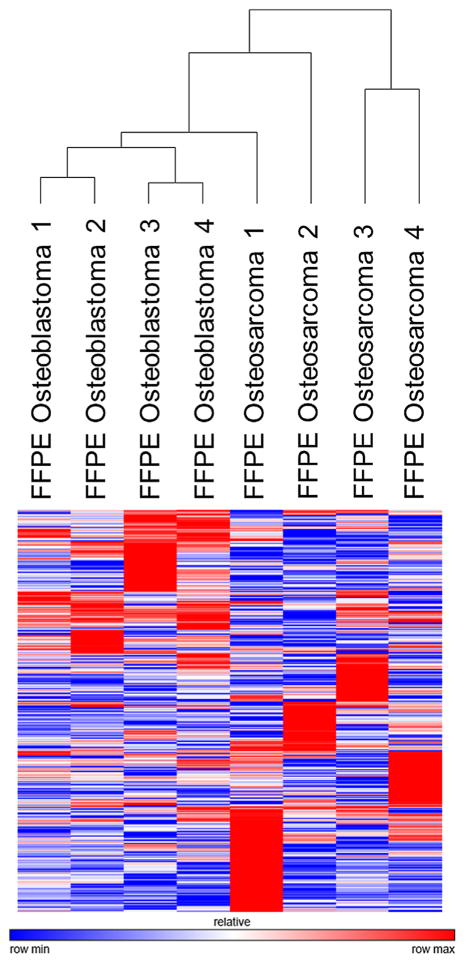
To determine if microRNA profiles were different between osteosarcoma and osteoblastoma, we performed unsupervised hierarchical clustering on tumor specimens using comprehensive microRNA profiles consisting of 2,252 human annotated microRNA sequences. The clustering dendrogram showed a trend toward independent grouping of the osteoblastomas and osteosarcomas (Fig. 1). A direct comparison of FFPE tumor specimens showed 76 microRNAs with greater than threefold expression in osteosarcoma compared to osteoblastoma (Supplementary Table S1), and 42 microRNAs enriched greater than threefold in osteoblastoma versus osteosarcoma (Supplementary Table S2).
Figure 1.
An overview of microRNA sequencing results. Unsupervised hierarchical clustering using the Pearson correlation method was performed on comprehensive microRNA sequencing data consisting of 2,252 microRNA sequences, from FFPE osteosarcoma and osteoblastoma tumor specimens. The clustering dendogram shows independent clustering of the osteosarcoma and osteoblastoma FFPE specimens, suggesting that microRNA biomarkers may be effective in discriminating between these two tumors.
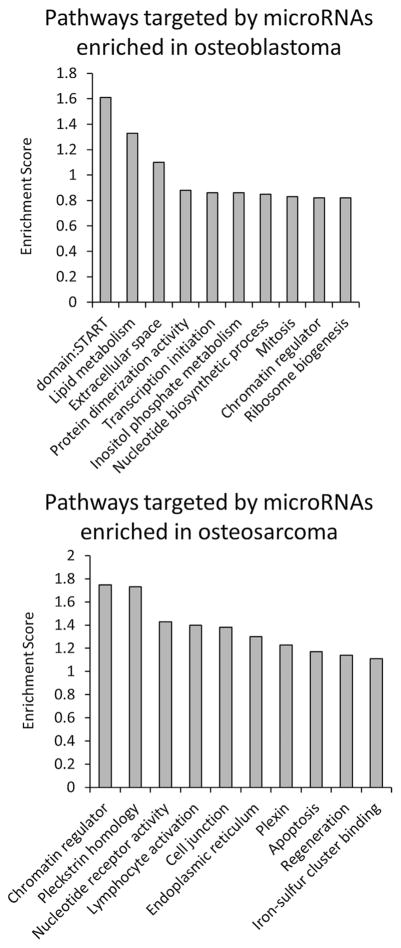
To determine if differentially expressed microRNAs are representative of the underlying tumor biology, we examined possible gene targets for differentially expressed microRNAs that may contribute to the benign versus malignant behavior in osteoblastoma and osteosarcoma, respectively.23 ComiR analysis was performed using microRNAs that showed at least a threefold difference in expression between osteoblastoma and osteosarcoma. We identified the top 1,000 genes that were predicted to be preferentially targeted in both osteoblastoma and osteosarcoma (Supplementary Table S3). Gene ontology analysis (DAVID 6.7) was used to identify regulatory pathways controlled by predicted gene targets. Genes linked to mitosis, lipid metabolism, transcription, and protein synthesis were predicted to be targeted by microRNAs enriched in osteoblastoma. In contrast, genes linked to apoptosis and lymphocyte activation were predicted to be inhibited by microRNAs enriched in osteosarcoma (Fig. 2). This preliminary analysis identifies microRNA regulatory mechanisms that may be acting to promote the benign and malignant behaviors of these two respective tumors, further work to validate underlying mechanisms is required before definitive conclusions can be made.
Figure 2.
ComiR analysis of microRNA targets in osteoblastoma and osteosarcoma. To determine if microRNAs differentially expressed between osteoblastoma and osteosarcoma reflect underlying tumor biology, we carried out functional gene annotation clustering using DAVID 6.7 for predicted microRNA targets generated using ComiR. Functional gene clusters were created for all gene targets in osteoblastoma and osteosarcoma combined, and then for osteosarcoma and osteoblastoma separately, to determine which tumor type contributes most toward each functional gene cluster. This analysis shows that genes controlling mitosis, lipid metabolism, transcription, and protein synthesis were predicted to be targeted by microRNAs enriched in osteoblastoma. In contrast, genes linked to apoptosis and lymphocyte activation were predicted to be inhibited by microRNAs enriched in osteosarcoma.
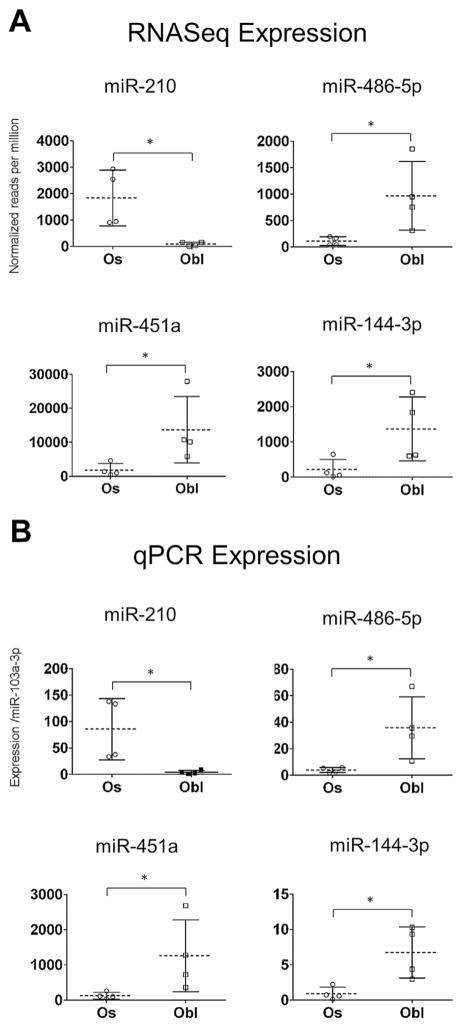
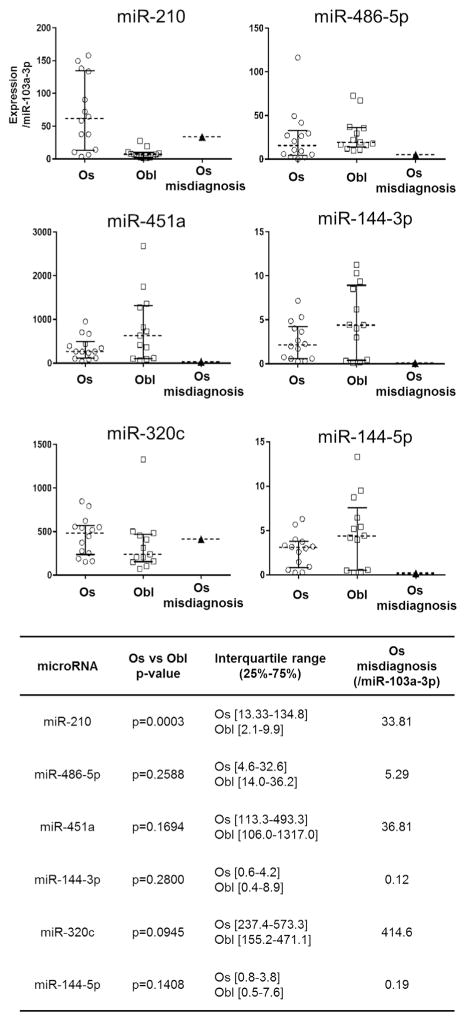
To refine the diagnostic accuracy of this initial analysis, we focused on microRNAs with the greatest ability to discriminate between osteosarcoma and osteoblastoma. Our approach ultimately aims to convert the high-throughput RNA sequencing data into a clinical test that can interrogate a select group of diagnostic microRNAs using qPCR and be readily performed at most medical centers. Such a test would be more affordable and less time consuming but not require specialized sequencing equipment. To develop this qPCR-based assay, we identified microRNA candidates that were abundantly expressed (>500 normalized reads in either osteoblastoma or osteosarcoma samples) with the greatest differential expression (>5-fold change) between osteosarcoma and osteoblastoma based on RNA sequencing data. Four microRNAs, miR-210, miR-486-5p, miR-451a, and miR-144-3p met these criteria (Fig. 3A).
Figure 3.
Identification of microRNA biomarker candidates using RNA-seq. (A) MicroRNA sequencing identified three micro-RNAs, miR-486-5p, miR-451a, and miR-144-3p, as having an average expression greater than 500 normalized reads per million, and a statistically significant fold change enrichment greater than fivefold compared with osteosarcoma. MiR-210 was the only microRNA to show a statistically significant fold change greater than fivefold in osteosarcoma, and was found to have an average expression level 20 times higher in osteosarcoma compared with osteoblastoma. Based on their differential expression patterns, these four microRNAs were selected for further qPCR validation. (B) qPCR validation of the same tumor samples used for RNA-seq confirmed differential expression of these four microRNA biomarker. Expression data showed good concordance with RNA-seq results, showing similar variability and distribution for each tumor type. Statistical significance was evaluated using the Wilcoxon rank-sum test, a p-value <0.05 is indicated by an “*”. Error bars are given as ±1 standard deviation from the mean.
qPCR Normalization
Sample normalization is critical for ensuring the consistency of a diagnostic qPCR test. Ribosomal RNA U6 is commonly used as a normalizer in microRNA studies. But because it is larger in size than micro-RNAs, it is more susceptible to degradation and may show variable expression across FFPE tumor samples because of differences in sample handling, processing, and storage. Therefore, we selected a microRNA for normalization that is highly expressed but shows relatively little variation across FFPE tumor samples. When analyzing the RNA-seq data, we limited our search to microRNAs that were robustly expressed at greater than 1,000 normalized reads per million, and then sorted these samples according to the coefficient of variation (Fig. 4). MiR-103a-3p showed the least amount of variation across tumor specimens. In addition, miR-103a-3p has also been reported as a reliable normalization factor in other publications, and has been applied to other tumor biomarker studies.24 Based on our sequencing data, and previous reports demonstrating its reliability, we chose to use miR-103a-3p for qPCR normalization in this investigation.
Figure 4.
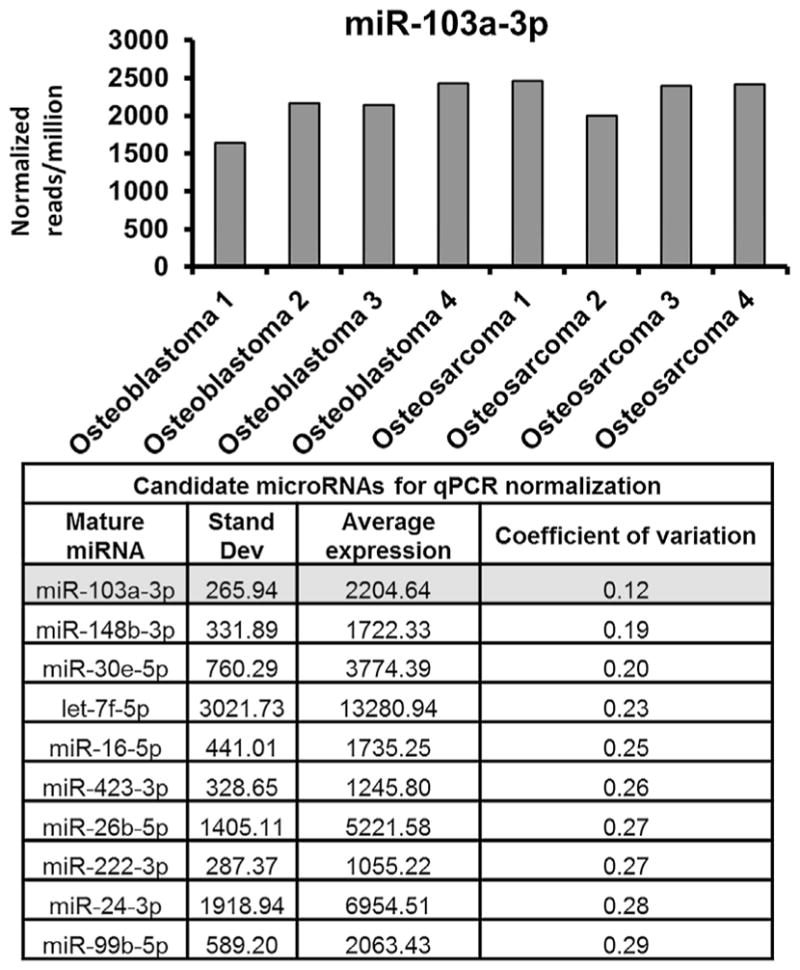
The established microRNA normalizer miR-103a-3p showed the least amount of variance among robustly expressed (>1,000 normalized reads per million) microRNAs across FFPE tumor specimens and was, therefore, chosen as the primary normalizer for this investigation.
qPCR Validation Using TaqMan Assays
Because archival tumor specimens are an exceedingly valuable resource with low RNA yields, we performed highly sensitive qPCR TaqMan assays to maximize the amount of molecular data that could be obtained from each tumor specimen. qPCR was initially performed on the eight tumor samples used for RNA sequencing to confirm the accuracy and differential expression of our candidate microRNA biomarkers. RNA-seq and qPCR quantification showed excellent concordance with very similar distributions across sample groups (Fig. 3B). These findings establish the reliability of qPCR as a diagnostic tool for discriminating microRNA biomarkers that are differentially expressed between osteosarcoma and osteoblastoma.
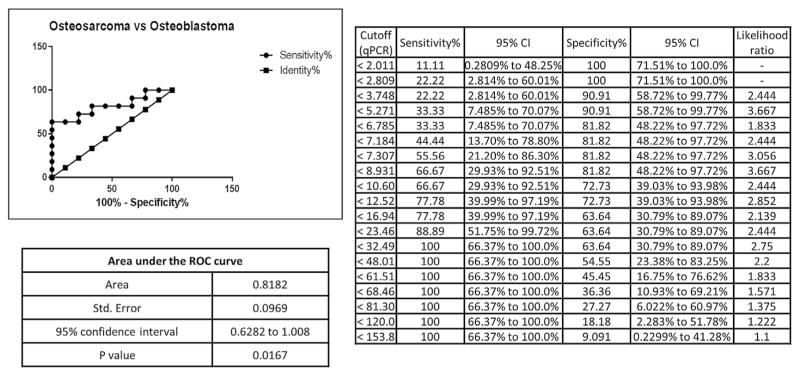
An independent set of 22 additional FFPE tumor samples (11 osteosacomas and 11 osteoblastomas) were obtained by combining archival specimens collected from two separate institutions. These specimens were used to validate the diagnostic accuracy of the four microRNA biomarker candidates. RNA from tumor specimens that did not amplify efficiently (Ct values greater than 35 for miR-103a-3p) were excluded from further analysis (in total two osteoblastoma specimens were excluded). Statistically significant differential expression of miR-210 was confirmed by qPCR validation (Fig. 5). A receiver operating characteristic (ROC) curve, displaying favorable biomarker characteristics, was generated for miR-210 using qPCR expression data, the curve displays favorable biomarker characteristics (Area under the curve (AUC) of 0.8182, p = 0.0167) (Fig. 6).
Figure 5.
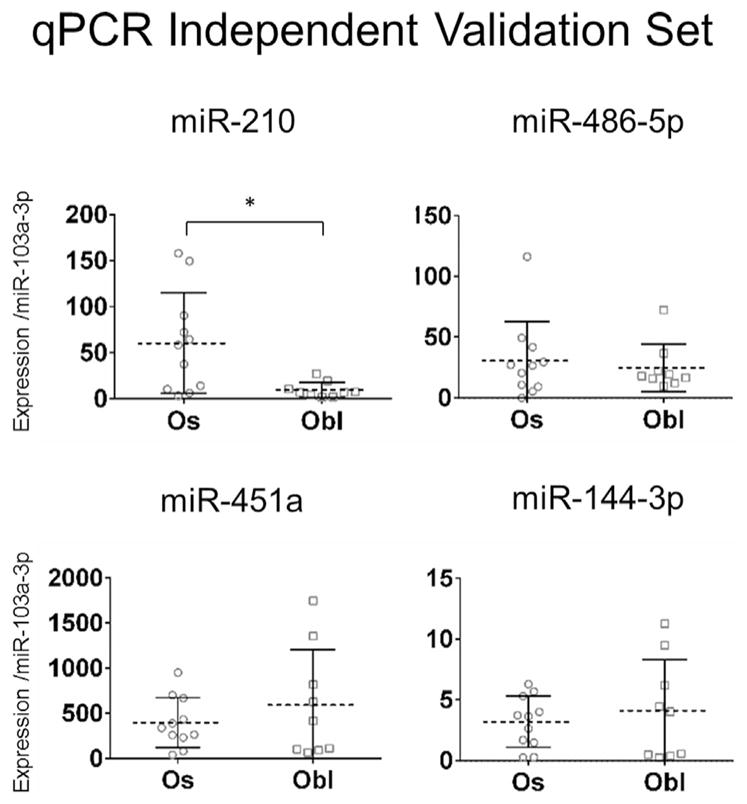
Validation of microRNA biomarkers using an independent data set of 20 FFPE tumor specimens. qPCR validation of microRNA biomarker candidates identified by our initial RNA-Seq screen, confirmed statistically significant differential expression of miR-210 between osteoblastoma and osteosarcoma. Statistical significance was evaluated using the Wilcoxon rank-sum test a p-value <0.05 is indicated by an “*”. Error bars are given as ±1 standard deviation from the mean.
Figure 6.
A receiver operating characteristic (ROC) curve was generated using all 28 tumor specimens that produced interpretable expression data for miR-210. The ROC curve displays favorable characteristics for a diagnostic biomarker with an AUC of 0.8182 (p = 0.0167).
To determine if miR-210 could be useful in discriminating between osteosarcomas and osteoblastomas in cases where diagnosis is especially challenging, we applied microRNA biomarkers to a historical test case. This specimen represents an osteoblastic osteosarcoma that was initially diagnosed as an osteoblastoma, and later confirmed to be an osteosarcoma after tumor recurrence and lung metastasis. qPCR analysis shows that miR-210 is elevated in this tumor specimen at a level that is not observed in any of the osteoblastoma specimens (Fig. 7), a finding that is also reflected in our specificity calculations (Fig. 5). The microRNAs miR-320c and miR-144-5p were included as outgroups in this evaluation, and as expected, did not show statistically significant differences in expression between tumor types. Based on these observations, the microRNA biomarkers from this investigation would have been potentially informative for this clinical case, and could have favorably altered this patient’s diagnostic workup and treatment plan.
Figure 7.
qPCR expression data for all 28 tumor specimens evaluated in this investigation. The diagnostic utility of miR-210 was tested on an osteoblastic osteosarcoma biopsy that was initially thought to be a vertebral osteoblastoma based on clinical, radiographic, and histologic criteria. The misdiagnosed osteosarcoma specimen had a qPCR expression level for miR-210 of 33.81 (normalized to miR-103a-3p), which is higher than any osteoblastoma we evaluated in this investigation, and corresponds to a specificity of 100% based on our ROC curve (Fig. 6). The additional microRNAs were evaluated as outgroups, and did not show differential expression between tumor types. Statistical significance was evaluated using the Wilcoxon rank-sum test, a p-value <0.05 is indicated by an “*”. Error bars represent the interquartile range (25–75%).
Recent studies have proposed β-catenin as a promising marker to distinguish between osteosarcomas and osteoblastomas.7 Osteosarcomas have been shown to have reduced Wnt signaling, and associated cytoplasmic beta-catenin staining in comparison with osteoblastomas which are more likely to display nuclear staining.6–8 To investigate whether miR-210 expression can be applied as a useful adjunct to beta-catenin staining, we compared miR-210 expression in various osteosarcoma and osteoblastoma specimens that we had stained for beta-catenin. Our studies reaffirm previous work8 that beta-catenin staining is a useful marker for differentiating between osteoblastomas and osteosarcomas. When we compared miR-210 expression across tumor specimens where beta-catenin staining did not indicate the proper diagnosis we found that miR-210 expression favored the correct diagnosis (Table 1). Thus, miR-210 expression is potentially a useful biomarker that can be used in conjunction with beta-catenin staining to help clarify nondiagnostic cases or provide a warning when beta-catenin staining may be favoring the incorrect diagnosis.
Table 1.
A Comparison of Beta-Catenin Staining and miR-210 as Discriminating Markers for Osteoblastoma and Osteosarcoma
| Tumor Type | Consensus Pathology Review | miR-210 Expression (/miR-103a-3p) | miR-210 Classification (Based on IQR 25–75%) |
|---|---|---|---|
| Osteosarcoma | Cytoplasmic | 72.25 | Osteosarcoma |
| Osteosarcoma | Cytoplasmic | 64.66 | Osteosarcoma |
| Osteosarcoma | Cytoplasmic | 37.65 | Osteosarcoma |
| Osteosarcoma | Cytoplasmic | 14.28 | Osteosarcoma |
| Osteosarcoma | Focal nuclear | 58.35 | Osteosarcoma |
| Osteosarcoma | Cytoplasmic | 6.39 | Osteoblastoma |
| Osteosarcoma | Cytoplasmic | 157.97 | Osteosarcoma |
| Osteoblastoma | Nuclear | 4.14 | Osteoblastoma |
| Osteoblastoma | Nuclear | 10.75 | Nondiagnostic |
| Osteoblastoma | Nuclear | 27.33 | Osteosarcoma |
| Osteoblastoma | Focal nuclear | 7.42 | Osteoblastoma |
| Osteoblastoma | Nuclear | 7.17 | Osteoblastoma |
These results confirm findings from previous studies showing beta-catenin as a discriminating marker for osteoblastoma and osteosarcoma. In cases where beta-catenin staining is equivocal (highlighted cases) miR-210 expression is able to indicate the proper diagnosis. These findings show that miR-210 expression levels can be used in conjunction with beta-catenin staining to improve the accuracy of tumor diagnosis.
DISCUSSION
Musculoskeletal tumors present unique challenges, because the differential diagnosis is often very broad and encompasses both benign and malignant lesions. Determining an accurate diagnosis and formulating an optimal treatment plan is critical for achieving good clinical outcomes for patients. This investigation provides evidence that microRNAs can be used as biomarkers to help distinguish between osteoblastomas and malignant osteosarcomas, and provides further support for the use of microRNAs as diagnostic markers for differentiating between other musculoskeletal tumors.
Our present findings can be further appreciated within the broad context of previous and current methods that define molecular biomarkers in osteosarcoma cells and clinical biopsies, as well as to refine diagnostics and treatment modalities. Collaborative studies from our group and others are representative of different molecular strategies that have been used for biochemical analyses of osteosarcomas. These studies include proteomic characterization of nuclear proteins,25,26 candidate analysis of changes in regulatory proteins or miRNAs linked to osteoblast growth and bone cancer,27–33 cytogenetic analysis of chromosomal aberrations,34,35 in situ analysis of gene expression for selected markers,36 as well as microRNA profiling.13,15 Together with many other studies that have been summarized in a large automated database focusing on the molecular pathology of osteosarcoma,37 there is now relatively broad (albeit not necessarily deep) knowledge of proteins, genes, and miRNAs that are phenotypically and/or causally linked to osteosarcoma. This paucity of molecular studies to date is presumably due to the extreme rarity of the disease and difficulties in procuring adequate tissue samples. Our present findings that leverage unique archived osteoblastoma specimens, which were derived from two tertiary referral centers, may have significant impact on diagnosis and future mechanistic studies of osteoblastoma as a still poorly understood bone tumor.
Elevated expression of miR-210, which is a hypoxia-induced microRNA controlled by hypoxia inducible factor 1α (HIF1A), is known to be enriched in osteosarcoma.38 Increased levels of this miRNA are a molecular proxy marker for reduced relative levels of oxygen in cells and tissues. Recent investigations suggest that miR-210 has important prognostic implications, with higher expression being associated with unfavorable long-term survival.39,40 Osteoblastomas are known to be highly vascular well-perfused tumors, and could be expected to express lower levels of miR-210 as we indeed observed in this study. Hypoxia inducible factor has been previously proposed as a biomarker for osteosarcoma, and our findings corroborate this concept. Yet, the advantage of miR-210 is its greater stability and reliable detection in archival FFPE specimens, even after treatment with harsh decalcifying agents.
Differences in surgical technique and tumor processing can potentially impact the expression of micro-RNAs in FFPE specimens. These technical issues have the potential to render microRNA expression signatures more variable across institutions, potentially limiting their diagnostic potential. However, the hypoxia-related miR-210, may indeed be highly specific for osteosarcoma compared to osteoblastoma. Osteoblastomas have a rich vascular supply and do not appear to express high-levels miR-210, while the majority of osteosarcomas we have analyzed exhibit elevated expression of miR-210. It is conceivable that biopsy sampling error and differences in tissue handling may lead to test results showing low levels of expression of miR-210 in an osteosarcoma biopsy (false negative result). The hypoxia-dependent expression of miR-210 ensures that false positive results for osteoblastoma detection are unlikely. While negative results remain inconclusive, positive identification of robust miR-210 expression provides a novel diagnostic indicator that favors radical surgical intervention. Because the hypoxia response is a highly conserved and ubiquitous cell autonomous response, anatomic location alone cannot account for the differential expression of miR-210 seen between osteosarcoma and osteoblastoma. The anatomy independent expression of miR-210 further supports its application as a discriminating tumor biomarker.
Even though miR-210 is a useful diagnostic aid, its expression remains a quantitative measurement and some osteosarcomas have levels that do not differ from osteoblastomas. Osteoblastomas occur preferentially in the posterior elements of the spine, which has a robust blood supply to support the spinal cord, which could potentially suppress miR-210 expression. The misdiagnosed osteoblastic osteosarcoma that we evaluated earlier (Fig. 7) was also located in the posterior elements of the spine, but the robust expression of miR-210 in this specimen is on the low range of expression levels detected in other osteosarcoma tissues, consistent with this tumor being derived from a highly vascularized environment.
It is important to note that microRNA expression profiles not only reflect what tumor cells are producing, they also provide important information regarding the tumor microenvironment, and neighboring cells that support tumor growth. Musculoskeletal tumors can appear histologically as a heterogeneous mixture of cells, and for many musculoskeletal lesions (i.e., chondroblastoma, giant cell tumor) the causative tumorigenic cell is often elusive. Thus, the microRNAs which take into account tumor microenvironment, as well as the tumor cells themselves are informative markers for tumor diagnosis. It would be premature to conclude that the tumor specific microRNAs identified in this investigation play a mechanistic role in tumor pathogenesis. However, miR-210 has been shown to suppress Wnt signaling41 and may play a role in the known downregulation of Wnt signaling observed osteosarcomas.8
MicroRNAs such as miR-210 identified in this investigation are well suited for use as clinical bio-markers. They have several advantages over traditional biomarkers in that they are highly stable and can be isolated from archived formalin fixed paraffin embedded tumor specimens that are routinely use in clinical practice. Our studies show that miRNAs maintain their integrity even after being treated with JVMGB—Provided surgical specimens and reviewed tumor histology for the study. Participated in the design of the study, interpreted data, prepared and approved final manuscript. AJvW—Participated in the design of the study, interpreted data, prepared and approved final manuscript.
Supplementary Material
Acknowledgments
Grant sponsor: NIH R01; Grant number: AR049069; Grant sponsor: NIH R03; Grant number: AR066342; Grant sponsor: NIH F32; Grant number: AR066508; Grant sponsor: Iniciativa Cientfíca Milenio; Grant number: P09/016-F; Grant sponsor: FONDECYT; Grant number: 1060772; Grant sponsor: FONDAP; Grant number: 15090007; Grant sponsor: Orthopaedic Research and Education Foundation.
We thank the members of our research group for stimulating discussions, as well as the Mayo Clinic Bioinformatics Core for their assistance with high-throughput RNA sequencing and bioinformatics support. This work was supported by NIH R01 grant AR049069 (to AJvW), NIH R03 grant AR066342 (to ANL), NIH F32 AR066508 (to AD), and a Resident Research Grant from the Orthopaedic Research and Education Foundation (to SMR). We also appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their name.
Footnotes
AUTHORS’ CONTRIBUTIONS
SMR—Participated in the design of the study, carried out experiments, interpreted data, prepared and approved final manuscript. JTM—Provided surgical specimens and reviewed tumor histology for the study, helped revise, and approve final manuscript. AD— Assisted with RNA extraction from tumor specimens as well as qPCR validation studies. Helped revise and approve final manuscript. ETC—Assisted with RNA extraction from tumor specimens as well as qPCR validation studies. Helped revise and approve final manuscript. WW—Assisted with RNA extraction from tumor specimens as well as qPCR validation studies. Helped revise and approve final manuscript. FX— Assisted with microRNA network analysis. Helped revise and approve final manuscript. RRT—Helped revise and approve final manuscript. JME—Carried out bioinformatics analysis of microRNA sequencing data. Helped revise and approve final manuscript. RZ—Assisted with tumor histology and tissue sectioning. Helped revise and approve final manuscript. BdBIH—Assisted with tumor histology and tissue sectioning. Helped revise and approve final manuscript. AM—Helped revise and approve final manuscript. ALF —Provided surgical specimens and reviewed tumor histology for the study, helped revise and approve final manuscript. CYI—Helped revise and approve final manuscript. PSR—Provided surgical specimens for the study, helped revise and approve final manuscript. TCS—Provided surgical specimens for the study, helped revise and approve final manuscript. MJY— Provided surgical specimens for the study, helped revise and approve final manuscript. FHS—Provided surgical specimens for the study, helped revise and approve final manuscript. DRD—Helped revise and approve final manuscript. ANL—Provided surgical specimens for the study. Helped revise and approve final manuscript. MAG—Helped revise and approve final manuscript. AGHC—Reviewed tumor histology for the study. Helped revise and approve final manuscript. AMO— Provided surgical specimens and reviewed tumor histology for the study. Helped revise and approve final manuscript. AMCJ—Reviewed tumor histology for the study. Helped revise and approve final manuscript. JVMGB—Provided surgical specimens and reviewed tumor histology for the study. Participated in the design of the study, interpreted data, prepared and approved final manuscript. AJvW—Participated in the design of the study, interpreted data, prepared and approved final manuscript.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Arndt CA, Rose PS, Folpe AL, et al. Common musculoskeltumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kansara M, Teng MW, Smyth MJ, et al. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–735. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- 3.Horvai A, Unni KK. Premalignant conditions of bone. J Orthop Sci. 2006;11:412–423. doi: 10.1007/s00776-006-1037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CD, Bridge JA, Hogendoorn PC, et al. WHO classificaiton of tumors of soft tissue and bone. 4. Lyon: International Agency for Research on Cancer (IARC); 2013. pp. 275–295. [Google Scholar]
- 5.Unni KK. Benign osteoblastoma (giant osteoid osteoma) In: Unni KK, editor. Dahlin’s bone tumours: general aspects and data on 11087 cases. 5. Philadelphia, PA: Lippincott-Raven; 1996. pp. 131–142. [Google Scholar]
- 6.Nord KH, Nilsson J, Arbajian E, et al. Recurrent chromosome 22 deletions in osteoblastoma affect inhibitors of the Wnt/beta-catenin signaling pathway. PLoS ONE. 2013;13:e80725. doi: 10.1371/journal.pone.0080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan Y, Zhao W, Jiang Y, et al. β-catenin is a valuable marker for differential diagnosis of osteoblastoma and osteosarcoma. Hum Pathol. 2014;45:1459–1465. doi: 10.1016/j.humpath.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y, Mohseny AB, Karperien M, et al. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- 9.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Meng W, McElroy JP, Volinia S, et al. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS ONE. 2013;8:e64393. doi: 10.1371/journal.pone.0064393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lulla RR, Costa FF, Bischof JM, et al. Identification of differentially expressed MicroRNAs in osteosarcoma. Sarcoma. 2011;2011:732690. doi: 10.1155/2011/732690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maire G, Martin JW, Yoshimoto M, et al. Analysis of miRNA-gene expression-genomic profiles reveals complex mechanisms of microRNA deregulation in osteosarcoma. Cancer Genet. 2011;204:138–146. doi: 10.1016/j.cancergen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Mintz MB, Sowers R, Brown KM, et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res. 2005;65:1748–1754. doi: 10.1158/0008-5472.CAN-04-2463. [DOI] [PubMed] [Google Scholar]
- 15.Jones KB, Salah Z, Del Mare S, et al. MiRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865–1877. doi: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J N Am. 2011 Available at: http://journal.embnet.org/index.php/embnetjournal/article/view/200.
- 17.Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedländer MR, Chen W, Adamidi C, et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol. 2008;26:407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 19.Coronnello C, Hartmaier R, Arora A, et al. Novel modeling of combinatorial miRNA targeting identifies SNP with potential role in bone density. PLoS Comput Biol. 2012;8:e1002830. doi: 10.1371/journal.pcbi.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronnello C, Benos PV. ComiR: combinatorial micro-RNA target prediction tool. Nucleic Acids Res. 2013;41:W159–W164. doi: 10.1093/nar/gkt379. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bidwell JP, Fey EG, van Wijnen AJ, et al. Nuclear matrix proteins distinguish normal diploid osteoblasts from osteosarcoma cells. Cancer Res. 1994;54:28–32. [PubMed] [Google Scholar]
- 26.Yang S, Quaresma AJ, Nickerson JA, et al. Subnuclear domain proteins in cancer cells support the functions of RUNX2 in the DNA damage response. J Cell Sci. 2015;128:728–740. doi: 10.1242/jcs.160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galindo M, Pratap J, Young DW, et al. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem. 2005;280:20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan SS, Pereira BP, Zhou YF, et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol Biol Rep. 2009;36:153–158. doi: 10.1007/s11033-008-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira BP, Zhou Y, Gupta A, et al. Runx2, p53, and pRB status as diagnostic parameters for deregulation of osteoblast growth and differentiation in a new pre-chemotherapeutic osteosarcoma cell line (OS1) J Cell Physiol. 2009;221:778–788. doi: 10.1002/jcp.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucero CM, Vega OA, Osorio MM, et al. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol. 2013;228:714–723. doi: 10.1002/jcp.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.San Martin IA, Varela N, Gaete M, et al. Impaired cell cycle regulation of the osteoblast-related heterodimeric transcription factor Runx2-Cbfbeta in osteosarcoma cells. J Cell Physiol. 2009;221:560–571. doi: 10.1002/jcp.21894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Deen M, Akech J, Lapointe D, et al. Genomic promoter occupancy of runt-related transcription factor RUNX2 in Osteosarcoma cells identifies genes involved in cell adhesion and motility. J Biol Chem. 2012;287:4503–4517. doi: 10.1074/jbc.M111.287771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Deen M, Taipaleenmäki H, Zhang Y, et al. MicroRNA-34c inversely couples the biological functions of the runt-related transcription factor RUNX2 and the tumor suppressor p53 in osteosarcoma. J Biol Chem. 2013;288:21307–21319. doi: 10.1074/jbc.M112.445890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvarajah S, Yoshimoto M, Ludkovski O, et al. Genomic signatures of chromosomal instability and osteosarcoma progression detected by high resolution array CGH and interphase FISH. Cytogenet Genome Res. 2008;122:5–15. doi: 10.1159/000151310. [DOI] [PubMed] [Google Scholar]
- 35.Martin JW, Zielenska M, Stein GS, et al. The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma. 2011;2011:282745. doi: 10.1155/2011/282745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin JW, Chilton-MacNeill S, Koti M, et al. Digital expression profiling identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as potential predictive biomarkers for neo-adjuvant chemotherapy response in paediatric osteosarcoma. PLoS ONE. 2014;9:e95843. doi: 10.1371/journal.pone.0095843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poos K, Smida J, Nathrath M, et al. Structuring osteosarcoma knowledge: an osteosarcoma-gene association database based on literature mining and manual annotation. Database (Oxford) 2014 doi: 10.1093/database/bau042. pii:bau042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicchillitti L, Di Stefano V, Isaia E, et al. Hypoxia-inducible factor 1-α induces miR-210 in normoxic differentiating myoblasts. J Biol Chem. 2012;287:44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai H, Lin L, Cai H, et al. Prognostic evaluation of microRNA-210 expression in pediatric osteosarcoma. Med Oncol. 2013;30:499. doi: 10.1007/s12032-013-0499-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Zhao J, Shi M, et al. Elevated expression of miR-210 predicts poor survival of cancer patients: a systematic review and meta-analysis. PLoS ONE. 2014;9:e89223. doi: 10.1371/journal.pone.0089223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin L, Chen Y, Niu Y, et al. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics. 2010;11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.