Introduction
Along with the increasing prevalence of heart failure (HF),1 the use of ventricular assist device (VAD) therapy for advanced HF has risen dramatically.2 Continuous-flow VAD therapy is used to both extend and improve life; hence, understanding person-oriented outcomes (e.g. quality of life, depression and anxiety) is important.3,4 Family caregivers of patients undergoing VAD implantation play an important role in most aspects of post-implant care.5 In fact, the absence of an appropriate family or informal (unpaid) caregiver is a relative contraindication to VAD implantation.6,7 Early and sustained improvements in patient quality of life (QOL) after VAD implantation are well known,8 and patient anxiety and depression also generally decrease.4,9 In contrast, there is qualitative evidence that family caregivers of VAD patients are at risk for compromised QOL and high anxiety and depression post-implant.10-12 Importantly, person-oriented outcomes of patients and their caregivers are typically interdependent – meaning that the outcomes for one member of the family caregiving dyad typically influence the other – both in the context of chronic illness in general,13 as well as in the context of HF14,15 and VAD implantation16 in particular. The inherent interrelatedness of person-oriented outcomes of both members of a patient-caregiver dyad has important implications for research design and interpretation. Specifically, research that examines patients and their caregivers together can better approximate the context in which patients and caregivers experience and manage illness,13 providing critical information to help clinicians support patients and families to cope with and manage HF and VAD therapy together. Thus, the purpose of this study was to characterize changes for both members of the VAD dyad on important person-oriented outcomes (QOL, depression, and anxiety) from pre-implantation to 3 months post-implantation.
Methods
Design and Population
This was a formal interim analysis of data from an ongoing prospective study of VAD patients and their family or informal (unpaid) caregivers, modeled after the dyadic framework proposed by Berg and Upchurch.13 A primary aim of this study is to examine concurrent change in person-oriented outcomes among VAD patients and their caregivers. Data were collected over two years of enrollment (2013-2015) from a sample recruited from an academic medical center in the Northwestern United States. Patients were part of a federally-sponsored study on biobehavioral responses to VAD implantation; the design and recruitment procedures have been published previously.17 Patients were included if they were ≥21 years of age and excluded for previous VAD/heart transplant or if they were unable to complete study requirements (e.g. concomitant terminal illness, major psychiatric illness, major cognitive impairment). Caregivers were eligible if they were the adult (≥21 years of age) primary caregiver of the enrolled patient, as identified by the patient and agreed upon by the caregiver and the advanced HF team. The center's Institutional Review Board reviewed and approved all procedures and all patient and caregiver participants provided written informed consent.
Data for this analysis were collected at three time points: a median of 5 days prior to VAD implantation, and again at 1 and 3 months post-implantation. Data on patient comorbid conditions (Charlson Comorbidity Index18), etiology and duration of HF, New York Heart Association Class, ejection fraction, and implant strategy were abstracted from the medical record. Both patients and caregivers completed surveys which included demographic data (age, gender, race/ethnicity, education, employment, and relationship to one another) and study instruments as described below. Caregivers self-reported their own comorbid conditions using a validated instrument.19
Measures
QOL in patients and caregivers was measured using the EuroQol 5 Dimensions Visual Analogue Scale (EQ-5D VAS). The EQ-5D VAS is a standard vertical visual analogue scale, on which participants rate their current health-related QOL from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”). The EQ-5D is widely used in both healthy and chronically ill populations, and has been recommended in VAD populations in particular for its reliability, validity and utility in quantifying general health-related QOL.3,20 A general, rather than HF-specific, measure of QOL was selected given the dyadic focus of this analysis, as parallel measures from both dyad members facilitate within-dyad comparisons on the construct of interest.
Depression in patients and caregivers was measured using the Patient Health Questionnaire (PHQ-8), an 8-item depression screen.21 Participants respond on a 0-3 Likert scale; scores were summed to produce a total score ranging from 0-24 with higher scores indicating greater depressive symptoms. The PHQ-8 is appropriate for self-administration; scores greater than 5, 10, 15, or 20 indicate mild, moderate, moderately severe, or severe depression, respectively.21 In this sample, Cronbach's α for patients and caregivers at all time points ranged from 0.84 to 0.90.
Anxiety in patients and caregivers was measured using the anxiety subscale of the Brief Symptom Inventory.22 The anxiety subscale contains 6 items on a 0-4 Likert scale. The mean of responses produces a total score ranging from 0-4 with higher scores indicating greater anxiety.22 In this sample, Cronbach's α for patients and caregivers at all time points ranged from 0.78 to 0.90.
Analysis
The sample was described using means and standard deviations for continuous data and frequency and percentages for categorical data. Trajectories of change for patients and caregivers on each variable of interest were estimated using latent growth modeling with parallel processes.23 Growth modeling with parallel processes allows for joint analysis of two interdependent processes (e.g. concurrent change in patient and caregiver depression), providing an estimation of the intercept (pre-implant assessment) and slope (change over time) for each member of the dyad, as well as how each process is correlated.24 Importantly, this approach includes random effects between intercepts and slopes of both members of the dyad while controlling for the dependent nature of these data, allowing us to characterize changes in person-oriented outcomes for both members of the VAD patient-caregiver dyad. We generated three separate growth models with parallel processes: one for QOL, depression, and anxiety respectively. Changes in person-oriented outcomes were quantified in the metric of Hedges g (standardized mean difference with a correction factor for small samples).25 Missing data were handled using full information maximum likelihood estimation.26 Descriptive statistics were conducted using Stata 14; parallel process models were generated using MPlus 7.
Results
Characteristics of the sample (n = 41 dyads) are presented in Table 1. Patients and caregivers were in their mid-fifties on average. Most patients were male and most caregivers were female, and the majority of patient and caregiver participants were Caucasian/non-Hispanic. Most caregivers were the patients' spouse; the next most common caregiver relationship was parental. The average length of relationship was 27.5 years (median 28.3, interquartile range 16.0 – 39.0). The majority of patients received VAD therapy as a bridge to transplant, and the duration of HF at baseline was 7.9 years on average (median 5.2, interquartile range 2.5 – 10.5). Patients were primarily INTERMACS Class 3 or higher at time of implant. There was a small amount of attrition due to patient death (n = 2) and dissolution of the caregiving relationship (n =2). Data on patient and caregiver QOL, depression, and anxiety are presented in Table 2.
Table 1. Characteristics of the Sample (n=41 dyads).
| Patient mean±SD or n(%) |
Caregiver mean±SD or n(%) |
|
|---|---|---|
| Age | 53.8±14.2 | 54.7±11.4 |
| Gender (Female) | 6(14.6) | 33(80.5) |
| Caucasian | 35(85.4) | 37(90.2) |
| Non-Hispanic | 40(97.6) | 39(95.1) |
| Relationship Type | ||
| Spouse | - | 30(73.2) |
| Parent of Patient | - | 7(17.1) |
| Relationship Length (Months) | 330.2±178.7 | |
| Employment | ||
| Full-time | 3(7.5) | 16(39.0) |
| Part-time | 1(2.5) | 6(14.6) |
| Unemployed/Retired | 21(51.2) | 17(41.5) |
| Quit due to health | 13(32.5) | 1(2.4) |
| Education | ||
| High School or Less | 19(46.3) | 17(41.5) |
| Bachelors or Some College | 21(51.2) | 20(48.8) |
| Masters/Professional | 1(2.4) | 4(9.8) |
| CCI Score* | 2.4±1.5 | 1.1±1.4 |
| Idiopathic Etiology of HF | 25(61.0) | - |
| Duration of HF (Months)† | 94.9±95.4 | - |
| NYHA Class | ||
| III | 21(51.2) | - |
| IV | 16(39.0) | - |
| Ejection Fraction (%) | 20.3±2.5 | - |
| VAD Implant Strategy | ||
| Bridge to Transplant | 25(62.5) | - |
| Destination Therapy | 12(30.0) | - |
| Bridge to Decision | 3(7.5) | - |
Table 2. Study Measures.
| Measure | Patient (Mean ± SD) |
Caregiver (Mean ± SD) |
Correlation* |
|---|---|---|---|
| Quality of Life† (possible range 0-100; higher scores indicate better quality of life) | |||
| Pre-Implant | 26.7 ± 20.8 | 52.9 ± 8.2 | 0.06 |
| 1-Month | 49.3 ± 23.7 | 51.5 ± 9.3 | 0.10 |
| 3-Months | 61.0 ± 20.1 | 49.8 ± 10.5 | -0.16 |
| Depression‡ (possible range 0-24; higher scores indicate greater depressive symptoms) | |||
| Pre-Implant | 10.2 ± 5.8 | 5.6 ± 4.9 | 0.02 |
| 1-Month | 6.8 ± 5.2 | 5.1 ± 4.7 | 0.40b |
| 3-Months | 4.4 ± 4.2 | 6.3 ± 5.5 | 0.31a |
| Anxiety§ (possible range 0-4; higher scores indicate greater anxiety) | |||
| Pre-Implant | 1.0 ± 0.8 | 0.7 ± 0.6 | -0.08 |
| 1-Month | 0.5 ± 0.7 | 0.6 ± 0.7 | 0.07 |
| 3-Months | 0.3 ± 0.4 | 0.6 ± 0.7 | 0.55c |
Note: Data collected pre-implant and at 1 and 3 months post-implant.
Model-based Pearson's correlations;
p<0.05,
p<0.01,
p<0.001
EuroQol Visual Analogue Scale
Patient Health Questionnaire-8
Brief Symptoms Inventory-Anxiety Subscale
Quality of Life
Patient and caregiver QOL was not correlated prior to implant or at 1 or 3 months post-implant. On average, patients had large, statistically significant improvements in QOL from pre- to post-implant (Figure 1). In contrast, caregiver QOL significantly worsened from pre- to post-implant. Based on the random effects modeling, worse caregiver QOL at baseline was correlated with greater improvements in patient QOL in response to VAD.
Figure 1. Patient and Caregiver Quality of Life.
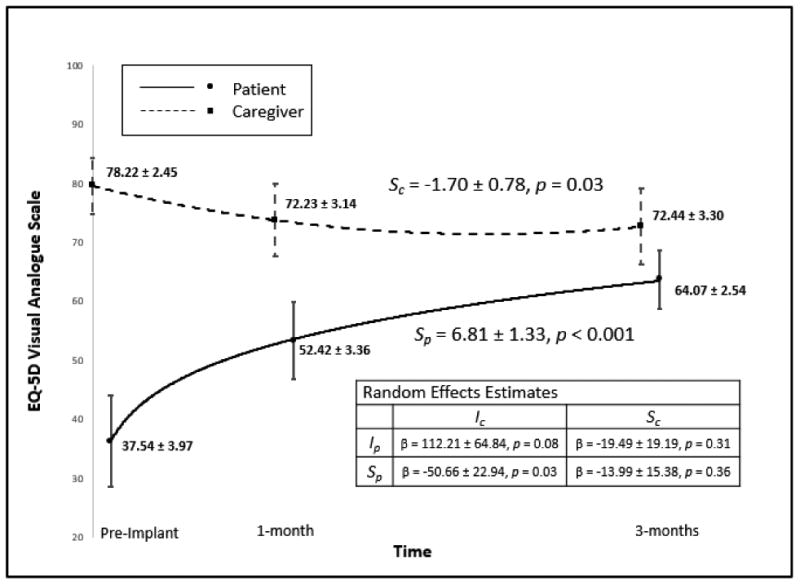
Change in patient and caregiver quality of life. Means and 95% confidence intervals are displayed for patients and caregivers at each time point. Note: Ip: patient intercept, Sp: patient slope (β ± SE), Ic: caregiver intercept, Sc: caregiver slope (β ± SE). Effect sizes for patient and caregiver slope in the metric of Hedges g were 0.80 and 0.34, respectively.
Depression
There was no significant correlation between patient and caregiver depressive symptoms prior to implant, but worse depression in patients was correlated with worse depression in caregivers at both 1 and 3 months post-implant (and vice versa). In response to VAD implantation, patients had large, statistically significant improvements in depressive symptoms from pre- to post-implant (Figure 2). In contrast, caregiver depressive symptoms remained relatively stable from pre- to post-implant; there was a small numerical increase (worsening depression) at 3 months that was not statistically significant. Finally, based on the random effects analysis there were no significant associations among pre-implant values or change over time in depression between patients and their caregivers.
Figure 2. Patient and Caregiver Depression.
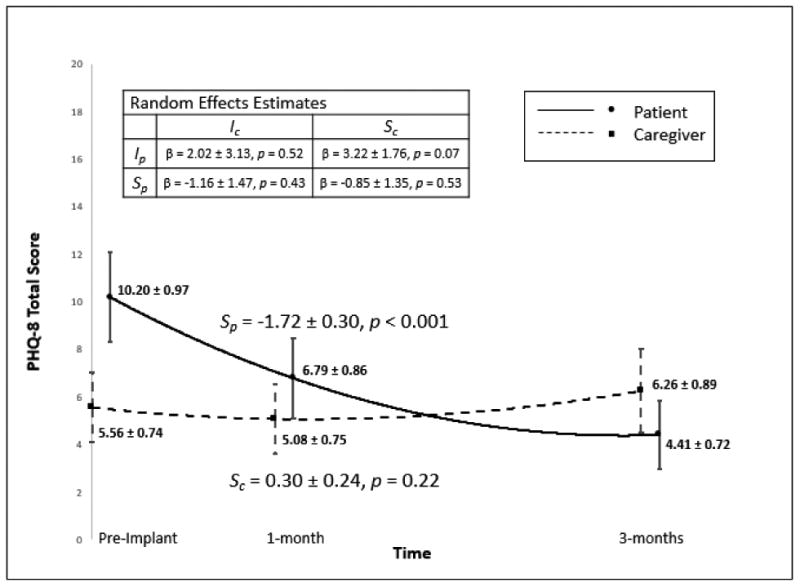
Change in patient and caregiver depression. Means and 95% confidence intervals are displayed for patients and caregivers at each time point. Note: Ip: patient intercept, Sp: patient slope (β ± SE), Ic: caregiver intercept, Sc: caregiver slope (β ± SE). Effect sizes for patient and caregiver slope in the metric of Hedges g were 0.87 and 0.19, respectively.
Anxiety
Patient and caregiver anxiety were not correlated prior to implant or at 1 month post-implant, but worse anxiety in patients was correlated with worse anxiety in caregivers at 3 months post-implant (and vice versa). On average, patients had moderate-to-large, statistically significant improvements in anxiety from pre- to post-implant (Figure 3). In comparison, caregiver anxiety remained relatively stable over time, as there was no statistical change from pre- to post-implant. Based on the random effects modeling, patients with worse anxiety pre-implant tended to have caregivers who reported less anxiety and vice-versa. Additionally, worse caregiver anxiety at baseline was associated with less reduction in patient anxiety in response to VAD.
Figure 3. Patient and Caregiver Anxiety.
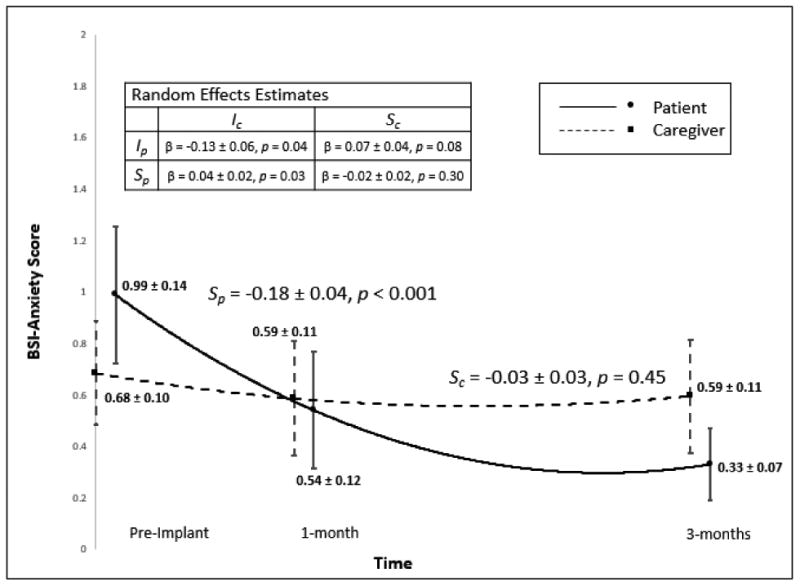
Change in patient and caregiver anxiety. Means and 95% confidence intervals are displayed for patients and caregivers at each time point. Note: Ip: patient intercept, Sp: patient slope (β ± SE), Ic: caregiver intercept, Sc: caregiver slope (β ± SE). Effect sizes for patient and caregiver slope in the metric of Hedges g were 0.68 and 0.12, respectively.
Discussion
In this sample of 41 patients undergoing VAD therapy and their caregivers, we observed improvement in person-oriented outcomes of QOL, depression, and anxiety among patients from pre- to post-VAD. Concurrently, patients' caregivers experienced worsening QOL, but relative stability in depression and anxiety. In this section, we discuss initial levels, change, and interdependence in person-oriented outcomes for patients and caregivers; implications for clinical practice and research; and study limitations.
While our findings of QOL improvement for VAD patients are consistent with the existing literature,8,9 this study adds novel quantitative information on how QOL changes for VAD caregivers from pre- to post-implantation. Pre-implant, caregivers in our sample had QOL scores that were equivalent to national norms.27 Over time, however, caregiver QOL significantly worsened, and at 1 and 3 months post-implant, average QOL scores were worse than healthy population averages.27 While we do not have an EQ-5D VAS comparator in HF caregiving, QOL scores in our sample were worse than those reported in stroke caregivers.28 Given that psychological distress is associated with poor QOL,29 it is possible that sustained depression and anxiety in our sample is a driver of compromised caregiver QOL. Additionally, substantial gains in VAD patient QOL alongside losses in caregiver QOL may signal a potential trade-off in patient and caregiver QOL post-VAD. This potential trade-off is also reflected in our random-effects findings, where worse caregiver QOL pre-implant was associated with greater improvements in patient QOL post-implant. These quantitative findings are consistent with qualitative reports of caregivers sacrificing their own health to care for the VAD patient,30 possibly as a mechanism for coping with elevated emotional distress post-VAD.31
Findings from this study are also consistent with what little has been published on depression and anxiety for VAD patients and caregivers. For example, patient depression and anxiety improve post-implantation,4,9,16 and similarly, we found substantial improvements for patients from pre- to post-VAD. For caregivers, VAD caregiving is characterized by high levels of anxiety from the point of decision onwards, and many caregivers report persistent fear of clinical events and uncertainty for the future (i.e. transplant potentiality or device failure).11,12,32-34 Additionally, patient physical symptoms have been previously associated with worse caregiver psychological health,35 and our sample – like the broader population of VAD patients – had advanced HF (NYHA Class III/IV) that is characterized by a heavy burden of symptoms. Hence, it not surprising that we observed caregiver depression and anxiety that were numerically worse than national norms and published averages in HF caregiving samples.36-39 However, it is important to note that we did not statistically compare these published samples with our own, and we observed a substantial amount of variability (i.e. large standard deviations) in both depression and anxiety in our caregiving sample. Furthermore, at 3 months post-VAD, caregiver depression and anxiety were numerically worse than patient depression and anxiety on average – a finding which is consistent with previous work in VAD.16,40 Additionally, we observed worse caregiver anxiety pre-implant was associated with less improvement in patient anxiety over time, and increasing correlations between patient and caregiver depression and anxiety.
Our correlational and random effects findings for patients and caregivers in regards to anxiety are novel and somewhat unintuitive, having not previously been reported in VAD dyads or similar caregiving contexts. In particular, we found that worse patient anxiety at the pre-implant stage of the trajectory was associated with less caregiver anxiety, but at 3 months post-implant worse patient anxiety was associated with worse caregiver anxiety. Given the pathogenesis of advanced HF, it is expected that patients would have worse anxiety than caregivers pre-implant. However, why an inverse relationship between patients and caregivers exists in our sample is less clear and requires further inquiry into patient HF symptom biomechanics and associated caregiver response across the trajectory of HF. It is possible that patients who are the most anxious are also the most sick, and caregivers of these patients (who face severe and life-threatening illness with few treatment options) respond to the prospect of VAD therapy with hope and optimism, both of which have been shown to buffer symptoms of anxiety.41 As HF improves in response to device therapy, however, patients likely return to the level of affective symptoms that existed in the dyadic relationship prior to HF becoming advanced, while caregivers appear to remain relatively stable. Thus, given the large proportion of spousal dyads in our sample, a return to some level of normalcy may also facilitate a return to within-dyad sharing of psychological distress, a phenomenon called emotional contagion42 that has also been observed in stroke dyads.43 It is also important to note that, given the small sample size, these analyses do not contain covariates. Other individual or dyadic effects may influence trajectories of change or relationships between trajectories, a limitation of this work that necessitates further research with larger and more heterogeneous samples. However, overall the results of this preliminary analysis demonstrate that the experience and outcomes of one member of the caregiving dyad also influence the other member, and vice versa, supporting a need to examine VAD patients and caregivers within the context of the caregiving relationship.13,44
This is the first study in continuous-flow devices to quantify change over time in caregiver person-oriented outcomes from pre-implant through post-implant. Given the dramatic changes in patient outcomes, it is important to discuss the contrasting finding of relative stability in caregivers. First, VAD caregivers typically transition into VAD caregiving with a substantial amount of HF caregiving experience,30 and have described VAD caregiving as different but equally challenging.12 Furthermore, caregivers have characterized the VAD experience as a “24/7” role even months after implant45 and report distress at all phases (from pre-implant evaluation through support).32 This may explain both the burden of depression and anxiety at baseline and lack of change over time. It is also important to note that “no average change” is not equivalent to “no change,” given that substantial variability in change impeded our ability to precisely estimate slope coefficients for caregiver depression and anxiety. In particular, we observed substantial variability in caregiver anxiety, depression, and QOL at each time point and over time, as evidenced by large standard deviations and wide 95% confidence intervals. Furthermore, it is important to note that, while the patient underwent a major clinical intervention (VAD), the caregiver did not; thus, we would not expect consistent caregiver response in a therapeutic direction. Rather, our findings of interdependence and variability in change for patients and caregivers provides support for examining and quantifying potential subgroups of dyads that respond well or poorly to VAD therapy together, in order to identify dyads that may be at particularly high risk for poor outcomes.
Implications
This study has several clinical and research implications. First, variability in responses to VAD implant limit our ability to provide anticipatory guidance to patients in general and family caregivers in particular. There remains an imminent need for research that provides clarity on which patients and caregivers are at highest risk, and how they can be supported together to optimize person-oriented outcomes. Second, we observed correlations in person-oriented outcomes within dyads over time. Given that VAD therapy is often a long-term intervention, research is needed that examines patients and caregivers together to inform clinical strategies that best approximate the real-world context in which patients and caregivers jointly manage the device. Third, we observed a substantial burden of emotional distress for caregivers and a significant decline in QOL. Lack of preparation for the psychological burden of caregiving and the necessity of support systems for caregivers and patients are well-documented,5,11,33 however, little is known about how support should be structured or potential costs/benefits. Importantly, despite difficulties, most patients and caregivers have minimal decisional regret.12,46 Thus, in order to advance the science towards supporting patients and caregivers together and identifying when and for whom psychosocial and dyadic interventions are most appropriate, we need investment in research that includes both patients and caregivers, and that employs robust methods to handle dyadic data.
Limitations
This study has limitations. First, this was a relatively small, single-site sample of primarily middle-aged, white male patients and female caregivers, almost all of whom were couples. Future work with analyses by age, gender, relationship type, and more diverse racial and ethnic backgrounds is needed. Second, out of necessity we excluded a small number of patients who did not have a primary caregiver at the time of implant; thus, these findings may not be generalizable to rare situations where a VAD is placed without clear caregiving support. Finally, this current analysis was unadjusted, and followed patients and caregivers through 3 months post-implant; our future work will contain follow-up through 6 months and will control for the effects of potential confounders.
Conclusions
In this study of person-oriented outcomes in VAD patients and caregivers, we found that VAD caregiver QOL worsened from pre- to post-implant, while patient QOL improved significantly. VAD patient depression and anxiety also improved, while caregivers reported substantial depression and anxiety pre-implant that did not improve over time. Given the morbidity and mortality associated with end-stage HF and VAD, it is likely normative for patients and caregivers to feel anxious and/or depressed prior to implant. For patients, these symptoms will most likely improve post-implantation, but for caregivers, it is reasonable to recommend supportive therapy (e.g. psychological services) across the spectrum of VAD support to manage persistent anxiety and depression and potentially mitigate compromises to QOL. Furthermore, given the relationships between patient and caregiver outcomes and the transactional nature of the caregiving dyad, there may be particular benefit to referring patients and caregivers together for supportive services (e.g. joint patient-caregiver counseling). However, in order to better identify and support patients and caregivers who are at greatest risk for poor outcomes, future research is needed that examines how patients and caregivers respond to VAD therapy together, within the context of the caregiving dyad.
Acknowledgments
Funding: This work was supported by the National Institutes of Health/ National Institute of Nursing Research F31NR014760 (Bidwell) and R01NR013492 (Lee).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Conflicts of Interest: None declared.
Contributor Information
Julie T. Bidwell, Oregon Health & Science University School of Nursing, Portland, OR.
Karen S. Lyons, Oregon Health & Science University School of Nursing, Portland, OR.
James O. Mudd, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Jill M. Gelow, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Christopher V. Chien, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
Kathleen L. Grady, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Christopher S. Lee, Oregon Health & Science University School of Nursing and Knight Cardiovascular Institute, Portland, OR.
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015 doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Grady KL, Warner Stevenson L, Pagani FD, et al. Beyond survival: recommendations from INTERMACS for assessing function and quality of life with mechanical circulatory support. J Heart Lung Transplant. 2012;31(11):1158–1164. doi: 10.1016/j.healun.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers C, Denollet J, de Jonge N, Caliskan K, Kealy J, Pedersen SS. Patient-reported outcomes in left ventricular assist device therapy: a systematic review and recommendations for clinical research and practice. Circ Heart Fail. 2011;4(6):714–723. doi: 10.1161/CIRCHEARTFAILURE.111.962472. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal-Barby JS, Kostick KM, Delgado ED, et al. Assessment of patients' and caregivers' informational and decisional needs for left ventricular assist device placement: Implications for informed consent and shared decision-making. J Heart Lung Transplant. 2015;34(9):1182–1189. doi: 10.1016/j.healun.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peura JL, Colvin-Adams M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126(22):2648–2667. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Allen JG, Weiss ES, Schaffer JM, et al. Quality of life and functional status in patients surviving 12 months after left ventricular assist device implantation. J Heart Lung Transplant. 2010;29(3):278–285. doi: 10.1016/j.healun.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady KL, Naftel D, Stevenson L, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2014;33(4):412–421. doi: 10.1016/j.healun.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlrup B, Ekstrom H, Nordell E, Elmstahl S. Coping as a caregiver: a question of strain and its consequences on life satisfaction and health-related quality of life. Arch Gerontol Geriatr. 2015;61(2):261–270. doi: 10.1016/j.archger.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick JN, Kellom K, Hull SC, et al. Caregivers and Left Ventricular Assist Devices as a Destination, Not a Journey. J Card Fail. 2015;21(10):806–815. doi: 10.1016/j.cardfail.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Kitko LA, Hupcey JE, Gilchrist JH, Boehmer JP. Caring for a spouse with end-stage heart failure through implantation of a left ventricular assist device as destination therapy. Heart Lung. 2013;42(3):195–201. doi: 10.1016/j.hrtlng.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychol Bull. 2007;133(6):920–954. doi: 10.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi RB, Piette J, Fihn SD, Edelman D. Examining the interrelatedness of patient and spousal stress in heart failure: conceptual model and pilot data. J Cardiovasc Nurs. 2012;27(1):24–32. doi: 10.1097/JCN.0b013e3182129ce7. [DOI] [PubMed] [Google Scholar]
- 15.Rohrbaugh MJ, Shoham V, Cleary AA, Berman JS, Ewy GA. Health consequences of partner distress in couples coping with heart failure. Heart Lung. 2009;38(4):298–305. doi: 10.1016/j.hrtlng.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers C, Denollet J, Caliskan K, et al. Psychological distress in patients with a left ventricular assist device and their partners: an exploratory study. Eur J Cardiovasc Nurs. 2015;14(1):53–62. doi: 10.1177/1474515113517607. [DOI] [PubMed] [Google Scholar]
- 17.Lee CS, Mudd JO, Gelow JM, et al. Background and design of the profiling biobehavioral responses to mechanical support in advanced heart failure study. J Cardiovasc Nurs. 2014;29(5):405–415. doi: 10.1097/JCN.0b013e318299fa09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43(6):607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 20.Maciver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation. 2012;126(7):866–874. doi: 10.1161/CIRCULATIONAHA.111.040279. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):1–7. [Google Scholar]
- 22.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 23.Willett JB, Sayer AG. Using covariance structure analysis to detect correlates and predictors of individual change over time. Psychological Bulletin. 1994;116(2):363–381. [Google Scholar]
- 24.Willett JB, Sayer AG. Cross-Domain Analysis of Change Over Time: Combining Growth Modeling and Covariance Structure Analysis. In: Marcoulides GA, Schumacker RE, editors. Advanced Structural Equation Modeling. New York, NY: Psychology Press; 1996. pp. 125–157. [Google Scholar]
- 25.Hedges L. Distribution theory for Glass' estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6(2):107–128. [Google Scholar]
- 26.Muthen BO, Kaplan D, Hollis M. On structural equation modeling with data that are not missing completely at random. Psychometrika. 1987;52(3):431–462. [Google Scholar]
- 27.Szende A, Janssen B, Cabases J. Self-reported population health: An international perspective based on EQ-5D. Springer Netherlands; 2014. Population norms for the EQ-5D. [PubMed] [Google Scholar]
- 28.McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke. 2005;36(10):2181–2186. doi: 10.1161/01.STR.0000181755.23914.53. [DOI] [PubMed] [Google Scholar]
- 29.Pinquart M, Sorensen S. Correlates of physical health of informal caregivers: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):P126–137. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- 30.Baker K, Flattery M, Salyer J, Haugh KH, Maltby M. Caregiving for patients requiring left ventricular assistance device support. Heart Lung. 2010;39(3):196–200. doi: 10.1016/j.hrtlng.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Egerod I, Overgaard D. Taking a back seat: support and self-preservation in close relatives of patients with left ventricular assist device. Eur J Cardiovasc Nurs. 2012;11(4):380–387. doi: 10.1177/1474515111435609. [DOI] [PubMed] [Google Scholar]
- 32.Kaan A, Young QR, Cockell S, Mackay M. Emotional experiences of caregivers of patients with a ventricular assist device. Prog Transplant. 2010;20(2):142–147. doi: 10.1177/152692481002000208. [DOI] [PubMed] [Google Scholar]
- 33.Akbarin M, Aarts C. Being a close relative of a patient with a left ventricular assist device. Eur J Cardiovasc Nurs. 2013;12(1):64–68. doi: 10.1177/1474515111432996. [DOI] [PubMed] [Google Scholar]
- 34.Marcuccilli L, Casida JJ, Bakas T, Pagani FD. Family caregivers' inside perspectives: caring for an adult with a left ventricular assist device as a destination therapy. Prog Transplant. 2014;24(4):332–340. doi: 10.7182/pit2014684. [DOI] [PubMed] [Google Scholar]
- 35.Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):P112–128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- 36.Kocalevent RD, Hinz A, Brahler E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2013;35(5):551–555. doi: 10.1016/j.genhosppsych.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Hwang B, Fleischmann KE, Howie-Esquivel J, Stotts NA, Dracup K. Caregiving for patients with heart failure: impact on patients' families. Am J Crit Care. 2011;20(6):431–441. doi: 10.4037/ajcc2011472. quiz 442. [DOI] [PubMed] [Google Scholar]
- 38.Pressler SJ, Gradus-Pizlo I, Chubinski SD, et al. Family caregivers of patients with heart failure: a longitudinal study. J Cardiovasc Nurs. 2013;28(5):417–428. doi: 10.1097/JCN.0b013e3182563877. [DOI] [PubMed] [Google Scholar]
- 39.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: testing dyadic dynamics using Actor-Partner Interdependence Model. J Psychosom Res. 2009;67(1):29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunzel B, Laederach-Hofmann K, Wieselthaler G, Roethy W, Wolner E. Mechanical circulatory support as a bridge to heart transplantation: what remains? Long-term emotional sequelae in patients and spouses. J Heart Lung Transplant. 2007;26(4):384–389. doi: 10.1016/j.healun.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 41.Rajandram RK, Ho SM, Samman N, Chan N, McGrath C, Zwahlen RA. Interaction of hope and optimism with anxiety and depression in a specific group of cancer survivors: a preliminary study. BMC Res Notes. 2011;4:519. doi: 10.1186/1756-0500-4-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatfield E, Cacioppo JT, Rapson RL. Emotional Contagion. Cambridge, MA: Cambridge University Press; 1994. [Google Scholar]
- 43.McCarthy MJ, Lyons KS, Powers LE. Expanding poststroke depression research: movement toward a dyadic perspective. Top Stroke Rehabil. 2011;18(5):450–460. doi: 10.1310/tsr1805-450. [DOI] [PubMed] [Google Scholar]
- 44.Bidwell JT, Lyons KS, Lee CS. Caregiver well-being and patient outcomes in heart failure: A meta-analysis. J Cardiovasc Nurs. doi: 10.1097/JCN.0000000000000350. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcuccilli L, Casida JM. From insiders' perspectives: adjusting to caregiving for patients with left ventricular assist devices. Prog Transplant. 2011;21(2):137–143. doi: 10.1177/152692481102100209. [DOI] [PubMed] [Google Scholar]
- 46.McIlvennan CK, Jones J, Allen LA, et al. Decision-making for destination therapy left ventricular assist devices: implications for caregivers. Circ Cardiovasc Qual Outcomes. 2015;8(2):172–178. doi: 10.1161/CIRCOUTCOMES.114.001276. [DOI] [PMC free article] [PubMed] [Google Scholar]


