Abstract
Several different lysosomal storage diseases, mainly mucopolysaccharidosis (MPS) type I, II, and VI, are complicated by severe obstruction of the upper airways, tracheobronchial malacia, and/or stenosis of the lower airways. Although enzyme replacement therapies (ERTs) are available, the impact of these on tracheobronchial alterations has not been reported. By extending the life expectancy of MPS patients with ERTs, airway problems may become more prevalent at advanced ages. These airway abnormalities can result in severe, potentially fatal, difficulties during anesthetic procedures. Usually, upper airway obstruction is treated by tracheostomy. However, with lower airway malacia and/or stenosis, there are no procedures available to date to address these difficulties. We report the first cases using a new technique of tracheal stenting in patients with MPS type VI (Maroteaux–Lamy syndrome) and type II (Hunter syndrome) who had almost complete tracheal occlusion and total airway collapse. An updated literature review is also reported.
Introduction
Mucopolysaccharidoses (MPS) are a heterogeneous group of different lysosomal storage disorders, resulting from the abnormal degradation of glycosaminoglycans (GAGs) (Muenzer 2004). The crude cumulative incidence is approximately 1 in 25,000 newborns (Baehner et al. 2005; Nelson 1997). The main accumulated storage products include GAGs containing heparan, dermatan, keratan, and chondroitin sulfates (Neufeld and Muenzer 2001). The wide clinical spectrum for MPS is related to the particular GAG products involved, the degree of enzyme deficiency, and the associated amount of accumulated storage products.
In patients with MPS types I, II, and VI (OMIM #607014, #309900, and #253200, respectively; each including dermatan sulfate as the storage product) and MPS types IV A and B (OMIM #253000 and #253010, respectively; including chondroitin and/or keratan sulfates as storage products) (Muenzer 2004), treating physicians have raised considerable attention concerning the development of airway obstructions (Shapiro et al. 1985; Semenza and Pyeritz 1988), which may lead to fatal complications in emergency cases or during planned ear, nose, and throat procedures, including death during intubation (Yoskovitch et al. 1998; Muhlebach et al. 2011; Muhlebach et al. 2013; Walker et al. 2013). Additionally, patients affected by MPS face a substantial number of surgical procedures (and therefore intubations) during childhood and adolescence (Young and Harper 1979; Arn et al. 2012).
A high postsurgical mortality rate due to respiratory and cardiac disease has been reported in MPS patients, with higher mortality among those with a more severe phenotype (Young and Harper 1979; Arn et al. 2012). Among 196 deceased patients with MPS type I, 32 had surgery within 1 month of death, including 20 who had surgery within 10 days of death (Arn et al. 2012).
Several reports have been published concerning the difficulties in airway management among MPS patients (Adachi and Chole 1990; Nicolson et al. 1992; Gaitini et al. 1998; Walker et al. 2003; Ard et al. 2005). Aside from the difficulties in airway management, the use of neuromuscular blocking agents, the prolongation of anesthesia and/or aggressive ventilator support will contribute to the deterioration of these findings.
Airway obstructions may be localized in any of the physiological airways, from the nose to the peripheral bronchiae (Steven Sims and Kempiners 2007). While the upper airways are usually obstructed by large amounts of storage material, the more common complications seen in the trachea and bronchiae are tracheomalacia and bronchomalacia, which sometimes lead to complete major airway collapse (Morehead and Parsons 1993). This malacia may occur in combination with bronchial and tracheal stenosis due to large storage material “tumors” in the mucosa of airways and surrounding tissues, leading to a compression of the airway (Morehead and Parsons 1993). Usually, these stenoses occur around adolescence and are progressive, and may lead to almost complete obstruction of a bronchus or the trachea. Because of upper airway obstruction, a substantial number of MPS patients are treated with tracheotomy (Jeong et al. 2006). But, tracheostomy itself may cause significant problems to the patients, when the auto-positive end-expiratory pressure (PEEP) function of the glottis is reduced or excluded, and an airway collapse caused by the malacia will become apparent. It is reported that the most common cause of death in Hunter syndrome (MPS type II, at least in the attenuated phenotype) is impairment of cardiorespiratory function, which may aggravate the neurological deterioration, and these patients do not usually survive beyond adolescence (Brama et al. 1986; Gaitini et al. 1998; Gross and Lemmens 2010). For MPS types I, II, and VI, enzyme replacement therapy (ERT) has been available for several years. ERT with recombinant human N-acetylgalactosamine 4-sulfatase has been shown to halt and partially reverse the decline in pulmonary function in patients with MPS type VI [Harmatz et al. 2010]. However, to date, there are no reports available regarding the impact of ERT on airway obstruction or malacia. In Hurler syndrome, even after early bone marrow transplantation and donor engraftment, tracheal stenosis and tracheomalacia may occur in the long term and complicate the course of the patient (Valayannopoulos et al. 2010). It is noteworthy that the reported Hurler patient developed 12 years after successful engraftment, without signs of graft-versus-host disease and with leukocyte enzyme activity of 50% of normal, severe pulmonary hypertension and tracheal and bronchial obstructions and malacia, which resolved after additional ERT with weekly infusions of 100 U/kg of α-l-ironidase.
It is therefore conceivable that by extending the life expectancy of the patients with ERT, tracheobronchial airway obstruction or malacia may become more prominent at advanced ages.
No conclusive management guidelines exist regarding the treatment of upper airway obstructions by tracheotomy to date or for the treatment of tracheomalacia in combination with severe tracheal stenosis in patients with MPS. Many patients therefore remain untreated, mandatory surgical procedures are withheld, and they ultimately die.
Presentation of Case 1
We report a 20-year-old female patient with severe MPS type VI (Maroteaux–Lamy syndrome). At the age of 17 years, the patient received a tracheotomy during a neurosurgical procedure because of complicated intubation and the inability to wean the patient off ventilation because of upper airway obstruction. At the same time, the patient started ERT. Besides her severe clinical phenotype, the patient’s situation was complicated by moderate mitral and aortic valve stenosis, and moderate mitral valve regurgitation. Since the age of 17 years, the patient developed progressive respiratory impairment with the need for home ventilation overnight. During an upper airway infection, the patient developed severe respiratory failure with sepsis and required continuous 24-h ventilation. During a 3-month period of intensive care, the patient’s respiratory function deteriorated further with the need for assisted spontaneous ventilation with a PEEP of 18 mmHg and a pressure support of 10 mmHg. Multiple attempts failed to wean the patient off ventilation.
Bronchoscopy revealed subtotal tracheal stenosis (beyond which the bronchoscope could not pass) and tracheomalacia. A three-dimensional computed tomography scan showed a large tumorous mass infiltrating and impressing the lower trachea proximal to the bronchial bifurcation and impressing the origin of the left main bronchus (Fig. 1a, b). Under a PEEP of 18 mmHg, the inner lumen of the tracheal stenosis was <3 mm. By reduction of PEEP to <10 mmHg, the trachea collapsed and showed a total occlusion of the airway. It was demonstrated during bronchoscopy that the long-term high PEEP ventilation may have aggravated the tracheomalacia above the stenosis by un-physiological high pressure ventilation. All assessments were done under mild sedation without deep anesthesia, and during bronchoscopy additional local anesthesia. These findings were judged as being untreatable by surgery.
Fig. 1.
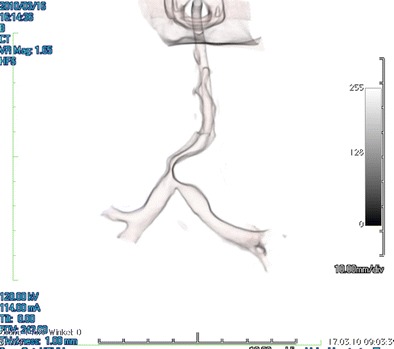
3D computerized tomography scans showing severe tracheal stenosis with tracheo-bronchomalacia of the left main bronchus during hold inspiration with a positive end-expiratory pressure (PEEP) of 18 mmHg. Note severe obstruction of the trachea and bifurcation, and the post-stenotic bronchomalacia. The patient was ventilated with intubation past the tracheostoma but with its end remaining proximal to the tracheal stenosis
After informed consent from the patient and her parents, it was decided to attempt a palliative stent implantation into the tracheal stenosis. The procedure was performed under fluoroscopic and bronchoscopic control. After passing a guide wire across the stenosis into the left main bronchus, a sizing of the internal diameter of the stenosis was performed with an inflatable balloon (Fig. 2a). Because the stenosis was in close proximity to the tracheal bifurcation, two guide wires were advanced through the stenosis into both the right and the left main bronchi. One 22-mm long, uncovered bare platinum/iridium stent (CP8Z22 Stent, NuMED Inc., Hopkinton, NY) was mounted on two balloon catheters (20-mm long and 6-mm diameter when fully inflated) simultaneously. This ensemble of stent and two balloons was advanced over both guide wires across the tracheal stenosis and placed into the bifurcation (Fig. 2b). Both balloon catheters were simultaneously inflated to their nominal diameter. Thereafter, each balloon catheter was exchanged to a 12-mm balloon catheter and alternately inflated to allow airflow to the left and right main bronchi (Fig. 2c). Immediately after stent implantation, PEEP could be reduced to 8 mmHg. No tracheal rupture, stent migration, or dysphagia occurred.
Fig. 2.
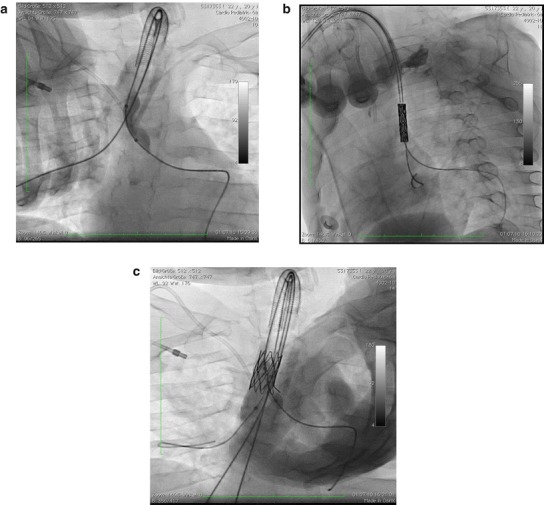
Balloon sizing of the true tracheal stenosis after placing a guide wire into the left lower bronchus. Note the severe narrowing of the trachea at the bifurcation (a). After placing guide wires into the right and left main bronchus the advanced ensemble of a stent mounted on two balloons immediately before inflation (b). Fully deployed stent with two balloons inflated: one balloon in the right main bronchus (12-mm diameter) and the other balloon in the left main bronchus (6-mm diameter). Note the stent opening to the left main bronchus to avoid tumorous obstruction of the main left bronchus (c)
Three weeks after the first stent implantation, a second stent was placed at the proximal end of the initial previously implanted stent and dilated to 12 mm. The patient recovered well and could be weaned off after 24-h ventilation. Bronchoscopy at discharge showed an open trachea (Fig. 3a) and a free origin to the left main bronchus (Fig. 3b). At 18 months after the second procedure, the patient needed re-dilatation of the implanted stents to 12-mm diameter because of granulomatous tissue formation. Two years after stenting of the trachea, the patient showed no signs of respiratory failure or requirement for single-sided ventilation.
Fig. 3.
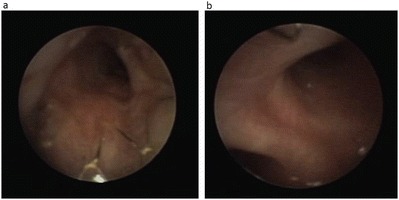
Bronchoscopy before discharge of the patient. The tracheal airway is almost free of stenosis, and the stent is in place with mild granulation tissue (a). Origin of the left main bronchus at 2 o’clock and distal stent ending at 12 o’clock. Note that the entrance of the left main bronchus is only minimally impressed (b)
Presentation of Case 2
A 23-year-old severely affected patient with Hunter syndrome without major neurological involvement on ERT since 2008 required an operation because of the failure of his implanted port system. The introduction of anesthesia was complicated by pharyngeal obstructions and the stiffness of the cranio-cervical junction. After successful oro-tracheal intubation and operation, the patient could not be weaned from ventilation anymore, requiring a PEEP of 15 mmHg and a pressure support of 10 mmHg. Even after tracheostomy, ventilation could not be reduced and the patient could not be weaned from the ventilator. Tracheography under fluoroscopic control and bronchoscopy, all procedures were performed under mild intravenous sedation with midazolam with doses between 0.02 and 0.05 mg/kg body weight, revealed a total tracheal collapse, once the PEEP was reduced below 8 mmHg. The tracheal collapse affected the whole trachea. Therefore, the above mentioned approach was used to stent the trachea from the bifurcation upwards to the tracheostoma. Here the trachea was stented to an internal diameter of 14 mm. After successful implantation of two CP stents over a total length of 48 mm the distal tip of the tracheal cannula was advanced 5 mm within the proximal end of the stent (Fig. 4). Immediately after stent deployment the ventilation could be reduced to a spontaneous mode with a PEEP of 5 mmHg and a pressure support of 10 mmHg. During follow-up, the patient could be weaned from ventilation. Aside from tracheostomy, the patient has not required mechanical ventilation and has had just one upper airway infection in 22 months of follow-up.
Fig. 4.
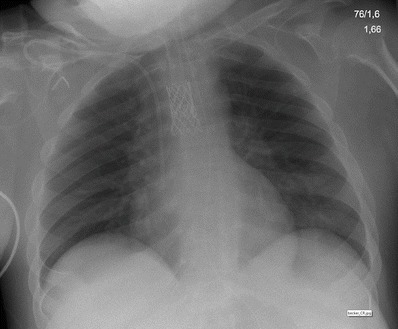
Chest radiogram of the second patient with complete tracheomalacia and after stenting of the trachea. Of note, the tracheal cannula reaches in the upper orifice of the stent. Patient is spontaneously breathing
Discussion
Tracheobronchial obstructions are infrequent in pediatric or adolescent subjects unaffected by MPS, but when they are seen, they are associated with significant morbidity and mortality (Anton-Pacheco et al. 2008). Airway obstruction is usually caused by malignant diseases in adults and occurs more frequently after reconstructive surgery in children (Nicolai 2008). Nevertheless, malacia and stenosis, either congenital or acquired, are the most frequent airway anomalies encountered (Davitt et al. 2002). Symptomatic airway stenosis is usually treated surgically with good results so intraluminal airway stenting remains a therapeutic approach for only very selected cases (Filler et al. 1998; Nicolai et al. 2001; Nicolai 2008), and may be accompanied by potential long-term complications, like chronic pulmonary infections, re-obstruction, and migration of the implanted stent into surrounding structures.
These cases are the first reports of palliative permanent tracheal stenting in a patient with severe Maroteaux–Lamy (MPS type VI) and Hunter (MPS type II) syndrome. Furthermore, the presented technique of stent implantation, using a double-wire double-balloon technique, has not been previously described. It offers the possibility of tracheal stenting in the immediate proximity to the tracheobronchial bifurcation with preservation of airflow to both the right and left main bronchi.
Following a review of the literature, airway stenting has only been reported in two MPS patients, although there are several reports of bronchio-tracheal stenting among non-malignant cases, for which indications were mainly tracheo- or bronchomalacia, but rarely stenosis (Furman et al. 1999). One patient with MPS type II (Hunter syndrome) had peripheral bronchial stenosis (Davitt et al. 2002) and another patient with MPS type IV (Morquio syndrome) had bronchial malacia (Anton-Pacheco et al. 2008). Unfortunately, follow-up data were not presented in either patient (Davitt et al. 2002; Anton-Pacheco et al. 2008).
Other therapeutic options for the treatment of tracheal stenosis in MPS patients might include the use of CO2 or Nd-Yak laser surgery for either upper or lower airway stenosis (Lin et al. 2000; Mierzwinski et al. 2006). However, laser surgery might fail with a combination of stenosis and malacia. Bronchio-tracheal stenosis with tracheomalacia has been reported for MPS types I, II, and VI, while tracheomalacia might occur predominantly in MPS type IV (Shapiro et al. 1985; Semenza and Pyeritz 1988). It seems that from all MPS patients, those with abnormal dermatan sulfate storage are at risk for developing tracheobronchial complications. It has been shown that in non-MPS patients approximately 14.8% of the dry weight of tracheal material consists of GAGs, which usually bind to hyaluronan (Roberts and Paré 1991, Rains et al. 1992). During aging, around 30% of the chondroitin sulfates are being replaced by keratan sulfate, which has less water binding capacity, and is therefore responsible for the decrease of elasticity of the airways during aging (Rains 1992). Nevertheless, except within the cartilage, dermatan sulfate accounts for 20–40% of the weight of the tracheal muscles, the adventitia, and the intercartilage tissue, while heparan sulfate plays a neglectible role (Binette et al. 1994). Therefore it can be suggested that in these tracheal structures a high turnover of dermatan sulfates takes place and in diseases with a disturbed dermatan sulfate metabolism a missing dermatan sulfate brake down will lead to a substantial storage of material within these structures.
Even after bone marrow transplant, a close follow-up of these patients is mandatory, and once those patients are showing signs of deterioration of pulmonary function, or the development of trachea bronchial complications or pulmonary hypertension, increasing the dose of ERT or initiating enzyme replacement should be considered. Furthermore, the use of neuromuscular blocking agents, prolonged anesthesia and/or aggressive ventilator support should be limited or avoided, because it may contribute to the deterioration of these trachea bronchial complications.
Nevertheless, stent implantation offers a palliative ultima ratio approach to treat bronchio-tracheal stenoses and/or with malacia and maintain open airways in these patients permanently.
Synopsis
We report the first cases and their follow-up of tracheal stenting using a new technique in patients with MPS type VI and II who had almost complete tracheal occlusion and total airway collapse.
Conflict of Interest
Christoph Kampmann declares that he has no conflict of interest.
Christiane M. Wiethoff declares that she has no conflict of interest.
Ralf G. Huth declares that he has no conflict of interest.
Eugen Mengel declares that he has no conflicts of interest.
Gundula Staatz declares that she has no conflicts of interest.
Michael Beck declares no conflicts of interest.
Stefan Gehring declares no conflicts of interest.
Torsten Mewes declares that he has no conflicts of interest.
Tariq Abu-Tair declares that he has no conflicts of interest.
Christoph Kampmann has received honoraria for lectures and compensation for travel and accommodation from Biomarin and Shire.
Eugen Mengel has received honoraria for lectures and compensation for travel and accommodation from Biomarin and Shire and an unrestricted grant from Genzyme.
Michael Beck has received honoraria for lectures and compensation for travel and accommodation and unrestricted grants from Shire, Genzyme, Actelion, and Biomarin.
All authors declare that they have no competing interests.
Informed Consent
Treated and reported patients were in end-stage disease with an acute life-threatening condition; it was the absolute wish of the parents and guardians to offer the patients a treatment strategy. Therefore, the basis of this report is on intention to treat.
Details of the Contribution of Individual Authors
CK, TW, RGH, and CMW have conducted the interventions,
CK and CMW have written the manuscript,
GS and TA-T delivered 3D scanning,
MB, EM, and SG taken care of the patients before and during procedures,
CK, CMW, and TA-T were planning and reporting the work described in this article, and
MB and EM made important contributions to the manuscript.
Contributor Information
Christoph Kampmann, Email: kampmann@mail.uni-mainz.de.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Adachi K, Chole RA. Management of tracheal lesions in Hurler syndrome. Arch Otolaryngol Head Neck Surg. 1990;116:1205–1207. doi: 10.1001/archotol.1990.01870100099022. [DOI] [PubMed] [Google Scholar]
- Anton-Pacheco JL, Cabezali D, Tejedor R, et al. The role of airway stenting in pediatric tracheobronchial obstruction. Eur J Cardiothorac Surg. 2008;33:1069–1075. doi: 10.1016/j.ejcts.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Ard JL, Jr, Bekker A, Frempong-Boadu AK. Anesthesia for an adult with mucopolysaccharidosis I. J Clin Anesth. 2005;17:624–626. doi: 10.1016/j.jclinane.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Arn P, Whitley C, Wraith JE, et al. High rate of postoperative mortality in patients with mucopolysaccharidosis I: findings from the MPS I Registry. J Pediatr Surg. 2012;47:477–484. doi: 10.1016/j.jpedsurg.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- Binette JP, Burgi W, Ohishi H, Grundboeck-Jusko J, Burki R, Maekawa Y, Tschopp FA, Kimura A, Schmid K. The glycosaminoglycan composition of human tracheas and the changes observed during aging and disease. Clin Chem Acta. 1994;225:179–185. doi: 10.1016/0009-8981(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Brama I, Gay I, Feinmesser R, Springer C. Upper airway obstruction in Hunter syndrome. Int J Pediatr Otorhinolaryngol. 1986;11:229–235. doi: 10.1016/S0165-5876(86)80034-9. [DOI] [PubMed] [Google Scholar]
- Davitt SM, Hatrick A, Sabharwal T, Pearce A, Gleeson M, Adam A. Tracheobronchial stent insertions in the management of major airway obstruction in a patient with Hunter syndrome (type-II mucopolysaccharidosis) Eur Radiol. 2002;12:458–462. doi: 10.1007/s003300100932. [DOI] [PubMed] [Google Scholar]
- Filler RM, Forte V, Chait P. Tracheobronchial stenting for the treatment of airway obstruction. J Pediatr Surg. 1998;33:304–311. doi: 10.1016/S0022-3468(98)90452-3. [DOI] [PubMed] [Google Scholar]
- Furman RH, Backer CL, Dunham ME, Donaldson J, Mavroudis C, Holinger LD. The use of balloon-expandable metallic stents in the treatment of pediatric tracheomalacia and bronchomalacia. Arch Otolaryngol Head Neck Surg. 1999;125:203–207. doi: 10.1001/archotol.125.2.203. [DOI] [PubMed] [Google Scholar]
- Gaitini L, Fradis M, Vaida S, et al. Failure to control the airway in a patient with Hunter’s syndrome. J Laryngol Otol. 1998;112:380–382. doi: 10.1017/s0022215100140526. [DOI] [PubMed] [Google Scholar]
- Gross ER, Lemmens HJ. Hunter syndrome in an adult: beware of tracheal stenosis. Anesth Analg. 2010;110:642–643. doi: 10.1213/ANE.0b013e3181c539ce. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Yu Z-F, Giugliani R, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: evaluation of long-term pulmonary function in patients treated with recombinant human N-acetylgalactosamine 4-sulfatase. J Inherit Metab Dis. 2010;33:51–60. doi: 10.1007/s10545-009-9007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HS, Cho DY, Ahn KM, Jin DK. Complications of tracheotomy in patients with mucopolysaccharidoses type II (Hunter syndrome) Int J Pediatr Otorhinolaryngol. 2006;70:1765–1769. doi: 10.1016/j.ijporl.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Lin CM, Hsu JC, Liu HP, Li HY, Tan PP. Anesthesia of CO2 laser surgery in a patient with Hunter syndrome: case report. Chang Gung Med J. 2000;23:614–618. [PubMed] [Google Scholar]
- Mierzwinski J, Zwierz A, Jaworski A, et al. The use of Nd-Yag laser in the treatment of tracheal tumor in 14 years old child with mucopolysaccharidosis type II. Otolaryngol Pol. 2006;60:603–606. [PubMed] [Google Scholar]
- Morehead JM, Parsons DS. Tracheobronchomalacia in Hunter’s syndrome. Int J Pediatr Otorhinolaryngol. 1993;26:255–261. doi: 10.1016/0165-5876(93)90096-L. [DOI] [PubMed] [Google Scholar]
- Muenzer J. The mucopolysaccharidoses: a heterogenous group of disorders with variable pediatric presentations. J Pediatr. 2004;144(5 Suppl):S27–S34. doi: 10.1016/j.jpeds.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Muhlebach MS, Wooten W, Muenzer J. Respiratory manifestations in mucopolysaccharidoses. Paediatr Respir Rev. 2011;12:133–138. doi: 10.1016/j.prrv.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Muhlebach MS, Shaffer CB, Georges L, Abode K, Muenzer J. Bronchoscopy and airway management in patients with mucopolysaccharidoses (MPS) Pediatr Pulmonol. 2013;48:601–607. doi: 10.1002/ppul.22629. [DOI] [PubMed] [Google Scholar]
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, editors. The metabolic and molecular basis of inherited disease. New York: McGraw Hill; 2001. pp. 3421–3452. [Google Scholar]
- Nicolai T. Airway stents in children. Pediatr Pulmonol. 2008;43:330–344. doi: 10.1002/ppul.20790. [DOI] [PubMed] [Google Scholar]
- Nicolai T, Huber RM, Reiter K, Merkenschlager A, Hautmann H, Mantel K. Metal airway stent implantation in children: follow-up of seven children. Pediatr Pulmonol. 2001;31:289–296. doi: 10.1002/ppul.1041. [DOI] [PubMed] [Google Scholar]
- Nicolson SC, Black AE, Kraras CM. Management of a difficult airway in a patient with Hurler-Scheie syndrome during cardiac surgery. Anesth Analg. 1992;75:830–832. doi: 10.1213/00000539-199211000-00032. [DOI] [PubMed] [Google Scholar]
- Rains JK, Bert L, Roberts CR, Paré PD. Mechanical properties of human tracheal cartilage. J Appl Physiol. 1992;72(1):219–225. doi: 10.1152/jappl.1992.72.1.219. [DOI] [PubMed] [Google Scholar]
- Roberts CR, Paré PD. Composition changes in human tracheal cartilage in growth and aging, including changes in proteoglycan structure. Am J Physiol. 1991;261:L92–L101. doi: 10.1152/ajplung.1991.261.2.L92. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine (Baltimore) 1988;67:209–219. doi: 10.1097/00005792-198807000-00002. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Strome M, Crocker AC. Airway obstruction and sleep apnea in Hurler and Hunter syndromes. Ann Otol Rhinol Laryngol. 1985;94:458–461. doi: 10.1177/000348948509400508. [DOI] [PubMed] [Google Scholar]
- Steven Sims H, Kempiners JJ. Special airway concerns in patients with mucopolysaccharidoses. Respir Med. 2007;101:1779–1782. doi: 10.1016/j.rmed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Valayannopoulos V, de Blic J, Mahlaoui N, et al. Laronidase for cardiopulmonary disease in Hurler syndrome 12 years after bone marrow transplantation. Pediatrics. 2010;126:e1242–e1247. doi: 10.1542/peds.2009-2843. [DOI] [PubMed] [Google Scholar]
- Walker PP, Rose E, Williams JG. Upper airways abnormalities and tracheal problems in Morquio’s disease. Thorax. 2003;58:458–459. doi: 10.1136/thorax.58.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Belani KG, Braunlin EA, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36:211–219. doi: 10.1007/s10545-012-9563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoskovitch A, Tewfik TL, Brouillette RT, Schloss MD, Der Kaloustian VM. Acute airway obstruction in Hunter syndrome. Int J Pediatr Otorhinolaryngol. 1998;44:273–278. doi: 10.1016/S0165-5876(98)00063-9. [DOI] [PubMed] [Google Scholar]
- Young ID, Harper PS. Long-term complications in Hunter’s syndrome. Clin Genet. 1979;16:125–132. doi: 10.1111/j.1399-0004.1979.tb00861.x. [DOI] [PubMed] [Google Scholar]


