Abstract
Goals
To evaluate provider knowledge, attitudes and barriers to HBV care and management practices across diverse primary care settings.
Background
Factors influencing adherence to recommended hepatitis B virus (HBV) screening and management guidelines are poorly defined.
Methods
Providers across various healthcare settings in San Francisco were surveyed. Multivariate analyses were used to identify factors associated with recommended HBV screening, vaccination, and disease monitoring.
Results
Of 277 (41.3%) responding providers, 42% reported performing HBV screening in >50% of at-risk patients, and 49%, HBV vaccination in >50% of eligible patients. Most reported appropriate monitoring of a majority of HBV-infected patients with ALT (79%) and HBV viral load (67%) every 6–12 months, but performed any hepatocellular carcinoma (HCC) screening in 49%. Provider factors significantly associated with HBV screening were speaking an Asian language (OR 3.27), offering HBV treatment (OR 3.00), having >25% of Asian patients in practice (OR 2.10), practicing in safety net settings (OR 7.51) and having higher barrier score (OR 0.74). Appropriate HBV monitoring was associated with provider speaking an Asian language (OR 3.43) and provider age (OR 0.68/decade). HCC screening was associated with having >25% of patients speaking English as a second language (OR 4.26) and practicing in safety net settings (OR 0.14).
Conclusions
Rates of adherence to HBV guidelines were suboptimal irrespective of practice setting and were influenced by certain provider, patient and practice factors. This study reinforces the importance of engaging primary care providers in development, dissemination, and implementation of evidence-based HBV practice guidelines.
Keywords: HBV guidelines, HBV screening and management, HCC surveillance, primary care, vulnerable populations
INTRODUCTION
Chronic hepatitis B virus (HBV) infection poses a significant public health burden, affecting 350 million individuals worldwide, with 1.25–2.2 million carriers in the United States alone.1, 2 Complications of HBV include development of cirrhosis, fulminant liver failure, and hepatocellular carcinoma (HCC).3, 4 It is estimated that nearly 20% of patients with chronic HBV will die from complications of liver disease.5 Therefore, screening practices are essential to ensure early patient identification and prompt treatment initiation.
As of 2014, the United States Preventive Services Task Force (USPSTF) recommends HBV screening in persons considered “high risk” for infection.6 This recommendation is now in line with the American Association for the Study of Liver Diseases (AASLD) and the Center for Disease Control and Prevention (CDC) guidelines.2, 7 Aside from individuals from endemic areas (prevalence ≥2%), those who practice risky behaviors (injection drug users, multiple sexual partners, men who have sex with men) and have potential exposure to HBV (household contacts, healthcare workers) are considered high-risk.6 According to AASLD, both HBV surface antigen (HBsAg) and antibody (anti-HBs) tests should be obtained to both screen for and assess HBV vaccination eligibility.2 Interval monitoring of ALT levels and HBV viral load are also recommended to help monitor disease course and identify those eligible for treatment initiation.2
HBV poses a major health disparity in the U.S., with many infected individuals being from predominantly immigrant, underinsured, and low income communities.8–10 Asian and Pacific Islanders (API) are a particularly vulnerable population, with disproportionately high rates of HBV infection. The San Francisco Bay Area is home to one of the largest API populations in the U.S., and it is estimated that approximately 9% of API in this region have chronic HBV.11 Previously, our group has published on HBV screening practices among providers of a San Francisco safety-net health care system that serves uninsured and underinsured populations.12–14 We showed that among APIs, less than two thirds underwent HBV testing and less than 50% of susceptible patients were vaccinated for HBV.12 Moreover, based on self-report, over 40% of providers were unfamiliar with HBV screening guidelines.13
With the recent enactment of the Affordable Care Act, public and private health systems alike are faced with treating the previously underinsured populations with HBV. As such, it has become important to understand provider knowledge and approach to HBV in this vulnerable population in an effort to minimize health disparity. The aim of this study is to describe provider practices, as well as knowledge, attitudes, and barriers to the prevention and management of HBV in a diverse patient population across a broad spectrum of healthcare systems.
MATERIALS and METHODS
Provider Survey
A survey was administered to primary care providers affiliated with several major health systems in San Francisco participating in the San Francisco Hepatitis B Quality Improvement Collaborative, organized by the San Francisco Health Improvement Partnership and Hepatitis B Free Campaign. This collaborative was created to facilitate a cooperative, citywide effort in San Francisco among health care organizations to systematically improve processes for Hepatitis B screening and clinical care. This survey was performed as part of an initial needs-assessment to identify gaps in HBV care in the city. Participating systems included Kaiser Permanente San Francisco (an HMO), Hill Physicians Medical Group (including the University of California, San Francisco Medical Group and private practice offices throughout San Francisco), the San Francisco Chinese Community Health Care Association, as well as the safety net health care system, the San Francisco Health Network (SFHN). The SFHN is administered by the San Francisco Department of Public Health and includes a network of 15 primary care clinics, San Francisco General Hospital (an academic medical center), and the San Francisco Community Clinic Consortium (which includes 11 federally qualified health centers).
The survey was sent to providers by postal or electronic mail, and a subsequent mailing to non-respondents were conducted after 4 weeks. Provider responses were de-identified for data analysis.
The survey instrument was developed by study investigators with input from experts in primary care, hepatology, survey design, and using a previously published related survey.14 Content domains included provider, practice and patient characteristics; HBV screening, vaccination, and management practices; familiarity with HBV management guidelines; HCC screening practices including modalities used; and provider attitudes about and perceived barriers towards HBV care. The survey was pilot-tested with 20 physicians and revised based on their feedback.
Data Analysis
Provider characteristics – demographic, training and experience, and practice features – are described using frequencies, overall and by setting. In order to assess overall provider knowledge, favorable attitudes, and barriers to HBV care, the percent correct (for knowledge items) and percent agreeing (for attitude and barrier items) for each question was calculated. For use in multivariable models, individual providers’ composite scores were formed from responses to these questions. Specifically, the knowledge score was computed as the number of correct responses to eleven questions assessing knowledge (1 for correct, 0 for incorrect; maximum score 11). The attitude score was determined by summing the numerical codes assigned to responses to eight questions designed to assess attitudes (1 for “agree” response, 0.5 for “unsure” response, and 0 for “disagree” response; maximum score 8). The barrier score was also determined by summing the numerical codes for nine questions regarding perceived barriers in their practice (1 for “agree” response, 0.5 for “unsure” response, and 0 for “disagree” response; maximum score 9). Higher composite scores represent higher knowledge, favorable attitude, and greater barriers to HBV care. The three mean ± SD composite scores were calculated and also expressed as mean percentages.
Seventy-five percent of providers reported all 18 characteristics and only 5% omitted more than three. SAS proc MI was used to generate 5 replicate datasets with imputed missing values. Multivariable forward stepwise regression modeling via SAS proc logistic (adjusted for provider age, gender, Asian race, and provider patient load, and stratified by dataset) was used to identify which of the 14 other provider characteristics are significantly associated with each of the 4 outcomes – HBV screening, vaccination, disease monitoring and HCC surveillance – with SAS proc mianalyze used to estimate the mean results across the 5 replicate datasets. The four binary outcome measures indicated providers’ self-reported performance of: 1) recommended HBV screening, defined as use of both HBsAg and anti-HBs, 2) HBV vaccination in greater than 50% of vaccine-eligible patients, 3) recommended HBV disease monitoring in those with chronic HBV, defined as use of ALT every 6 months and HBV DNA every 6–12 months, and 4) recommended HCC surveillance in eligible patients with chronic HBV, defined as performing abdominal ultrasound every 6 months. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 671 providers were sampled, with 277 respondents (148 from the safety net and 129 from non-safety net systems) for a response rate of 41.3%. The majority of providers were female, MDs, and approximately half self-identified as Caucasian and one-third, API (Table 1). Over 40% of providers were in practice for over 10 years, and approximately half of providers served a patient panel comprised of over 25% Asians and also patients with limited English proficiency. Half of the practices served patients with a significant proportion of patients that were uninsured or underinsured, with the majority of these providers (91%) practicing in the safety net setting.
Table 1.
Provider and Practice Characteristics.
| Characteristic | All Providers (N=277) (% (N)) |
|---|---|
|
| |
| Age, years | |
| 20–29 | 10.8 (30) |
| 30–39 | 30.3 (84) |
| 40–49 | 26.4 (73) |
| 50–59 | 18.4 (51) |
| ≥ 60 | 8.7 (24) |
| Not reported | 5.4 (15) |
|
| |
| Female gender | 58.8 (163) |
|
| |
| Race/Ethnicity | |
| Caucasian | 50.2 (139) |
| African-American | 1.8 (5) |
| Hispanic/Latino | 5.8 (16) |
| Asian | 30.7 (84) |
| Other/Not reported | 11.9 (33) |
|
| |
| U.S. Born | 70.8 (196) |
|
| |
| Providers with Asian language proficiency | 22.4 (62) |
|
| |
| Post-Graduate Degree | |
| MD | 78.0 (216) |
| Non-MD | 16.2 (45) |
| Not reported | 5.8 (16) |
|
| |
| Specialty | |
| Internal Medicine | 60.2 (157) |
| Family Medicine | 32.1 (89) |
| Infectious Diseases | 2.8 (8) |
| Other/Not reported | 8.3 (23) |
|
| |
| Years in practice | |
| <= 10 years | 51.6 (143) |
| >10 years | 43.3 (120) |
| Not reported | 5.1 (14) |
|
| |
| Number of patients seen per week, patients | |
| 0–20 | 27.1 (75) |
| 21–40 | 22.0 (61) |
| 41–60 | 19.1 (53) |
| 61–80 | 11.6 (32) |
| >80 | 14.8 (41) |
| Not reported | 5.4 (15) |
|
| |
| Provider practice consists of more than 25% Asian patients | 48.4 (134) |
|
| |
| Provider practice consists of more than 25% of patients with limited English proficiency | 52.7 (146) |
|
| |
| Provider practice consists of more than 25% of patients uninsured | 53.1 (147) |
|
| |
| Provider practice consists of more than 25% of patients with chronic HBV infection | 3.2 (9) |
|
| |
| Provider offers HBV treatment in their practice | 15.5 (43) |
Although 40% of providers felt they were unfamiliar with AASLD guidelines for HBV management (48% somewhat familiar and 7% very familiar), overall provider knowledge scores were favorable (Supplemental Table 1). However, nearly a third of providers were not aware that high levels of HBV viral load are associated with an increased incidence of cirrhosis and that HBV-induced liver cancer can occur in the absence of cirrhosis. Moreover, one third of providers thought that all patients with hepatitis B should be treated, suggesting a lack of familiarity with treatment eligibility criteria. However, providers were motivated to screen for HBV when it was evidence driven, recommended by a national organization, and if it was used as a quality measure by their institution or insurance companies. The most commonly cited barriers to HBV screening were lack of clarity of guidelines, uncertainty or unawareness of guidelines, and patient financial barriers.
HBV Screening and Vaccination Practices Based on Provider Self-Report
HBV prevention practices as reported by providers are summarized in Figure 1. Only 40% of providers reported HBV screening rates of greater than 50% among their at-risk adult patients. For HBV testing, 95% of providers used HBsAg, with 78% ordering both HBsAg and anti-HBs (with or without anti-HBc); 16% did not include anti-HBs as part of their screening. Only half of providers reported vaccinating greater than 50% of eligible patients, and 19% of providers reported vaccinating more than 75% of their eligible patients against HBV.
Figure 1.
Proportion of adult patients in practice screened for and vaccinated against HBV by provider self report.
HBV Management Practices Based on Provider Self-Report
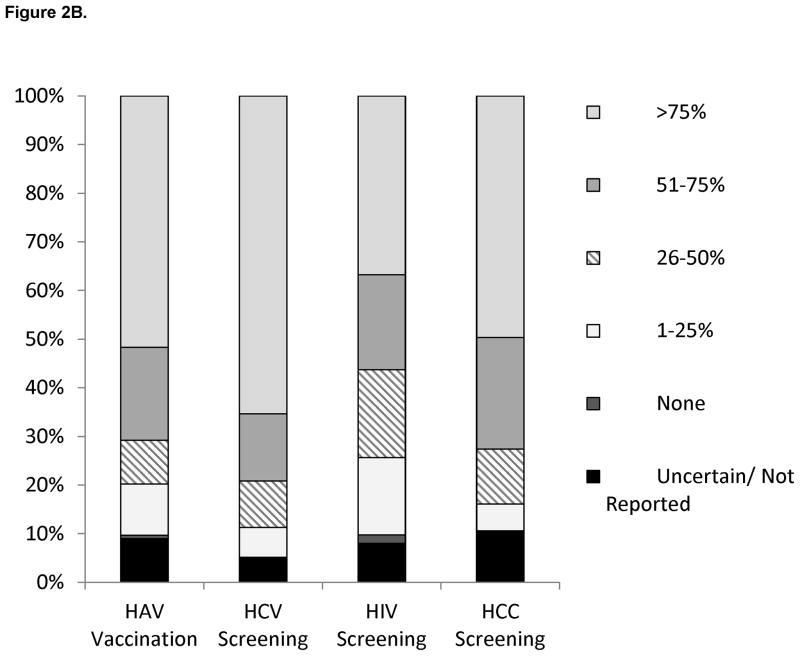
Provider self-reported management practices of HBV-infected patients are shown in Figure 2. With respect to monitoring of HBV disease activity (Figure 2A), the majority of providers reported appropriate monitoring of ALT and HBV viral load every 6–12 months in their HBV-infected population; ALT levels were most often monitored every 6 months and HBV viral load levels were most often monitored every 12 months. However, HBeAg testing was less frequently performed or not performed at all as part of HBV disease monitoring. More than 70% of providers performed hepatitis A virus (HAV) vaccination and testing for hepatitis C virus (HCV) coinfection in greater than 50% of their HBV-infected patients (Figure 2B). Treatment for HBV infection was offered by only 16% of primary providers. Although most providers performed HCC surveillance, only half performed HCC surveillance in >75% of HBV-infected patients (Figure 2B). The type and frequency of HCC screening tests used by providers are shown in Figure 3. As shown, AFP and US were the most frequent HCC screening modalities used, but a small proportion reported using CT or MRI scans.
Figure 2.
Figure 2A. Type and frequency of tests ordered for disease monitoring in patients with chronic HBV by provider self report.
Figure 2B. Proportion of HBV-infected patients in practice that received HAV vaccination, screening for HIV and HCV co-infection, and hepatocellular carcinoma surveillance by provider self-report.
Figure 3.
Type and frequency of tests performed for hepatocellular screening in HBV-infected patients by provider self report.
Factors Associated with HBV Screening and Vaccination
On multivariate analysis adjusted for provider age, gender, race and patient load in practice (Table 2), HBV screening was positively associated with provider speaking an Asian language (OR 3.27, 95%CI 1.19–8.96, p=0.021), offering HBV treatment within their clinical practice (OR 3.00, 95%CI 1.34–6.72, p=0.0076), and serving a patient panel with >25% API patients (OR 2.10, 95%CI 1.07–4.09, p=0.030) and negatively associated with a higher provider barrier score (OR 0.74, 95%CI 0.57–0.96, p=0.025). Moreover, practicing in the safety net setting was associated with about 7 times higher odds of screening for HBV (OR 7.51 95%CI 3.75–15.07, p<0.0001). With respect to HBV vaccination, offering HBV treatment in practice and serving a patient panel of >25% APIs were positively associated, while provider being foreign-born was negatively associated with HBV vaccination.
Table 2.
Multivariable logistic regression models* of factors associated with HBV prevention and management.
| HBV Screening | HBV Vaccination | HBV Monitoring | HCC Surveillance | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P-Value† | Odds Ratio (95% CI) | P-Value† | Odds Ratio (95% CI) | P-Value† | Odds Ratio (95% CI) | P-Value† |
| Provider Age (per decade) | 0.98 (0.74–1.29) | 0.87 | 0.99 (0.77–1.27) | 0.93 | 0.68 (0.53–0.87) | 0.0026 | 0.70 (0.48–1.01) | 0.060 |
| Female Provider | 0.82 (0.44–1.54) | 0.54 | 1.30 (0.76–2.25) | 0.34 | 0.79 (0.45–1.36) | 0.39 | 1.63 (0.71–3.72) | 0.25 |
| Asian Provider (vs. non-Asian) | 0.79 (0.31–2.02) | 0.62 | 0.82 (0.40–1.69) | 0.59 | 1.02 (0.47–2.20) | 0.96 | 1.60 (0.52–4.89) | 0.41 |
| Patient Load (per 20 patients) | 1.21 (0.94–1.56) | 0.13 | 1.04 (0.84–1.28) | 0.74 | 1.18 (0.96–1.45) | 0.12 | 1.29 (0.90–1.84) | 0.17 |
| Provider speaks Asian language | 3.27 (1.19–8.96) | 0.021 | 3.43 (1.38–8.53) | 0.0081 | ||||
| Provider works in Safety net | 7.51 (3.75–15.07) | <0.0001 | 0.14 (0.05–0.41) | 0.0002 | ||||
| Provider offers HBV treatment | 3.00 (1.34–6.72) | 0.0076 | 4.77 (2.12–10.73) | 0.0002 | ||||
| >25% of patient panel API | 2.10 (1.07–4.09) | 0.030 | 2.03 (1.12–3.66) | 0.019 | ||||
| Provider Barrier Score (per unit) | 0.74 (0.57–0.96) | 0.025 | ||||||
| Provider is foreign-born | 0.42 (0.19–0.94) | 0.034 | ||||||
| >25% of patient panel ESL | 4.26 (1.76–10.30) | 0.0013 | ||||||
Each model was adjusted for four characteristics: provider’s age, sex, race (Asian/non-Asian), and patient load.
Statistical significance is at p <0.05.
Factors Associated with Recommended HBV Monitoring and HCC surveillance in HBV infected patients
On multivariable analysis adjusted for provider age, gender, race and patient load in practice (Table 2), the only factor with a positive significant association with performing recommended HBV monitoring was provider speaking an Asian language (OR 3.43, 95%CI 1.38–8.53, p=0.0081), while provider age (OR per decade 0.68, 95%CI 0.53–0.87, p=0.0026) was negatively associated. Serving a patient panel with >25% patients speaking English as a second language (OR 4.26, 95%CI 1.76–10.30, p=0.0013) was positively associated with performing appropriate HCC surveillance, while practicing within the safety net setting (OR 0.14, 95%CI 0.05–0.41, p<0.001) was negatively associated.
DISCUSSION
This study provides insight into HBV preventive and management practices among primary care providers across a broad spectrum of health care settings serving an urban population. Provider reported rates of HBV screening and vaccination and HCC surveillance among eligible HBV-infected patients were suboptimal. We found that in addition to patient and practice factors, certain provider factors, including provider age, foreign birth, speaking an Asian language and perceived barriers to HBV care, were associated with HBV prevention and management practices. Moreover, practicing in the safety net health care system was strongly associated with HBV screening, but was negatively associated with performing recommended HCC surveillance.
The prevalence of HBV varies nationwide largely as a function of immigration patterns, and nearly one-fourth of the San Francisco Bay area population is from endemic Asian and Pacific Island regions.15 Motivated by high rates of undiagnosed chronic HBV infection in the API population, community efforts through the “Hepatitis B Free” Campaign were launched, possibly resulting in a heightened awareness among both providers and patients in the city 16, 17. Indeed, in this study, providers serving a large API population in practice had an increased odds of performing HBV screening and vaccination, irrespective of healthcare setting or medical group affiliation. Moreover, providers that spoke an Asian language had an increased odds of performing both HBV screening and appropriate HBV monitoring. In addition, practicing in the safety net setting was also independently associated with higher odds of HBV screening which may in part be related to the exposure of providers to a high proportion of at risk immigrant populations served in this network.
However, despite these community efforts, self-reported HBV prevention and management practices were suboptimal, and this study highlights several gaps in provider knowledge. Nearly half of providers were unfamiliar with current AASLD guidelines for the management of HBV that includes liver cancer screening 2. Notably, a third of providers were unaware that HBV therapy reduces the risk for liver disease progression, while a similar proportion reported that all patients with HBV should be treated, both suggesting a lack of familiarity with indications for HBV treatment. Approximately 15% of providers did not evaluate for evidence of immunity in at risk populations, which represents a missed opportunity to identify vaccine-eligible patients served by these providers. Moreover, many providers were unaware that HBV infection can induce HCC development in the absence of cirrhosis, highlighting limited awareness of HCC screening guidelines. Greater collaboration between primary care providers and liver disease specialists in developing and distributing evidence-based guidelines attuned to the context of primary care practice may help to address these gaps in knowledge and potentially improve HBV prevention and management in primary care practices.
Several factors apart from provider knowledge also appear to influence HBV practice patterns. Provider perceived barriers to HBV care have a clear impact on HBV prevention in this study, as a higher perceived barrier score was associated with a 25% reduction in the odds of performing HBV screening. In addition, practicing in the safety net setting was negatively associated with HCC surveillance, and this may indeed be a reflection of perceived barriers, particularly resource constraints such as access to abdominal imaging. These findings highlight that in addition to provider education, addressing provider perceived barriers to HBV care is critical. Provider foreign birth and older age were also negative predictors of HBV vaccination and HBV monitoring, respectively. While the reasons for these associations are not known and may be related to unmeasured factors, these findings suggest that targeted intervention among these provider groups is warranted.
The findings of this study support the notion that a complex interplay of factors shape HBV care, and additional systematic quality improvement strategies to implement guideline-driven HBV recommendations represent an important strategy to improve HBV care. This approach was recently demonstrated by the Northern California Kaiser Permanente group, who developed a HBV Liver Care Program that was informed by the finding of suboptimal adherence to HBV guidelines in a large cohort of HBV patients.18 The program uses many elements of the Chronic Care Model,19 such as a registry of infected patients, automated reminders to patients to perform recommended tests, and access to a dedicated care team that can order tests and administer therapy when indicated. While data regarding the impact of this intervention are currently not available, it is hoped that similar implementation of systematic solutions at the institutional level may help improve management of patients with HBV.
The strengths of this study includes the evaluation of provider knowledge, attitudes, and barriers to HBV care among a large number of primary care providers across diverse healthcare settings in an urban area, including those serving privately and publicly insured and uninsured populations. The limitations of the study include use of provider self-report and survey response rates of 40%, though this response rate is comparable to other previously reported provider survey studies. 13, 20, 21 Provider self-report tends to overestimate adherence to quality of care guidelines.22 However, the suboptimal rates of HBV screening, HBV vaccination, and HCC surveillance observed in this study suggest that many providers candidly acknowledged that they did not consistently implement screening and management practices for all appropriate patients. Moreover, in a previously published study of providers and patients in the San Francisco safety net, we found that provider self-report of HBV screening and immunization practices generally aligned with objective measures of clinical practice derived from electronic medical record data, adding further value to these findings.12 The generalizability of our findings may be limited by the possible heightened awareness of HBV among providers and patients in the San Francisco bay area as a result of community outreach efforts. However, the fact that HBV prevention and management practices remained suboptimal suggests that these efforts alone do not address the gaps in HBV care and reflects a potential for even higher gaps in HBV care in other regions of the country.
In summary, certain provider, patient, and practice settings influence HBV prevention and management practices in primary care settings. Rates of adherence to HBV screening and management guidelines appear to be suboptimal for this group of providers irrespective of practice setting. This study highlights the importance of engaging primary care providers in development, dissemination, and implementation of evidence-based HBV guidelines and identifies important topic areas and provider groups for targeted educational efforts. Given the complex interplay of factors influencing HBV care, implementation of systematic HBV quality improvement initiatives may help improve HBV prevention and care.
Supplementary Material
Acknowledgments
Declaration of funding sources: This work was in part supported by the National Institutes of Health grant number K24AA022523 (MK), BMS Virology Fellows Research Training Program (NM), and the National Center for Advancing Translational Sciences, National Institutes of Health through UCSF-CTSI Grant Number UL1 TR000004 (MK).
Footnotes
Conflict of interest disclosure: The authors have no conflicts of interest to report.
References
- 1.Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610–4. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinall EJ, Hawkins G, Fraser A, et al. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–40. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- 6.LeFevre ML Force USPST. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]
- 7.Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology. 2009;49:S35–44. doi: 10.1002/hep.22882. [DOI] [PubMed] [Google Scholar]
- 8.Lai M, Liaw YF. Chronic hepatitis B: past, present, and future. Clin Liver Dis. 2010;14:531–46. doi: 10.1016/j.cld.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Pollack H, Wang S, Wyatt L, et al. A comprehensive screening and treatment model for reducing disparities in hepatitis B. Health Aff (Millwood) 2011;30:1974–83. doi: 10.1377/hlthaff.2011.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag H, McGlynn KA, Graham GN, et al. Achieving health equity to eliminate racial, ethnic, and socioeconomic disparities in HBV- and HCV-associated liver disease. J Fam Pract. 2010;59:S37–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034–40. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 12.Mukhtar NA, Toy BC, Burman BE, et al. Assessment of HBV preventive services in a medically underserved Asian and Pacific Islander population using provider and patient data. J Gen Intern Med. 2015;30:68–74. doi: 10.1007/s11606-014-3057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burman BE, Mukhtar NA, Toy BC, et al. Hepatitis B management in vulnerable populations: gaps in disease monitoring and opportunities for improved care. Dig Dis Sci. 2014;59:46–56. doi: 10.1007/s10620-013-2870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56:1516–23. doi: 10.1007/s10620-010-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQuillan GM, Coleman PJ, Kruszon-Moran D, et al. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–8. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gish RG, Cooper SL. Hepatitis B in the Greater San Francisco Bay Area: an integrated programme to respond to a diverse local epidemic. J Viral Hepat. 2011;18:e40–51. doi: 10.1111/j.1365-2893.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey MB, Shiau R, Zola J, et al. San Francisco hep B free: a grassroots community coalition to prevent hepatitis B and liver cancer. J Community Health. 2011;36:538–51. doi: 10.1007/s10900-010-9339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar M, Shvachko VA, Ready JB, et al. Characteristics and management of patients with chronic hepatitis B in an integrated care setting. Dig Dis Sci. 2014;59:2100–8. doi: 10.1007/s10620-014-3142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 20.Lubega S, Agbim U, Surjadi M, et al. Formal hepatitis C education enhances HCV care coordination, expedites HCV treatment and improves antiviral response. Liver Int. 2013;33:999–1007. doi: 10.1111/liv.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan CE, Edwards TP, Luong MU, et al. Suboptimal surveillance for and knowledge of hepatocellular carcinoma among primary care providers. Clin Gastroenterol Hepatol. 2015;13:799–804. doi: 10.1016/j.cgh.2014.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–57. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.