Abstract
We tested the hypothesis that brain white matter integrity mediates the relationship between phenylalanine (Phe) control and executive abilities in children with phenylketonuria (PKU; N = 36). To do so, we examined mean diffusivity (MD) from diffusion tensor imaging (DTI) in two white matter brain regions (posterior parietal–occipital, PPO; centrum semiovale, CSO) and lifetime phenylalanine (Phe) exposure; the executive abilities examined included verbal strategic processing, nonverbal strategic processing, and working memory. Mediation modeling showed that MD in the PPO and CSO mediated the relationship between Phe exposure and nonverbal strategic processing, MD in the CSO mediated the relationship between Phe exposure and verbal strategic processing, and MD in the PPO mediated the relationship between Phe exposure and working memory. These exploratory findings demonstrate the importance of using sophisticated modeling procedures to understand the interplay among metabolic control, neural factors, and functional outcomes in individuals with PKU.
Keywords: Brain, Executive abilities, Mediation, Neuroimaging, Phenylketonuria, White matter
Introduction
Phenylketonuria (PKU; 261600) is an inherited metabolic disorder associated with a deficiency in or absence of the phenylalanine hydroxylase enzyme (EC 1.14.16.1). As a consequence, the amino acid phenylalanine (Phe) is improperly metabolized, which leads to higher than normal Phe levels (De Groot et al. 2010). Although serious cognitive sequelae are generally avoided through early detection and dietary treatment to limit Phe intake (Mitchell et al. 2011; Paine 1957), individuals with early and continuously treated PKU often have lower than expected intellectual abilities (Waisbren et al. 2007), as well as impairments in processing speed (Janos et al. 2012) and executive abilities (Christ et al. 2010; DeRoche and Welsh 2008).
For decades it has been hypothesized that PKU-related cognitive impairment is associated with dopamine deficiency (De Groot et al. 2010), because elevations in Phe disrupt the neurochemical cascade by which Phe is converted to tyrosine, a precursor of dopamine, and other catecholaminergic neurotransmitters (Scriver 2007). Compromised white matter integrity in the brain, however, is another possible mechanism underlying PKU-related cognitive impairment and represents the focus of the current study. Recent research using diffusion tensor imaging (DTI) indicates that white matter compromise is widespread throughout the brain in individuals with early and continuously treated PKU (Antenor-Dorsey et al. 2013; Hood et al. 2014a). More specifically, although fractional anisotropy (FA; reflecting the degree of water diffusion asymmetry) is relatively normal, mean diffusivity (MD; reflecting degree of displacement of water molecules) is significantly decreased (Antenor-Dorsey et al. 2013; Scarabino et al. 2009; White et al. 2010, 2013).
In terms of relationships between white matter integrity, cognition, and Phe control in individuals with PKU, a number of studies have shown that higher Phe is associated with both decreases in MD (e.g., Vermathen et al. 2007; Hood et al. 2014a) and poorer cognition (e.g., Christ et al. 2010; Weglage et al. 2013). Our knowledge of relationships between DTI findings and cognition is quite limited, but the three relevant studies to date point to associations between MD across a range of brain regions and IQ (Peng et al. 2004, 2013; Antenor-Dorsey et al. 2013). There is, however, no research in which the interplay among white matter integrity, cognition, and Phe control has been modeled within a single study. The purpose of this exploratory study was to address this substantial gap in our knowledge of PKU. Specifically, we tested the hypothesis that white matter integrity mediates the relationship between Phe control and executive abilities.
Material and Methods
Participants
Children with PKU (n = 36; 17 males, 19 females) were diagnosed soon after birth and received early dietary management to limit Phe intake. Phe levels were obtained from heparinized plasma done via MS/MS in children fasted for a minimum 2.5–3 h before the blood draw. Lifetime Phe levels, with gaps of no more than 2 years prior to neuroimaging and cognitive evaluation, were available for all children and ranged from 0 to 2742 μmol/L (sample; M = 371.1, SD = 282.5, males; M = 420.3, SD = 303.9, females; M = 352.6, SD = 271.9). Age ranged from 6 to 18 years (sample; M = 12.2, SD = 3.8, males; M = 12.3, SD = 3.7, females; M = 12.2, SD = 4.1), education ranged from 0 to 13 years (M = 6.4, SD = 3.8), and IQ ranged from 75 to 122 (sample; M = 102.1, SD = 10.9, males; M = 75, SD = 117, females; M = 86, SD = 122). No child had a reported history of major medical, psychiatric, or learning disorder unrelated to PKU, and no child was treated with sapropterin dihydrochloride at the time of study.
Procedures
Approval to conduct this study was obtained from institutional review boards for the protection of human subjects at Washington University in St. Louis (WU) and Oregon Health & Science University (OHSU). All participants and/or guardians provided written informed consent prior to initiation of study procedures. Referring metabolic clinics provided blood Phe levels over the lifetime based on medical records. Executive and neuroimaging procedures were administered during a single session lasting approximately 4 h. Some data reported here were used in previous reports (e.g., Hood et al. 2014a, b) but not in relation to mediation modeling.
Index of Phe Control
Mean Phe exposure over the lifetime was computed based on all available Phe levels prior to evaluation. The number of lifetime blood Phe levels for individual children who participated in our study ranged from 86 to 466 (M = 215.0, SD = 98.0). The rationale for examining this index is detailed elsewhere (Hood et al. 2014a). Briefly, mean Phe exposure was computed to take into account the duration (i.e., years) and accumulative effects of exposure to elevations in Phe, because older children with PKU have experienced more prolonged exposure to elevated Phe than younger children. As the first step in calculating mean exposure, we obtained the mean and standard deviation of lifetime Phe and age across the entire sample of children with PKU. Z scores for lifetime Phe and age were then computed for each child based on the means and standard deviations of the sample. Mean exposure for each child was then calculated by summing the z scores for lifetime Phe and age. This method of calculation for mean exposure (M = 0, SD = 1.8, range = −2.5–4.5) results in scores that approximate a normal distribution, with higher scores indicating greater exposure.
Executive Abilities
The Matrix Reasoning subtest from the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999) was used to measure nonverbal strategic processing. During this task, children viewed series of incomplete matrices and selected which of five solutions best completed each matrix. The number of correct completions was recorded. A verbal wordlist learning task was used to measure verbal strategic processing. Children listened as a list of 18 words was read aloud then orally recalled as many words as possible in any order over five learning trials. The list comprised six words from each of three semantic categories (e.g., furniture, food, and parts of the body). A ratio reflecting the number of words reported serially in semantic clusters to the total number of words recalled over the five trials was recorded. An n-back task with two conditions, location and letter, was used to measure working memory. Children observed 1 of 8 letters (C, F, H, J, N, P, Q, and S) appearing alone at 1 of 8 locations along an imaginary circle on a computer monitor. In the location condition, children pressed a button when any letter appeared in the same location as two trials previously; in the letter condition, children pressed a button when the letter presented was identical to the letter presented two trials earlier. Otherwise, children withheld responses. The mean number of correct nonresponses averaged across location and letter conditions was recorded.
Standard scores (M = 100, SD = 15) from each task were used in mediation analyses. Matrix Reasoning standard scores in children with PKU (M = 99.3, SD = 11.1, range = 74.5–116.5) were based on age-referenced normative data that accompanied the subtest. Normative data were not available for the experimental wordlist learning and n-back tasks. However, to provide a similar interpretative context, we computed age-referenced standard scores for each task for children with PKU (wordlist learning: M = 99.4, SD = 8.2, range = 86.2–116.5; n-back task: M = 100.6, SD = 7.2, range = 89.0–113.1) based on data previously collected in our laboratory from a group of 80 healthy control children who ranged in age from 7 to 18 years (M = 12.4, SD = 3.2).
White Matter Integrity
Children were scanned with a 3.0T Siemens Trio at OHSU and with a 1.5T Siemens Sonata at WU. DTI was acquired using an echo planar imaging (EPI) sequence (TR = 9,000 ms, TE = 84 ms (OHSU) and 78 ms (WU), 2.5 mm (OHSU) and 3.0 mm (WU) isotropic voxels, conventional hexahedral (6 direction) encoding with diffusion sensitization of b-values = 0 and 1,000 s/mm2). Four complete DTI datasets were acquired for each participant, with a total imaging time of approximately 1 h. The first image-processing step was registration of all images. We defined the spatial relationships between all images in terms of affine transforms. T2W image registration was accomplished using vector gradient measure maximization. The first acquired, unsensitized (b = 0 s/mm2; I0) DTI volume was registered to the T2W image; stretch and shear were enabled (12 parameter affine transform) to partially compensate for EPI distortion. The remaining DTI images were then registered to the unsensitized DTI volume. The diffusion tensor and its three eigenvalues were calculated using log-linear regression in each voxel for each ROI (Shimony et al. 1999). Using standard methods, the DTI parameters were computed from the eigenvalues.
To minimize false-positives, we focused on two white matter brain regions: posterior parietal–occipital (PPO) and centrum semiovale (CSO) (see Fig. 1). These regions were selected based on a well-established DTI atlas (Oishi et al. 2008), and placement of ROIs was compared on each participant’s FA map and TW2 images simultaneously. ROIs were shifted by a few voxels as necessary by a trained neuroradiology technician to better conform to each individual’s native anatomy. Finalized ROIs were then applied to each subject’s mean diffusivity, axial diffusivity, and radial diffusivity parametric maps and sampled using Analyze 8.0 software similar to the methods of Shimony et al. 1999. Raters have established interrater correlation coefficients above 0.90 for mean diffusivity values for all ROIs.
Fig. 1.

ROI placement
We have previously shown that MD in these ROIs was significantly lower in individuals with PKU than in age-matched controls (White et al. 2013) and that MD in these ROIs was related to a range of indices of Phe control (Hood et al. 2014b). In addition, visually observable white matter abnormalities often occur in the PPO and CSO in individuals with PKU (Citton et al. 2012). For mediation analyses, standard scores were generated for MD based on data from a subset (N = 62; not all children completed neuroimaging) of the healthy control children whose data were used to generate executive abilities standard scores.
Data Analyses
As a starting point in our analyses, we conducted Pearson correlations to determine the simple bivariate relationships between mean Phe exposure over the lifetime, MD in the PPO and CSO, and executive abilities. Statistical rigor was increased by considering findings significant only if p < 0.05 and effect sizes were either medium or large (Cohen 1988).
For mediation analyses, we used a bootstrapping approach, which is a non-parametric resampling procedure for the assessment of indirect effects (Preacher and Hayes 2004, 2008). This method used an ordinary least squares regression-based path analytic framework for estimating direct and indirect effects. Mediation analyses indicate whether the total effect (weight c) of an independent variable (IV; mean Phe exposure) on a dependent variable (DV; nonverbal strategic processing, verbal strategic processing, or working memory) comprises a direct effect (weight c’) of an IV on a DV and an indirect effect (weight a × b) of an IV on a DV through a predicted mediator (MD). Weight a denotes the effect of an IV on a mediator, whereas weight b denotes the effect of a mediator on a DV. From our original dataset of 36 cases, random sampling with replacement generated a bootstrap sample of 36 cases. Repeating the process 5,000 times provided the basis for the bootstrap estimates. Means and standard errors of the 5,000 samples were then calculated.
Current recommendations indicate that inferences should not be based on the significance of paths a and b; instead, inferences should be an explicit quantification of the indirect effect, and significant indirect effects can occur in the absence of significant total or direct effects (Preacher and Hayes 2004, 2008). Given our small sample size, we focused on Kappa-squared (κ 2) to measure effect size because it is standardized and insensitive to sample size. Kappa-squared represents the proportion of the total possible effect in the sample and it can be interpreted analogous to R 2, with a kappa-squared of 0.01, 0.09, and 0.25 representing small, medium, and large effects, respectively. In addition, due to small sample size and the exploratory nature of the current study, there was no correction for multiple statistical comparisons. To increase statistical rigor, indirect effects were considered significant only if zero was not within the 95% confidence interval (CI), p < 0.05 two tailed, and effect size was medium or large. Given that three aspects of executive abilities and two ROIs were examined, a total of six mediation analyses were conducted.
We would also note that exploratory mediation analyses were conducted to determine whether subcomponents of MD, including axial diffusivity (AD) and radial diffusivity (RD), mediated the relationship between Phe exposure and executive abilities. Because results from these analyses were not explanatory beyond those conducted using MD, they are not discussed further.
Results
Correlation Analyses
Pearson correlations indicated that MD in the PPO was significantly related to nonverbal strategic processing and working memory, whereas MD in the CSO was significantly related to nonverbal and verbal strategic processing. MD in both the PPO and CSO was significantly related to mean Phe exposure. Executive abilities were not significantly related to mean Phe exposure, but because significant indirect effects may be present in the absence of total or direct relationships, we next conducted mediation analyses (Preacher and Hayes 2004; Rucker et al. 2011) (Table 1).
Table 1.
Correlations between MD, Phe exposure, and executive abilities
| Variable | PPO | CSO |
|---|---|---|
| Nonverbal strategic processing | 0.34* | 0.35* |
| Verbal strategic processing | 0.22 | 0.41* |
| Working memory | 0.36* | 0.19 |
| Mean Phe exposure | −0.65* | −0.41* |
Note: * = p < 0.05 with medium or large effect sizes
Mediation Analyses
Of the six mediation analyses conducted, four yielded the statistically significant results of interest that are discussed here. As shown in Fig. 1, neither the total (weight c) nor direct (weight c’) effects of mean Phe exposure on nonverbal strategic processing were significant in analyses including either PPO or CSO as mediators (panels a and b, respectively).
Of greater interest, the indirect effects of mean Phe exposure on nonverbal strategic processing through both the PPO (panel a; 95% CI entirely below zero, κ 2 = 0.24, medium effect) and CSO (panel b; 95% CI entirely below zero, κ 2 = 0.14, medium effect) were statistically significant. In terms of verbal strategic processing (panel c), with CSO as the mediator, the total effect (weight c) of mean Phe exposure on verbal strategic processing was not significant, although the direct effect (weight c’) was significant. More importantly, the indirect effect of mean Phe exposure on verbal strategic processing through the CSO was statistically significant (95% CI entirely above zero, κ 2 = 0.13, medium effect). Turning to working memory (panel d), with PPO as the mediator, neither the total (weight c) nor direct (weight c’) effects of mean Phe exposure on working memory were significant. The indirect effect of mean Phe exposure on working memory through PPO, however, was statistically significant (95% CI below zero, κ 2 = 0.26, large effect) (Fig. 2).
Fig. 2.
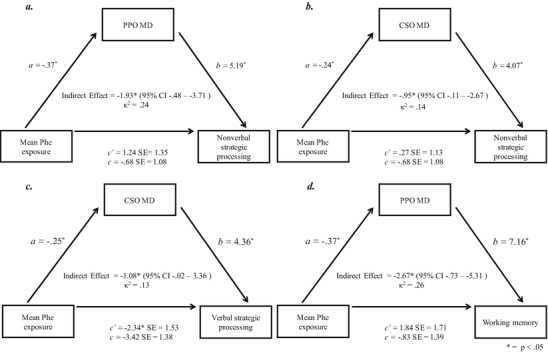
PPO and CSO as mediators of the relationships between mean Phe exposure and executive abilities
Discussion
White matter compromise in individuals with PKU has been related to poorer Phe control (Anderson et al. 2007; Das et al. 2013; Hood et al. 2014a), and this relationship has been shown more consistently when examined across the lifetime rather than at discrete points (Viau et al. 2011). More rarely, significant relationships have been shown between white matter integrity and cognition in individuals with PKU (Peng et al. 2004; Antenor-Dorsey et al. 2013). This is somewhat surprising, as compromised white matter integrity has been related to poorer cognition in many other neurological disorders (Chiaravalloti and DeLuca 2008).
There are a number of possible reasons why direct relationships between white matter integrity and cognition have rarely been found in studies of PKU. First, cognition has most often been examined in relation to observable white matter abnormalities using structural MRI, which makes it difficult to identify subtle relationships (Pietz et al. 1996; Weglage et al. 2013). In contrast, DTI permits detection of subtle disruptions in microstructural white matter integrity that are not detectable on structural images. Second, in previous studies, cognition has often been investigated using only IQ (Rupp et al. 2001). Such global measures, although useful, are less specific and do not address relationships with executive abilities, which are often impaired in individuals with PKU (Anderson et al. 2007; Christ et al. 2006). Finally, other studies have assessed relationships between cognition and white matter compromise in adults rather than children. It is possible that white matter compromise particularly affects cognition during earlier development.
In our exploratory study, mediation was used to assess the indirect effects of microstructural white matter integrity in PPO and CSO brain regions on the relationship between Phe exposure over the lifetime and executive abilities. Although both Phe and MD have been related to cognitive outcomes, such direct effects are not necessarily predictive of indirect effects. In fact, in our study, only one of the four significant mediation models showed a significant direct effect of Phe on cognition. Overall, results from our study are the first to show that white matter integrity mediates the relationship between Phe and executive abilities and suggest that white matter integrity may be a sensitive marker of cognitive dysfunction. Our results also suggest that to fully understand the complex interplay between metabolic control, white matter integrity, and executive abilities in children with PKU, all of these domains should be analyzed in conjunction.
In addition to the strengths of our study, such as the use of mediation and DTI, there are limitations that should be acknowledged. For example, our exploratory study had a small sample size, which could have resulted in decreased power to detect additional significant effects, and so requires replication in a larger sample. In addition, because the study was cross-sectional, causality was implied rather than determined. Future longitudinal research will be helpful in determining whether white matter integrity in childhood predicts executive abilities later in life and whether improvements in white matter integrity and in turn executive abilities can be obtained through lower lifetime Phe exposures.
Despite these limitations, our study provides unique information about neural processes that may affect cognition in children with PKU. Mediation analyses are rare in PKU research and have not previously been conducted in relation to executive abilities. We suggest that these types of analyses are crucial if we are to understand the complex relationships between metabolic control, neural factors, and functional outcomes in individuals with PKU.
Acknowledgments
The authors wish to thank those who participated in our research for their contributions. We also thank Suzin Blankenship and Laurie Sprietsma for their contributions to study management, as well as the physicians and staff of Washington University and Oregon Health & Science University who generously contributed to the study through recruitment and phenylalanine monitoring.
Funding
This research was supported by the National Institute of Child Health and Human Development (R01HD044901 and U54HD087011), an Investigator Sponsored Trial grant from BioMarin Pharmaceutical Inc., the Intellectual and Developmental Disabilities Research Center at Washington University with funding from the National Institute of Child Health and Human Development (P30HD062171) and the James S. McDonnell Foundation.
Compliance with Ethics Guidelines
Conflict of Interest
Anna Hood, Jerrel Rutlin, and Joshua Shimony declare that they have no conflict of interest. Desiree White and Dorothy Grange have received research grants from BioMarin Pharmaceutical Inc. and have served as consultants for BioMarin Pharmaceutical Inc.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients included in the study.
Contribution of Authors
Desiree White designed the study, wrote the protocol, trained research staff, supervised data collection, interpreted data, and co-wrote the manuscript. Anna Hood conducted literature review, analyzed, and interpreted data, and co-wrote the manuscript. Jerrel Rutlin analyzed and interpreted data and provided input on the writing of the paper. Joshua Shimony contributed to study design and provided statistical analysis, neuroimaging consultation, and input on the writing of the paper. Dorothy Grange contributed to study design, participant recruitment, and provided input on the writing of the paper. All authors contributed to and have approved the final manuscript.
Contributor Information
Anna Hood, Email: annahood@wustl.edu.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Dev Neuropsychol. 2007;32(2):645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- Antenor-Dorsey JAV, Hershey T, Rutlin J, Shimony JS, McKinstry RC, Grange DK, White DA. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab. 2013;109(2):125–131. doi: 10.1016/j.ymgme.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Christ SE, Steiner RD, Grange DK, Abrams RA, White DA. Inhibitory control in children with phenylketonuria. Dev Neuropsychol. 2006;30(3):845–864. doi: 10.1207/s15326942dn3003_5. [DOI] [PubMed] [Google Scholar]
- Christ SE, Huijbregts SCJ, de Sonneville LMJ, White DA. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol Genet Metab. 2010;99:S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Citton V, Burlina A, Baracchini C, Gallucci M, Catalucci A, Dal Pos S, Burlina A, Manara R. Apparent diffusion coefficient restriction in the white matter: going beyond acute brain territorial ischemia. Insights Imaging. 2012;3(2):155–164. doi: 10.1007/s13244-011-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- Das AM, Goedecke K, Meyer U, Kanzelmeyer N, Koch S, Illsinger S et al (2013) Dietary habits and metabolic control in adolescents and young adults with phenylketonuria: self-imposed protein restriction may be harmful. In: JIMD reports-case and research reports, vol 13. Springer, Heidelberg, pp 149–158 [DOI] [PMC free article] [PubMed]
- De Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, Van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99:S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Dev Neuropsychol. 2008;33(4):474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- Hood A, Antenor-Dorsey JAV, Rutlin J, Hershey T, Shimony JS, McKinstry RC et al (2014) Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol Genet Metab 114(1):19–24 [DOI] [PMC free article] [PubMed]
- Hood A, Grange DK, Christ SE, Steiner R, White DA. Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol Genet Metab. 2014;111(4):445–451. doi: 10.1016/j.ymgme.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janos AL, Grange DK, Steiner RD, White DA. Processing speed and executive abilities in children with phenylketonuria. Neuropsychology. 2012;26(6):735. doi: 10.1037/a0029419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med. 2011;13(8):697–707. doi: 10.1097/GIM.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X et al (2008) Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43(3):447–457 [DOI] [PMC free article] [PubMed]
- Paine RS. The variability in manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria) Pediatrics. 1957;20(2):290–302. [PubMed] [Google Scholar]
- Peng SS-F, Tseng W-YI, Chien Y-H, Hwu W-L, Liu H-M. Diffusion tensor images in children with early-treated, chronic, malignant phenylketonuric: correlation with intelligence assessment. Am J Neuroradiol. 2004;25(9):1569–1574. [PMC free article] [PubMed] [Google Scholar]
- Peng H, Peck D, White DA, Christ SE. Tract-based evaluation of white matter damage in individuals with early-treated phenylketonuria. J Inherit Metab Dis. 2013;37(2):237–243. doi: 10.1007/s10545-013-9650-y. [DOI] [PubMed] [Google Scholar]
- Pietz J, Kreis R, Schmidt H, Meyding-Lamade UK, Rupp A, Boesch C. Phenylketonuria: findings at MR imaging and localized in vivo H-1 MR spectroscopy of the brain in patients with early treatment. Radiology. 1996;201(2):413–420. doi: 10.1148/radiology.201.2.8888233. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/BF03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: current practices and new recommendations. Soc Personal Psychol Compass. 2011;5(6):359–371. doi: 10.1111/j.1751-9004.2011.00355.x. [DOI] [Google Scholar]
- Rupp A, Kreis R, Zschocke J, Slotboom J, Boesch C. Variability of blood–brain ratios of phenylalanine in typical patients with phenylketonuria. J Cereb Blood Flow Metab. 2001;21(3):276–284. doi: 10.1097/00004647-200103000-00011. [DOI] [PubMed] [Google Scholar]
- Scarabino T, Popolizio T, Tosetti M, Montanaro D, Giannatempo GM, Terlizzi R et al (2009) Phenylketonuria: white-matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. Radiol Med 114(3):461–474 [DOI] [PubMed]
- Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum Mutat. 2007;28(9):831–845. doi: 10.1002/humu.20526. [DOI] [PubMed] [Google Scholar]
- Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF et al (1999) Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 212(3):770–784 [DOI] [PubMed]
- Vermathen P, Robert‐Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn Reson Med. 2007;58(6):1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- Viau KS, Wengreen HJ, Ernst SL, Cantor NL, Furtado LV, Longo N. Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J Inherit Metab Dis. 2011;34(4):963–971. doi: 10.1007/s10545-011-9329-1. [DOI] [PubMed] [Google Scholar]
- Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, Levy H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab. 2007;92(1–2):63–70. doi: 10.1016/j.ymgme.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Weglage J, Fromm J, van Teeffelen-Heithoff A, Möller HE, Koletzko B, Marquardt T et al (2013) Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol Genet Metab 110:S44–S48 [DOI] [PubMed]
- White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ et al (2010) Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: A DTI study of the corpus callosum. Mol Genet Metab 99:S41–S46 [DOI] [PMC free article] [PubMed]
- White DA, Antenor-Dorsey JAV, Grange DK, Hershey T, Rutlin J, Shimony JS et al (2013) White matter integrity and executive abilities following treatment with tetrahydrobiopterin (BH4) in individuals with phenylketonuria. Mol Genet Metab 110(3):213–217 [DOI] [PMC free article] [PubMed]


