Abstract
Oro-pharyngeal dysphagia commonly occurs in patients with infantile onset Pompe disease (IOPD), which is a rare recessive neuromuscular disorder caused by deficiency of the lysosomal enzyme acid alpha-glucosidase. Without treatment, death occurs by 1 year of age from cardiorespiratory failure. Enzyme replacement therapy (ERT) has been used to increase life expectancy, however emerging developmental and medical morbidities have become apparent. A case file review of the feeding outcomes of 12 patients with IOPD, managed at a single tertiary centre, was undertaken. Two types of assessment had been completed: clinical feeding assessment (CFA) and instrumental videofluoroscopy swallow study (VFSS). A rating of functional oral intake at every Speech and Language Therapy feeding assessment from initial diagnosis to the most recent assessment was applied using the functional oral intake scale (FOIS).
Results indicate, initial diagnosis VFSS predicts long-term feeding outcomes. Even if a patient had an improvement in oral feeding after diagnosis, over a period of time their oral intake returned to the initial diagnosis VFSS level or below. All patients (8/8) who required non-oral feeding support under 6 months of age went on to require non-oral feeding support, even if they had periods of full oral feeding. CRIM negative status predicted significant oral feeding difficulties. An evidence-based follow-up protocol was developed. The information is used at diagnosis to counsel families regarding feeding prognosis and consideration of early gastrostomy when cardiac status allows safe anaesthesia. The results reinforce that feeding changes over time and patients require on-going dysphagia monitoring.
Keywords: Enzyme replacement therapy, Feeding, Functional oral intake scale, Infantile onset Pompe disease, Oro-pharyngeal dysphagia
Introduction
Pompe disease (glycogen storage disease type II, acid maltase deficiency, OMIM 232300) is a rare recessive neuromuscular disorder caused by deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA, EC 3.2.1.20). Patients with the most severe form, infantile onset Pompe disease (IOPD), typically present with symptoms within the first 6 months of life. Symptoms are characterised by progressive muscle weakness, manifesting as hypotonia, motor developmental delay, hypertrophic cardiomyopathy, respiratory difficulties, and feeding and swallowing problems. If untreated, death by 1 year of age ensues from cardiorespiratory failure (Chakrapani et al. 2010). Enzyme Replacement Therapy (ERT) has been available since 2006 in this population. Alglucosidase alfa (Myozyme, Genzyme, Cambridge, MA) is a recombinant enzyme which significantly improves survival rates, cardiomyopathy, and motor development (Kishnani et al. 2007; Reuser and van der Ploeg 2012).
Since patients are now surviving longer, impacts on functional capacities and the development of other co-morbidities are being identified. Respiratory compromise, reduced mobility, speech and language delay/disorders, and oro-pharyngeal dysphagia have all been documented (van Gelder et al. 2011; Chakrapani et al. 2010). Oro-pharyngeal dysphagia commonly presents in typically affected children, ranging from mild to severe impairment, characterised by weak oral stage skills, delayed swallow initiation, and residue in the pharynx post-swallow (Jones et al. 2009; Prater et al. 2012; van Gelder et al. 2011). Oro-pharyngeal dysphagia can lead to aspiration of food or fluid into the airway, causing chest infections, lung damage, poor nutrition, and dehydration (Wallis and Ryan 2012; Young 1993). Currently, there are two published long-term follow-up studies regarding swallow prognosis in this population, both indicating an improvement in swallow function once starting ERT and over time (Fecarotta et al. 2013; van Gelder et al. 2011). The reports included a single-case study followed for a period of 3 years and the assessment of 6 patients over time. There are no international or national guidelines for swallowing management.
In the authors’ clinical practice it was anecdotally observed that patients were experiencing more significant dysphagia and poorer long-term outcomes than previously reported in the literature. Thus this study had two aims: to document the progression of oral feeding skills in a cohort of children with IOPD from a single centre and to determine the frequency and modes of dysphagia follow-up that are required in the optimal management of such patients.
Methods
The medical records of 12 patients with IOPD currently managed in a single tertiary centre in the UK were reviewed. The study was approved by the local Research and Audit team as a retrospective review of case file documents that did not require patient consent. Inclusion criteria were defined as any active patient with a confirmed diagnosis of IOPD (on the basis of abnormal GAA enzyme activity in bloodspot or leucocyte assay, compatible GAA genotype, elevated urine tetrasaccharide, and presence of typical vacuolated lymphocytes on blood film), and receiving ERT.
Each Speech and Language Therapy feeding assessment, from diagnosis until May 2015, documented in the medical records was reviewed. Inpatient and outpatient assessments were included. Two different types of swallowing assessment were completed: clinical feeding assessment (CFA) and instrumental videofluoroscopy swallow study (VFSS). No data from any other centres were used.
CFAs were completed by one of three speech and language therapists. Every CFA involved the completion of a detailed case history, oro-motor examination, and direct observation of the patient eating and/or drinking while the speech and language therapist used cervical auscultation (Leslie et al. 2004). During this assessment, clinical signs of dysphagia were identified and the amount and type of oral intake were documented. The outcome of the CFA was used to guide oral feeding management and make recommendations for VFSS (Arvedson and Christensen 1993). VFSS was also carried out as routine assessment at initial diagnosis/start of ERT and 3–6 months after commencing ERT.
VFSS was completed by a speech and language therapist with expertise in dysphagia alongside a consultant radiologist. A VFSS is a radiological assessment of the swallowing process, by which a patient is observed drinking and/or eating using fluoroscopic screening with the addition of a contrast material, e.g. E-Z-Paque. During the assessment, oro-pharyngeal structure and function were assessed to identify aspiration and risk of aspiration. In addition, different strategies were trialled in order to optimise oral feeding and inform clinical management. The oesophageal phase was routinely reviewed by the radiologist.
The paediatric version of the functional oral intake scale (FOIS) was used to rate the oral intake of each patient at every assessment (Crary et al. 2005). The tool is a 7-point scale providing information on what the patient is eating and drinking, but does not describe aspiration (Table 1).
Table 1.
Functional oral intake scale (FOIS) paediatric version (Crary et al. 2005)
| Level 1—Nothing by mouth. |
| Level 2—Tube dependent with minimal attempts at food or liquids. For example, dummy dips, tastes of puree or small specified volumes of certain textures. |
| Level 3—Tube dependant with consistent intake of food or liquids, e.g. one or more textures ad lib but still requires tube feeding. |
| Level 4—Total oral intake of a single consistency. This classification is not used in paediatrics. |
| Level 5—Total oral intake with multiple consistencies but requiring special preparation or compensations, e.g. thickened formula, puree with syrup thick fluids. |
| Level 6—Total oral diet with multiple consistencies without special preparation, but with specific food limitations, e.g. no mixed consistencies. |
| Level 7—Total oral diet with no restrictions. |
Baseline demographic data, age of diagnosis, GAA genotype, cross-reactive immunologic material (CRIM) assay status, and treatment regimen were determined from medical records for all patients. Serial echocardiographic studies were reviewed and degree of cardiac hypertrophy estimated by calculation of the left ventricular mass index (LVMI) derived from M-mode echocardiograms (Khoury et al. 2009) at the time of initial diagnosis and at the most recent assessment. Requirement for respiratory support (supplemental oxygen, non-invasive or invasive ventilation) and gross motor function at the same time points was determined.
Results
Patients
Twelve subjects, three females and nine males, of different ethnicities were included in the study. No patient currently on the caseload was excluded from the study. Table 2 outlines patient information and age at initial VFSS and the most recent assessment. 11/12 patients were diagnosed at the centre. One patient (patient 5) was transferred with a diagnosis and receiving treatment from an overseas centre as an older child. The median age at diagnosis was 4.9 months and the median age of ERT initiation was 5.0 months. The age range at the most recent assessment was 19–144 months of age with a mean age of 61.4 months.
Table 2.
Patient information
| Patient | Sex | Age at diagnosis (months) | GAA genotype | CRIM status (Pos/Neg) | Age ERT started (months) | Immunomodulation (Y/N) | Age at initial diagnostic VFSS (months) | Age at the most recent assessment (months) | Cardiac hypertrophy (LVMI, g/m2) | Respiratory support | Gross motor status | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start of ERT | Recent assessment | Start of ERT | Recent assessment | Start of ERT | Recent assessment | |||||||||
| 1 | F | 4.9 | c.784G > A / c.784G > A | Pos | 5 | N | 5.1 | 56 | 613 | 60 | Oxy | Nil | Hypotonia | Walking independently |
| 2 | M | 0.4 | c.2744A > C / c.2744A > C | Pos | 0.4 | N | N/A | 89 | 96 | 48 | Nil | Nil | Normal | Walking independently |
| 3 | M | 0.2 | c.877G > A / c.877G > A | Pos | 2.2 | Y | 3.7 | 55 | 50.6 | 36 | Nil | nNIV | Hypotonia | Antigravity UL |
| 4 | F | 6.2 | c.2078dup / c.2078dup | Neg | 6.9 | Y | 6.6 | 24 | 773 | 55.7 | Oxy | cNIV | Hypotonia | Antigravity UL |
| 5 | M | 6.9 | n/a | Pos | NK | N | N/A | 82 | n/a | 55.6 | Nil | Nil | Hypotonia | Walk with support (left hemiplegia from neonatal stroke) |
| 6 | M | 3.1 | c.1933G > A / c.1933G > A | Pos | 3.4 | N | 7 | 40 | 355 | 178.6 | NIV | Nil | Hypotonia | Walking independently |
| 7 | M | 4.6 | c.1933G > A / c.1933G > A | Pos | 4.8 | N | 5.3 | 20 | 114.3 | 49.8 | Nil | Nil | Hypotonia | Walking independently |
| 8 | M | 6.2 | c.2608C > T / c.2608C > T | Neg | 6.7 | Y | 6.3 | 19 | 367 | 128 | Oxy | cNIV | Hypotonia | UL + LL antigravity movements |
| 9 | M | 6.4 | c.1798C > T / c.2105G > T | Pos | 9.2 | N | 10.1 | 124 | n/a | 49.1 | Nil | Nil | Hypotonia | Bottom shuffling |
| 10 | F | 5 | c.2560C > T / c.2560C > T | Neg | 5.1 | Y | 4.9 | 40 | 665 | 69 | Nil | Nil | Hypotonia | Walking independently |
| 11 | M | 3.6 | c.525del / c.2481 + 102_c.2646 + 31del535 | Neg | 4 | Y | 4 | 44 | 343 | 60.2 | Nil | Vent | Hypotonia | Minimal movement UL |
| 12 | M | 31.1a | c.1082C > T / c.1933G > A | Pos | 31.2 | N | 29.8 | 144 | 140.4 | 70 | nNIV | nNIV | Walk with support | Walking independently |
F female, M male, ERT enzyme replacement therapy, VFSS videofluoroscopy swallow study, CRIM cross-reactive immunologic material, NK not known, N/A not applicable, LVMI left ventricular mass index, Oxy oxygen, nNIV nocturnal non-invasive ventilation, cNIV continuous non-invasive ventilation, vent invasive ventilation, UL upper limbs, LL lower limbs
aPatient 12, classified as infantile onset as symptomatic with hypotonia, heart failure from 10.2 months age
Treatment
Four patients were CRIM negative and were treated with a standardised immunomodulation protocol (rituximab and methotrexate) at time of initiation of ERT. Eight patients were CRIM positive, of whom one received immunomodulation. All patients were treated with a standardised ERT schedule with a 12-week induction with weekly intravenous alglucosidase alfa 20 mg/kg/dose followed by alternate week dosing.
Swallow Assessments
10/12 patients had an initial baseline VFSS at time of diagnosis or when starting ERT. Of the two patients who did not have a baseline VFSS, one (patient 2) was fully orally fed since birth and has remained fully orally feeding with no concerns, and with no clinical indications to complete a VFSS. The other (patient 5) arrived in the UK as an older child and had already been receiving treatment. 5/10 patients had a repeat VFSS, which was completed on average 3.4 months after the initial VFSS (range from 2 to 4 months).
Serial FOIS scores for each patient are summarised in Table 3. When comparing the most recent assessment to the initial diagnosis VFSS (n = 10 patients), 50% of patients maintained the same level of oral feeding and 50% of patients deteriorated. No patient demonstrated increased oral intake over time. No clinically significant improvement in oral intake was seen after starting ERT. Only 1/5 patients (patient 4) had an increase in oral intake after starting ERT, as shown on VFSS; however, this was not maintained as seen at the most recent assessment.
Table 3.
FOIS scores over time
| Patient | FOIS score at initial diagnosis VFSS | FOIS score at VFSS approx. 3–6 months post-ERT | FOIS score at CFA approx. 3–6 months post-ERT | Most recent FOIS score (CFA or VFSS) |
|---|---|---|---|---|
| 1 | 3 | N/A | 2 | 2 |
| 2 | N/A | N/A | 7 | 7 |
| 3 | 3 | N/A | 2 | 2 |
| 4 | 2 | 3 | 3 | 2 |
| 5 | N/A | N/A | N/A | 6 |
| 6 | 3 | N/A | 7 | 2 |
| 7 | 2 | 2 | 2 | 2 |
| 8 | 2 | 2 | 2 | 2 |
| 9 | 7 | N/A | 7 | 7 |
| 10 | 3 | 3 | 3 | 2 |
| 11 | 2 | 2 | 2 | 2 |
| 12 | 5 | N/A | 3 | 2 |
FOIS functional oral intake scale, VFSS videofluoroscopy swallow study, ERT enzyme replacement therapy, CFA clinical feeding assessment, N/A not applicable
One patient (patient 9) who was fully orally feeding with no restrictions at the initial diagnosis VFSS was able to maintain full oral feeding. 100% of patients (8/8) who required non-oral feeding support before 6 months of age went on to require non-oral feeding support at their most recent assessment even if they had periods of full oral feeding.
CRIM negative status predicted significant oral feeding difficulties. All patients who were CRIM negative (n = 4) had long-term poor prognosis with limited oral intake. No patient had an FOIS score over level 2 at the most recent assessment. CRIM positive patients (n = 8) presented with a range of oral feeding skills, predicted at initial diagnosis VFSS.
At the most recent assessment all patients who were nonambulatory had an FOIS score of 2, however there was wider variability in feeding status among patients with preserved ambulation. Similarly, all patients requiring invasive or non-invasive ventilation had an FOIS score of 2, with wider variability among those not requiring ventilation. All patients showed at least partial resolution of cardiac hypertrophy, hence this did not appear to be a major factor driving feeding issues.
At the most recent assessment, 75% of patients were tube dependant, requiring a nasogastric tube or gastrostomy tube to meet their nutrition and hydration needs. All patients had some oral feeding, even if only small amounts, e.g. lip swipes/tastes of puree. Three patients were fully orally feeding. Two of those patients were feeding with no oral restrictions. One patient required thickened fluids.
Discussion
The long-term oral feeding outcomes for patients with IOPD in this study were poor and differ to findings in the existing literature. 75% of the cohort is dependent on non-oral enteral feeding, with all patients displaying significantly limited oral intake, predominantly due to oro-pharyngeal dysphagia. The data suggest that initial diagnosis VFSS is prognostic in determining long-term oral intake.
The results of this study are in contrast to previous published work that reported an improvement in oral feeding once commencing ERT (Fecarotta et al. 2013; van Gelder et al. 2011). The data presented here represents the first large longitudinal cohort study examining oral feeding over time, capturing a current caseload of all ERT-treated patients at this tertiary centre. Patients reported here have benefited from a routinely high level of speech and language therapy involvement, having routine assessments for an average of 3:8 years (range 0:9–9:6 years) resulting in accurate and complete clinical data in all patients. The cohort described here included four CRIM negative patients, who are known to have worse clinical outcomes in other areas such as mobility and respiratory function (Chakrapani et al. 2010). This study has shown that CRIM negative status is also associated with poorer feeding outcomes.
Although long-term oral feeding prognosis is poor the results indicate that over time oral intake fluctuates and patients require on-going monitoring by a dysphagia trained speech and language therapist to ensure safe management. Subsequently, a tertiary level care pathway was developed (Fig. 1). The care pathway outlines management from initial diagnosis to transition to adult services. Routine VFSS at diagnosis is completed to provide prognostic information and accurate dysphagia management. This information is used to educate and counsel parents about long-term oral feeding outcomes and non-oral feeding options. When no dysphagia is identified in the VFSS, patients continue with a full oral diet with no restrictions. They are then reviewed every 6 months at the tertiary centre and no referral to local Speech and Language Therapy feeding services are completed.
Fig. 1.
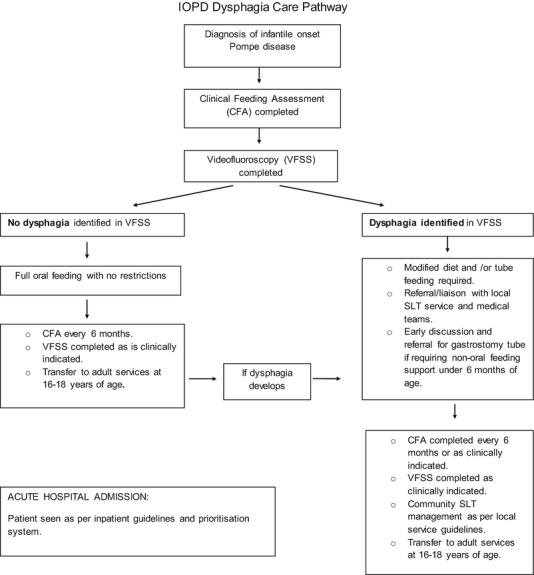
Tertiary level care pathway. IOPD infantile onset Pompe disease, VFSS videofluoroscopy swallow study, CFA clinical feeding assessment, SLT speech and language therapy
Modified diet and/or non-oral feeding support are recommended to those patients presenting with dysphagia in the VFSS. Dysphagic patients are then routinely seen every 6 months and as clinically indicated at the tertiary centre for monitoring. Patients requiring non-oral feeding support prior to 6 months of age will be referred for gastrostomy when medically stable. All dysphagic patients will be referred to local Speech and Language Therapy feeding services for community follow-up, to ensure liaison with school/nursery. VFSS is completed as clinically indicated for all patients.
Further long-term studies of feeding outcomes are required to ensure robust longitudinal data are gathered, and to validate the proposed care pathway. A multi-centre study should be considered in order to increase the number of participants. It is also recommended that national and international guidelines are developed to ensure evidence-based standardised care is available.
Acknowledgements
We thank the members of the Department of Metabolic Medicine, Lysosomal Storage Disorders team for their assessment and management of the patients included in this study that led to the robust data available. We thank members of the Department of Speech and Language Therapy for their contribution to ideas and revisions of the paper.
Single Sentence Synopsis
Oro-pharyngeal dysphagia is common among patients with IOPD and oral intake at diagnosis predicts long-term feeding outcomes.
Conflict of Interest
Gyani Swift, Maureen Cleary, Stephanie Grunewald, Sonia Lozano, Martina Ryan, and James Davison declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This article does not contain any studies with human or animal subjects performed by any of the authors. The study was approved by the local Research and Audit team as a retrospective review of case file documents that did not require patient consent.
Contribution of Authors
GS: Responsible for the study’s conception and design, collated and analysed all data and wrote the manuscript.
MC: Involved in critical revision and editing of the article.
SG: Involved in critical revision and editing of the article.
SL: Involved in the study’s conception, design, analysis of data, critical revision, and editing.
MR: Involved in critical revision and editing of the article.
JD: Oversaw the critical revision and editing of the article.
Guarantor
G Swift
Funding
None
Contributor Information
Gyani Swift, Email: gyani.swift@gmail.com.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Arvedson J, Christensen S (1993) Instrumental evaluation. In: Arvedson JC, Brodsky L (eds) Pediatric swallowing and feeding: assessment and management. Singular Publishing Group, California, pp 293–326
- Chakrapani A, Vellodi A, Robinson P, Jones S, Wraith JE. Treatment of infantile Pompe disease with alglucosidase alpha: the UK experience. J Inherit Metab Dis. 2010;33(6):747–750. doi: 10.1007/s10545-010-9206-3. [DOI] [PubMed] [Google Scholar]
- Crary M, Carnaby Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–1520. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Fecarotta S, Ascione S, Montefusco G, et al. Improvement in dysphagia in a child affected by Pompe disease treated with enzyme replacement therapy. Ital J Pediatr. 2013;39:30–35. doi: 10.1186/1824-7288-39-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Muller C, Lin M, et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2009;25(4):277–83. doi: 10.1007/s00455-009-9252-x. [DOI] [PubMed] [Google Scholar]
- Khoury PR, Mitsnefes M, Daniels SR, Kimballs TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid alpha-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Leslie P, Drinnan MJ, Finn P, Ford GA, Wilson JA. Reliability and validity of cervical auscultation: a controlled comparison using videofluoroscopy. Dysphagia. 2004;19:231–240. [PubMed] [Google Scholar]
- Prater SN, Banugaria S, De Armey PA-C, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012;14(9):800–810. doi: 10.1038/gim.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser AJJ, van der Ploeg T. Pompe disease. In: Mehta A, Winchester B, editors. Lysosomal storage disorders: a practical guide. West Sussex: Wiley; 2012. pp. 101–106. [Google Scholar]
- van Gelder CM, Van Capelle CI, Ebbink BJ, et al. Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy. J Inherit Metab Dis. 2011;35(3):505–511. doi: 10.1007/s10545-011-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C, Ryan M. Assessing the role of aspiration in pediatric lung disease. Pediatr Allergy Immunol Pulmonol. 2012;25(3):132–142. doi: 10.1089/ped.2012.0148. [DOI] [Google Scholar]
- Young C. Nutrition. In: Arvedson JC, Brodsky L, editors. Pediatric swallowing and feeding: assessment and management. California: Singular Publishing Group; 1993. pp. 157–208. [Google Scholar]


