Abstract
Purpose
Danger-associated molecular patterns (DAMPs) released of trauma could contribute to an immune suppressed state that renders patients vulnerable towards nosocomial infections. We investigated DAMP release in trauma patients, starting in the prehospital phase, and assessed its relationship with immune suppression and nosocomial infections.
Methods
Blood was obtained from 166 adult trauma patients at the trauma scene, emergency room (ER), and serially afterwards. Circulating levels of DAMPs and cytokines were determined. Immune suppression was investigated by determination of HLA-DRA gene expression and ex vivo lipopolysaccharide-stimulated cytokine production.
Results
Compared with healthy controls, plasma levels of nuclear DNA (nDNA) and heat shock protein-70 (HSP70) but not mitochondrial DNA were profoundly increased immediately following trauma and remained elevated for 10 days. Plasma cytokines were increased at the ER, and levels of anti-inflammatory IL-10 but not of pro-inflammatory cytokines peaked at this early time-point. HLA-DRA expression was attenuated directly after trauma and did not recover during the follow-up period. Plasma nDNA (r = −0.24, p = 0.006) and HSP70 (r = −0.38, p < 0.0001) levels correlated negatively with HLA-DRA expression. Ex vivo cytokine production revealed an anti-inflammatory phenotype already at the trauma scene which persisted in the following days, characterized by attenuated TNF-α and IL-6, and increased IL-10 production. Finally, higher concentrations of nDNA and a further decrease of HLA-DRA expression were associated with infections.
Conclusions
Plasma levels of DAMPs are associated with immune suppression, which is apparent within minutes/hours following trauma. Furthermore, aggravated immune suppression during the initial phase following trauma is associated with increased susceptibility towards infections.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-015-4205-3) contains supplementary material, which is available to authorized users.
Keywords: Trauma, Injury, Immune suppression, DAMPs, Infection
Introduction
The survival of multiple trauma patients has improved significantly during the past decades [1]. However, despite improvements in both traffic safety and pre- and in-hospital management, severe trauma remains a main cause of death among young people worldwide [2]. In 2014, 25,845 people were killed and over 203,500 seriously injured in road accidents in the EU alone [3]. Roughly, trauma-related mortality can be divided into two categories. Early deaths are mainly attributed to neurological damage or severe blood loss directly related to the trauma. The patients that survive the initial trauma often develop nosocomial infections or sepsis [4], representing a significant cause of late mortality in trauma patients. The increased susceptibility of trauma patients to develop infections is mediated by a suppressed state of the immune system that develops after trauma [4–9]. Two frequently used hallmarks of the immune-suppressed state after trauma are attenuated production of cytokines by leukocytes ex vivo stimulated with pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), and decreased leukocyte HLA-DR expression [6, 8, 10–13].
Release of danger-associated molecular patterns (DAMPs), which can elicit an immune response very similar to the response to PAMPs from invading pathogens in sepsis [14, 15], could contribute to immune suppression in trauma patients. DAMPs can both be actively released by ischemic cells as danger signals or originate from damaged or dead cells as debris [16, 17]. An example of a DAMP that can be released in the case of cell damage is mitochondrial DNA (mtDNA), which can trigger an immune response via Toll-like receptor 9 [18, 19]. Moreover, heat shock protein (HSP)-70 is released following trauma [20] and has been shown to induce immune cell deactivation [21]. Furthermore, previous studies have indicated that free nuclear DNA (nDNA) in plasma is a marker for cell damage or death, because it is one of the many cell components released if a cell is ruptured [19, 22]. Therefore, it might be an indicator of general DAMP release. However, the role of these DAMPs in the immune response after trauma and the possible development of a suppressed state of the immune system is unknown.
Taken together, although immune suppression and nosocomial infections are frequently described phenomena in trauma patients, the role of DAMPs that trigger pro- and anti-inflammatory responses remains elusive. The aim of this study was to investigate the release of DAMPs following trauma, starting in the very early (prehospital) phase, and to assess its relationship with immune suppression and nosocomial infections.
Parts of this work were presented at the 33rd International Symposium on Intensive Care and Emergency Medicine, held on 19–22 March 2013 in Brussels, Belgium [23] and at the European Society of Intensive Care Medicine (ESICM) Lives Annual Congress, held on 27 September–1 October 2014 in Barcelona, Spain [24].
Methods
Study population
Adult trauma patients (n = 166) admitted to the trauma care unit at the emergency room (ER) of the Radboud University Nijmegen Medical Centre were included in the study. Exclusion criteria were expected risk of blood sampling at the trauma scene (e.g., jeopardizing the clinical handling of the patient), known HIV/AIDS, known malignancies, and use of steroids (all dosages and types of administration) or other immunomodulatory medication previously to the trauma. Selective digestive tract decontamination (SDD) was administered to all patients who were admitted to the ICU (n = 101), as part of standard ICU protocol. Therefore, comparisons between ICU patients who did and did not receive antibiotics could not be made. Of the patients who were not admitted to the ICU (n = 65), only seven received (prophylactic) antibiotics; this group size does not allow for statistical analysis. Furthermore, comparing patients that did not receive antibiotics (and thus by definition were not admitted to the ICU) with patients that did receive antibiotics (all ICU patients and the seven non-ICU patients that received antibiotics) does not yield meaningful information, because of major differences in trauma/disease severity, placement of catheters, intubation etc.
The study was carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent (CMO2011/380, NL38169.091.11). All patients or legal representatives were informed about the study details at the first opportunity, usually within 1 day after admission. The local ethical committee that approved the study protocol agreed that it was not possible to do this at an earlier stage. Written informed consent was obtained from the patient or his/her legal representative if vena puncture was necessary to obtain blood samples. All determinations and data handing were performed under the guidelines of The National Institutes of Health and in accordance with the declaration of Helsinki and its later amendments.
Control samples (n = 12) were obtained from healthy male volunteers (median age 22 [interquartile range 19–27] years) participating in an experimental human endotoxemia trial (CMO2012/455, NCT01835457). Samples were obtained from the control group at baseline, before administration of endotoxin. Written informed consent was obtained from all of these volunteers prior to screening and inclusion in the study.
Sample and data collection
Blood was sampled shortly after trauma at the trauma scene by the Helicopter Emergency Medical Services (HEMS) before hospital admission (“pre-hospital”) if applicable, at arrival at the ER, and at days 1, 3, 5, 7, and 10 following trauma. The HEMS response time (time between notification of the HEMS team and arrival at the trauma scene) was 16 [12–19] min. Time spent at the trauma scene by the HEMS team was 23 [15–29] min and the interval between sampling at the trauma scene (time-point HEMS) and sampling at the ER was 39 [33–45] min.
Lithium heparin (LH) anti-coagulated blood was obtained for ex vivo stimulation experiments as described below, which were performed immediately after sampling. Ethylenediaminetetraacetic acid (EDTA) and LH anti-coagulated blood was centrifuged after withdrawal at 1600×g at 4 °C for 10 min, after which plasma was stored at −80 °C until further analysis. EDTA plasma for real-time quantitative PCR (qPCR) analysis was centrifuged again at 16,000×g at 4 °C for 10 min to remove potential remaining cells and cell debris. The supernatant was stored at −80 °C until further analysis. Blood for mRNA analysis was sampled in PAXgene blood RNA tubes (Qiagen, Valencia, CA, USA) and stored according to the manufacturer’s instructions.
Clinical parameters and demographic data were obtained from electronic patient files. Injury severity scores (ISS) were supplied by the Regional Emergency Healthcare Network. Infection within 28 days was defined as the presence of fever and/or other infectious symptoms (pain, swelling, erythema) with leukocytosis and positive cultures and/or another visible or otherwise proven infection focus corresponding to the symptoms of the patient. The attending physicians were blinded to the immune investigation results as these assays were performed after collection of all samples from each patient.
Plasma DAMP levels
Plasma from doubly centrifuged EDTA anti-coagulated blood was diluted 1:1 with phosphate buffered saline solution (PBS) after which DNA was isolated using the QIAamp DNA Blood Midi Kit (Qiagen, Valencia, CA, USA), using the “Spin Protocol” as described by the manufacturer. Isolated DNA was stored at −20 °C until further analysis. qPCR was performed using iQ SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA, USA) on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). A primer pair specific for the GAPDH gene present in all nucleated cells of the body was used for quantification of nuclear (n)DNA levels: forward 5′-AGCACCCCTGGCCAAGGTCA-3′, reverse 5-CGGCAGGGAGGAGCCAGTCT-3′. For quantification of mitochondrial (mt)DNA levels, the following primer pair specific for the mitochondrially encoded NADH dehydrogenase 1 (MT-ND1) gene was used: forward 5′-GCCCCAACGTTGTAGGCCCC-3′ and reverse 5′AGCTAAGGTCGGGGCGGTGA-3′. Primer pairs were obtained from Biolegio (Nijmegen, the Netherlands). Samples were analyzed in duplicate and DNA isolated from blood obtained from a healthy volunteer was used on each plate as a calibrator [CV % of 1.48 % (GAPDH) and 0.41 % (mtDNA) between plates]. Plasma nDNA and mtDNA levels are expressed as fold change relative to the calibrator sample using the formula 2ΔCt.
Plasma concentrations of HSP70/HSPA1A were determined batchwise using ELISA according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA).
Plasma cytokine concentrations
Plasma concentrations of the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8, and the anti-inflammatory cytokine IL-10 were analyzed batchwise in plasma obtained from EDTA anti-coagulated blood using a simultaneous Luminex assay according to the manufacturer’s instructions (Milliplex; Millipore, Billerica, MA, USA).
Ex vivo cytokine production
Leukocyte cytokine production capacity was determined by challenging whole blood from the patients with LPS ex vivo using an in-house developed system with prefilled tubes described in detail elsewhere [25]. Briefly, 0.5 mL of blood was added to tubes prefilled with 2 mL culture medium as negative control or 2 mL culture medium supplemented with 12.5 ng/mL Escherichia coli LPS [serotype O55:B5 (Sigma Aldrich, St Louis, MO, USA), end concentration 10 ng/mL]. Cultures were incubated at 37 °C for 24 h, centrifuged, and supernatants were stored at −80 °C until analysis. Concentrations of TNF-α, IL-6, and IL-10 were determined batchwise by ELISA according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA). Ex vivo cytokine production data were censored at time of infection diagnosis, because infections can induce immune alterations.
HLA-DRA mRNA expression
RNA was isolated from blood collected in Paxgene blood RNA tubes (Qiagen, Valencia, CA, USA). cDNA was synthesized from total RNA using the iScript cDNA Synthesis Kit (Bio-rad, Hercules, CA, USA). Subsequent qPCR analysis was performed using TaqMan gene expression assays (Life Technologies, Paisley, UK) for the reference gene peptidylpropylisomerase B (PPIB) (#Hs00168719_m1) and HLA-DRA (#Hs00219575_m1) on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). We chose PPIB on the basis of its stability in inflammatory conditions in peripheral whole blood [26] and previous use as a reference gene for HLA-DRA [27]. We chose the HLA-DRA gene because it was shown to correlate well with flow cytometric analysis of mHLA-DR [27–29], an established marker of immune suppression. HLA-DRA expression levels are expressed as fold change relative to the expression of PPIB in the same sample using the formula 2ΔCt. HLA-DRA data were censored at time of infection diagnosis, because infections can decrease HLA-DR expression.
Statistical analysis
Data presented in tables and text are expressed as median [interquartile range] and data in figures as geometric mean ± 95 % CI. Mann–Whitney U and Fischer exact tests were used to investigate differences between two groups as appropriate. Differences between patient data at the various time-points and data of healthy controls were performed using Kruskal–Wallis with Dunn’s post hoc tests. Differences between time-to-infection curves were tested using log-rank (Mantel–Cox) tests. A Cox proportional hazard model was used to adjust the relationship between HLA-DRA expression and time-to-infection for the usual clinical confounders age and ISS [10]. Correlations were calculated using Spearman correlation. All analyses were performed with available data of the corresponding time-points. As a result of missing values at certain time-points or patients that were lost to follow-up, patient numbers in the analyses vary. Principal component analysis (PCA) was performed to explore the expected covariation between multiple laboratory variables and their relationship with injury severity, thereby preventing the need to list all individual correlations [30]. No imputation was used, as missing values were judged to be non-random, i.e., blood for [X] was sampled at day [Y] and could therefore not be obtained in patients who died early. Instead, a core data set of variables and patients without missing values was established. Measurements were log-transformed, mean-subtracted, and z-score was calculated on which PCA was performed on the basis of the singular value distribution in a Python script. All other statistical analyses were performed using SPSS statistics version 22 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism version 5 (Graphpad Software, La Jolla, USA). A p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 166 patients were included between August 2010 and May 2013, the characteristics of which are listed in Table 1. The majority of patients suffered from head/neck or chest injury.
Table 1.
Patient characteristics
| Total (n = 166) | |
|---|---|
| Gender | |
| Male | n = 123 (74 %) |
| Female | n = 43 (26 %) |
| Age (years) | 50 [31–67] |
| Injury severity score | 26 [17–37] |
| Head/neck injury (ISS region 1) | n = 129, 78 % |
| Face injury (ISS region 2) | n = 47, 28 % |
| Chest injury (ISS region 3) | n = 96, 58 % |
| Abdomen or pelvic contents injury (ISS region 4) | n = 44, 27 % |
| Extremities or pelvic girdle injury (ISS region 5) | n = 78, 47 % |
| External injury (ISS region 6) | n = 77, 46 % |
| ICU admissiona | n = 101 (61 %) |
| Mechanical ventilationa | n = 96 (95 %) |
| Vasopressor therapya | n = 35 (35 %) |
| ICU length of stay (days) | 3 [1–7] |
| Corticosteroids administereda | n = 8 (5 %) |
| Transfusion of blood productsa | n = 24 (14 %) |
| Hospital length of stay (days) | 8 [1–17] |
| 28-day survival | n = 127 (77 %) |
| 28-day survival (among initial trauma survivors) | n = 127/147 (86 %) |
Initial trauma survivors were defined as patients who survived the initial 7 days following trauma. Vasopressor therapy always consisted of noradrenaline. Corticosteroids included all types of administration. Blood products included erythrocytes, thrombocytes, and fresh frozen plasma
aAdmitted to or used within 28 days after hospital admission
Plasma DAMPs
Plasma nDNA levels were profoundly increased at prehospital and ER time-points compared with healthy controls (Fig. 1a). Although levels decreased in the later phase, they remained elevated during the entire follow-up period. Plasma mtDNA levels were also elevated in trauma patients compared with healthy controls (Fig. 1b), although this increase was not as pronounced and only reached statistical significance at two time-points. Similar to nDNA, plasma HSP70 concentrations in trauma patients were highest shortly after trauma and decreased later on, but nevertheless remained elevated compared with healthy controls at all time-points (Fig. 1c).
Fig. 1.
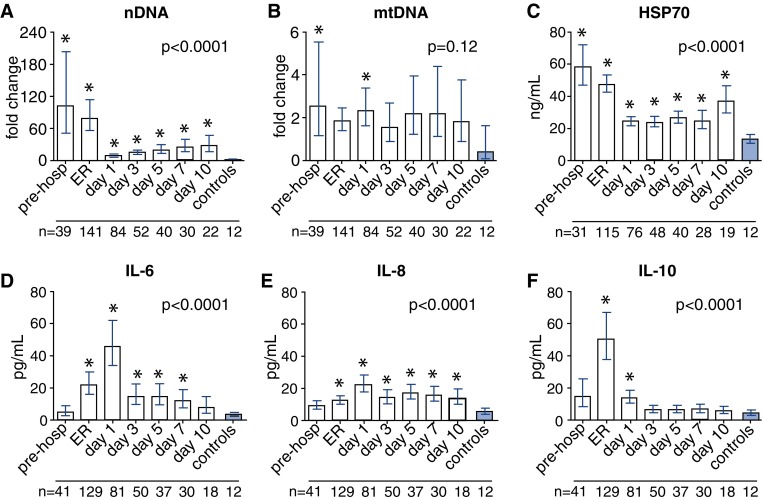
Plasma DAMP and cytokine levels. Plasma levels of nDNA (nuclear DNA, indicator of general DAMP release, a), mtDNA (mitochondrial DNA, DAMP, b), HSP70 (DAMP, c), and cytokines (d–f) in trauma patients and healthy controls. The number of patients/controls included at each time-point is indicated below each time-point. *p < 0.05 compared with healthy controls, pre-hosp prehospital, Er emergency room
Plasma cytokines
Plasma TNF-α concentrations in trauma patients were not elevated at any time-point compared with levels found in healthy controls and did not change over time (Supplementary Fig. 1). Plasma IL-6 levels were elevated from time-point ER until day 7 post-trauma (Fig. 1d), while IL-8 levels were slightly but significantly increased compared with healthy controls from time-point ER and remained elevated during the entire follow-up period (Fig. 1e). Both cytokines showed highest levels at day 1. Plasma IL-10 concentrations in trauma patients showed a distinct peak at the ER and remained significantly higher compared with healthy controls until day 1 (Fig. 1f). Plasma nDNA levels measured at the ER correlated with plasma IL-8 (r = 0.40, p < 0.0001, n = 121), IL-6 (r = 0.47, p < 0.0001, n = 121), and IL-10 (r = 0.45, p < 0.0001, n = 121) concentrations at the same time-point. Plasma HSP70 levels at the ER correlated with plasma IL-8 (r = 0.40, p < 0.0001, n = 100), IL-6 (r = 0.45, p < 0.0001, n = 100), and IL-10 (r = 0.48, p < 0.0001, n = 100) levels at that time-point.
Immune-suppressed state
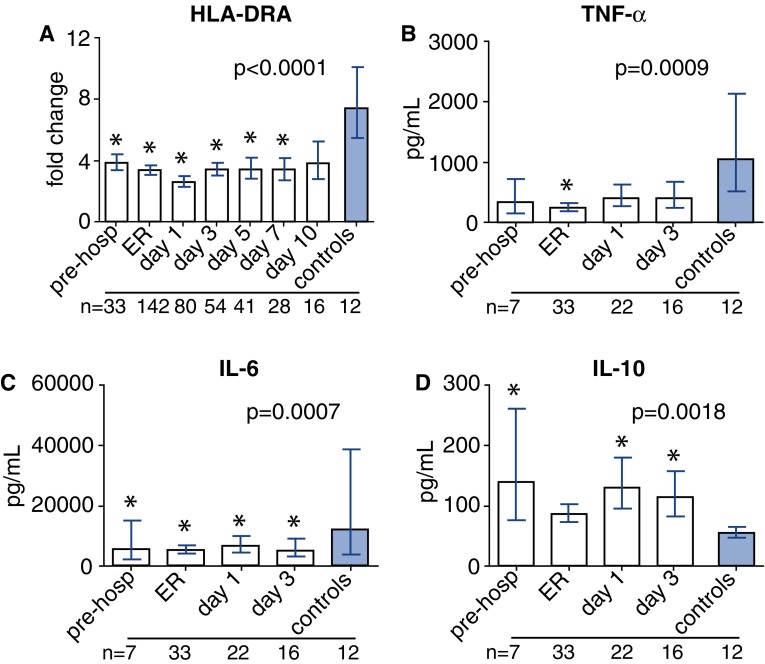
HLA-DRA mRNA expression in trauma patients was profoundly suppressed at all time-points compared with healthy controls (Fig. 2a). Plasma nDNA and HSP70 levels negatively correlated with HLA-DRA expression at time-point ER (r = −0.24, p = 0.006, n = 130, and r = −0.38, p < 0.0001, n = 106, respectively), while plasma mtDNA levels did not correlate with HLA-DRA expression (r = −0.09, p = 0.33, n = 130).
Fig. 2.
Markers of immune suppression. Leukocyte HLA-DRA mRNA expression in trauma patients and healthy controls, expressed as fold change compared with PPIB (a). Cytokine concentrations in supernatants after 24 h of whole-blood LPS stimulation in patients during the first 3 days after trauma and in healthy controls (b–d). The number of patients/controls included at each time-point is indicated below each time-point. *p < 0.05 compared with healthy controls, pre-hosp prehospital, Er emergency room
Ex vivo cytokine production capacity was investigated in a subgroup of patients (n = 36), whose characteristics were comparable to the entire patient cohort (supplementary Table 1). The capacity of leukocytes to produce pro-inflammatory cytokines TNF-α and especially IL-6 upon ex vivo stimulation with LPS was severely suppressed at the trauma scene and during the first days of hospital admission compared with healthy controls (Fig. 2b, c). In sharp contrast, ex vivo production of the anti-inflammatory cytokine IL-10 was augmented in the first days after trauma compared with healthy controls (Fig. 2d). This effect remained evident during the entire 10-day follow-up period (data not shown). Ex vivo TNF-α and IL-6 production at the ER correlated positively with HLA-DRA expression (r = 0.43, p = 0.02, n = 30, and r = 0.58, p = 0.001, n = 30, respectively). This was not the case for ex vivo IL-10 production (r = 0.22, p = 0.25, n = 30).
Relationship between injury severity, DAMPs, cytokines, and HLA-DRA
To comprehensively investigate the relationship between injury severity and the mediators measured, we PCA on data of nDNA, mtDNA, HSP70, IL-10, IL-6, IL-8, TNF-α, and HLA-DRA expression at the ER. In concordance with the individual correlations shown, the first principal component (PC1) had high loadings in the same direction of plasma nDNA, HSP70, IL-10, IL-6, IL-8, and TNF-α levels, while HLA-DRA had a smaller negative loading. PC1 had a total explained variance of 46 % and correlated with ISS (r = 0.64 p < 0.0001, supplementary Figure 2).
Susceptibility towards infections
Thirty-three patients (20 %) developed an infection during the first 28 days following trauma (characteristics of infected and non-infected patients provided in supplementary Table 2, upper part). Time until infection was 7 [4–12] days. Types of infection were pneumonia (n = 21), wound infection (n = 5), meningitis (n = 4), urinary tract infection (n = 2), central line infection (n = 1), empyema (n = 1), bacteremia (n = 1), paronychia (n = 1), and unknown (n = 1). Four patients suffered from two infections. Eighteen out of 21 patients with pneumonia were intubated at hospital admission and 15 at the moment of pneumonia diagnosis. Gender, age, and injury severity and injury location were comparable between patients who developed an infection within 28 days and those who did not. However, patients who developed an infection following trauma were more frequently admitted to the ICU, received more transfusions, and required a longer length of stay, both at the ICU and in the hospital. Furthermore, vasopressor therapy and corticosteroid use tended to be higher in patients who developed an infection. The 28-day survival was higher in patients who developed an infection compared with those who did not, likely due to direct trauma-related deaths. Indeed, when analyzing the data of patients who survived the initial phase after trauma, no difference in 28-day survival was observed (supplementary Table 2, lower part). ISS was slightly higher in patients who survived the initial phase after trauma and developed an infection, and these patients were more frequently admitted to the ICU. Furthermore, vasopressor therapy, transfusions, and corticosteroids were more frequently used, and ICU and hospital length of stay were increased in these patients.
Plasma mtDNA and nDNA levels at ER were higher in patients who developed an infection within 28 days compared with patients who did not (2.5 [1.4–6.6] vs. 1.4 [0.5–4.0] fold change, p = 0.046 for mtDNA, and 265.7 [30.7–1131.3] vs. 61.9 [11.7–367.5] fold change, p = 0.02 for nDNA). For HSP70, no differences were observed between these two groups (57.4 [26.0–79.8] vs. 47.2 [33.9–72.0] ng/mL, p = 0.89).
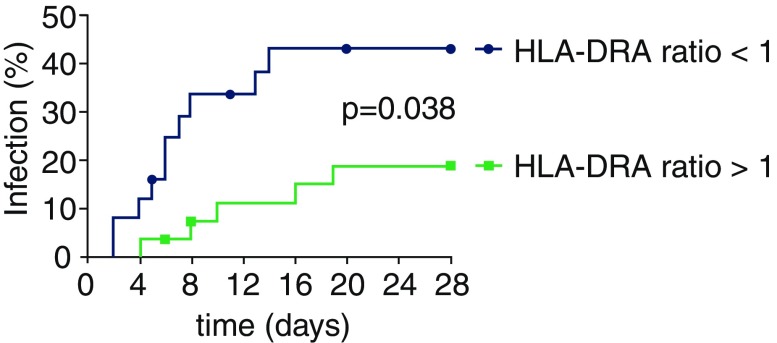
Previous studies indicate that the change in HLA-DR expression over time better predicts outcome and/or development of infections than absolute values of HLA-DR [10, 29, 31]. Accordingly, we investigated the relationship between change in HLA-DRA expression (increase or decrease between ER and day 3) and infections in a subgroup of patients from our cohort for which HLA-DRA data was available on these time-points. Patients exhibiting a decrease in HLA-DRA expression (ratio <1) more likely developed an infection compared with patients who showed an increase (ratio >1, Fig. 3). The relationship between a decrease in HLA-DRA expression and development of infection remained apparent after correcting for age and ISS (hazard ratio [95 % CI] of 3.02 [1.02–8.93], p = 0.046). Furthermore, ICU and hospital length of stay were increased in patients with decreasing HLA-DRA expression, while other characteristics were not significantly different (supplementary Table 3).
Fig. 3.
Relationship between change in HLA-DRA expression and development of infections during the first 28 days following trauma. Thirty-three (20 %) patients developed an infection within 28 days following trauma. Patients exhibiting a decrease in HLA-DRA expression between ER and day 3 (ratio <1) were more likely to develop an infection compared with those who showed an increase (ratio >1). Symbols placed on lines indicate censored patients because of death
Discussion
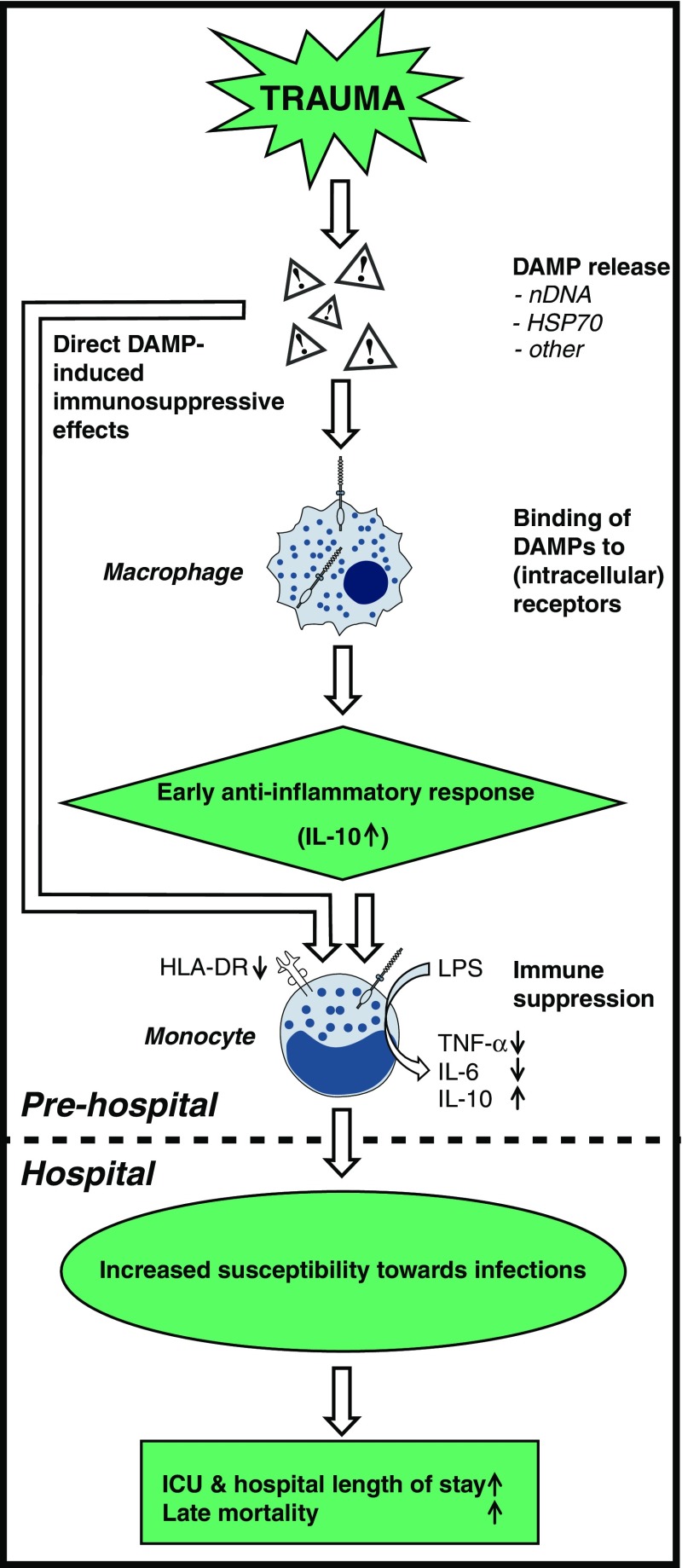
This study demonstrates that multi-trauma patients exhibit a suppressed state of the immune system already at the trauma scene, thus before admission of the patient to the hospital. This is characterized by low HLA-DRA expression and an anti-inflammatory cytokine pattern, both in vivo and ex vivo. Furthermore, we show that DAMPs are present in large quantities in the circulation during the prehospital phase and shortly after admission, and that DAMP levels are associated with the extent of immune suppression. Finally, our data demonstrate that further aggravation of immune suppression in the initial phase after trauma is associated with increased susceptibility towards infections. A conceptual representation of how DAMP release may lead to increased susceptibility towards infections following trauma is presented in Fig. 4.
Fig. 4.
Conceptual figure of how DAMP release may lead to increased susceptibility towards infections following trauma. Trauma results in the release of DAMPs including, but not limited to, nDNA and HSP70. Subsequently, DAMPs bind to (intracellular) receptors on immune cells such as macrophages, which induces a predominantly anti-inflammatory response characterized by IL-10 release. In turn, this leads to immune suppression, indicated by decreased monocytic HLA-DR expression as well as reduced production of TNF-α/IL-6 and increased production of IL-10 upon ex vivo stimulation with LPS. Alternatively, DAMPs can exert direct immunosuppressive effects, such as HSP70-induced LPS tolerance in monocytes. All these events take place in the very early (prehospital) phase following trauma. In the hospital, aggravated immune suppression is associated with increased susceptibility towards infections, consequent prolonged ICU and hospital length-of-stay, and increased late mortality
The pronounced general release of DAMPs, reflected by plasma nDNA levels, in the prehospital phase of trauma was associated with the immune suppression observed in our cohort of trauma patients. Although an observational study such as the current does not allow one to draw conclusions concerning cause and effect, our data suggests that DAMPs play a role in the suppressed state of the immune system. There are some data in support of this. HSP70 is known to induce LPS tolerance in monocytes, an in vitro phenomenon showing similarities to in vivo immune suppression [21]. Accordingly, we found a inverse relation between plasma HSP70 levels and HLA-DRA expression. Moreover, previous studies have suggested an immunomodulatory role for mtDNA in trauma patients [18, 32]. Of interest, while levels of mtDNA were increased after trauma, this increase was relatively modest. Levels of circulating nDNA were much higher and, unlike mtDNA, correlated with HLA-DRA expression, indicating that mtDNA release is not one of the major factors behind immune suppression in trauma patients. Previous studies that demonstrated much higher mtDNA concentrations in plasma had much smaller patient numbers (n = 15 [18] and n = 38 [32]) and used only a single 1600g centrifugation step [18, 32]. Chiu et al. demonstrated that double centrifugation of plasma (at 1600g and 16,000g, as performed in our study) is necessary to remove residual cells, each containing thousands of copies of the mitochondrial genome, making the results from one-spin protocol studies less reliable [33]. One could argue that a difference in injury severity could explain the difference, as Zhang et al. included only patients with ISS >25 [18]. However, Lam et al. included a majority of patients with ISS <16 [32], making it unlikely that the lower injury severity in our study population (median ISS of 26) explains the lower levels of mtDNA found.
Our study further shows that the early immune response following trauma has a distinct anti-inflammatory phenotype. Plasma levels of the archetypal pro-inflammatory mediator TNF-α were not increased whatsoever and increases in other pro-inflammatory cytokines such as IL-8 and IL-6 were relatively modest and peaked at later time-points. In sharp contrast, the anti-inflammatory cytokine IL-10 was produced rapidly following trauma and already reached peak levels at arrival in the ER. Of interest, in a previous study, trauma patients that were considered “immunoparalytic” based on HLA-DR expression on alveolar macrophages displayed higher IL-10 levels in BAL fluid [34]. IL-10 attenuates the immune response in several ways, e.g., through inhibition of the production of proinflammatory cytokines, such as TNF-α and IL-6 [35]. In our study, the initial IL-10 peak was followed by a peak in IL-6, which reached highest levels at day 1 following trauma. IL-6 is most renowned for its pro-inflammatory properties, although in trauma it is suggested that continuous IL-6 release accounts for the upregulation of anti-inflammatory mediators, such as prostaglandin E2, IL-1 receptor antagonist, IL-10, and transforming growth factor (TGF)-β and thereby also exhibits anti-inflammatory properties [36, 37]. These findings indicate that immune suppression sets in directly after the injury. The mechanisms initiating this immediate anti-inflammatory response remain to be elucidated, although these findings are in agreement with the current paradigm of the immune response during sepsis. In sepsis, it is now generally accepted that, instead of a previously assumed biphasic inflammatory response, consisting of an initial pro-inflammatory response and a subsequent compensatory anti-inflammatory response, a simultaneously occurring pro- and anti-inflammatory response is present [38]. Others have shown that the production of pro-inflammatory cytokines by leukocytes ex vivo stimulated with LPS is severely attenuated following trauma [11, 13]. Herein, we confirm these findings and demonstrate that the production of IL-10 is increased in these patients, with both phenomena already apparent at the trauma scene. This distinct anti-inflammatory phenotype ex vivo in the early phase following trauma corroborates our in vivo findings.
In keeping with previous work, our data reveal that HLA-DRA expression is decreased following trauma [6, 8, 10, 12, 13]. We importantly extend these findings by showing that this event already takes place before hospital admission and that DAMPs are associated with this phenomenon. The increased IL-10 levels early on following trauma might play a role in the decreased HLA-DRA expression, as IL-10 is known to reduce macrophage function. Furthermore, in accordance with an earlier study [13], we demonstrate that low HLA-DRA levels were associated with decreased production of pro-inflammatory cytokines in response to ex vivo stimulation of leukocytes with LPS. Several studies on small cohorts of trauma patients have investigated the relationship between HLA-DR expression and infectious complications [6, 8, 12, 13]. Some have found (trends towards) associations between low HLA-DR expression and infections [6, 13], while others have not [12]. One study showed that reduced expression of HLA-DR on alveolar macrophages, but not on circulating leukocytes, was associated with nosocomial pneumonia [8]. However, concerning the relation to outcome and/or development of infections, studies in trauma patients, septic patients, and in a cohort of ICU patients with various conditions have revealed that recovery of HLA-DR, rather than absolute values, is important [10, 29, 31]. In keeping with this, we found that a further decrease of HLA-DRA expression between admission and day 3 predicts development of infections. Taken together, these data suggest that aggravated immune suppression following the initial hit increases the risk of infection after trauma. Nevertheless, the anti-inflammatory phenotype present directly after trauma might also have beneficial effects through limiting excessive inflammation and thereby organ damage. As such, whether this phenotype is solely detrimental or has homeostatic features as well remains to be determined.
Our study has several limitations. First, inherent to this type of study, a substantial number of patients were lost to follow-up, e.g., because of discharge from the hospital or transfer to another hospital (in most cases due to recovery), or death (although mortality was low in our cohort). Therefore, if alterations in parameters observed initially in patients improve in those who recover, this could be missed. However, this does not affect the main conclusions of the manuscript as these are based on data obtained at early time-points and/or data of a subgroup of patients with a follow-up of several days. Another weakness of the current work typical for the multi-trauma patient population studied is the heterogeneity of the patients.
Second, the use of plasma nDNA levels as a marker of general DAMP release could be debated, as it is possible that specific DAMPs display other release or clearance patterns and do not necessarily follow plasma nDNA concentrations. Future studies focusing on the extensive range of DAMPs important in trauma could shed more light on this phenomenon and the importance of individual DAMPs in trauma.
Third, we used expression of the HLA-DRA gene in whole blood leukocytes as a marker of immune suppression, while most studies have used HLA-DR expression on the surface of monocytes determined using flow cytometry for this purpose. Flow cytometric analysis requires rapid analysis after sampling and the constant availability of a flow cytometer, which was not feasible in our setting, especially with regard to the samples obtained at the trauma scene. Nevertheless, the use of gene expression data is a limitation, as post-transcriptional effects can also affect HLA-DRA expression. Furthermore, next to monocytes, other cells present in whole blood may also express the HLA-DRA gene to various extents and/or may exhibit different kinetics of expression, although there is little known on this subject in the context of immune suppression. In several studies in septic patients, a population which is, similar to ours, highly heterogeneous and likely exhibiting profound changes in leukocyte counts and differentiation over time, HLA-DRA gene expression was not corrected for leukocyte counts/differentiation [27–29, 39]. Also, we do not have adequate data on daily leukocyte counts and differentiation in our cohort, as these were not regularly measured in the majority of patients. Nevertheless, the aforementioned studies in septic patients have shown that gene expression of HLA-DRA correlates well with flow cytometric analysis of mHLA-DR, with correlation coefficients ranging from 0.74 to 0.84 [27–29]; however, in one of these, a more moderate but still highly significant correlation coefficient of 0.53 was found [39]. Therefore, we feel qPCR analysis of HLA-DRA in our study is a reliable indicator of HLA-DR expression and immune suppression. Yet, we acknowledge that the lack of data on leukocyte counts is a limitation, because, next to possible effects on HLA-DR gene expression data, lymphopenia also represents a hallmark of immune suppression.
Finally, our control group consisted of solely young male volunteers. Especially with regard to the phenomenon of immunosenescence [40], this could have biased our results. However, when we compared levels of DAMPs and immunological parameters across five age categories [<28 (n = 33), 28–42 (n = 33), 43–56 (n = 34), 57–70 (n = 32), and >70 (n = 32)], or between males and females within our patient cohort, we found no differences in any of the parameters at any of the measured time-points. Also, we did not assess the functionality of the adaptive immune system, e.g., using functional assays such as proliferation or cytokine release by T cells.
In conclusion, we demonstrate that trauma results in release of DAMPs and that this is associated with an acute predominantly anti-inflammatory response and a suppressed state of the immune system. In trauma patients, these events take place already before hospital admission and the observed immune suppressed state is not preceded or accompanied by a pronounced pro-inflammatory phase. Aggravated immune suppression, as indicated by further decrease of HLA-DRA expression, is associated with the development of nosocomial infections in this patient population.
Electronic supplementary material
Plasma levels of TNF-α in trauma patients and healthy controls. The number of patients/controls included at each time-point is indicated below each time-point. The number of patients/controls included at each time-point is indicated below each time-point. * indicates p < 0.05 compared with healthy controls. pre-hosp prehospitally, Er emergency room (TIFF 550 kb)
Correlation between injury severity and the first principal component (PC1), to which IL-10, IL-6, IL-8, TNF-α, nDNA, and HSP70 contributed more than the expected average (TIFF 831 kb)
Supplementary material 5 (DOCX 17 kb)Additional file 5: Table. S3.
Acknowledgments
The authors would like to thank the Helicopter Emergency Medical Services team Lifeliner 3 coordinated by Nico Hoogerwerf for their help with the sample collection in the prehospital phase and Jelle Gerretsen for his help with the sample analysis.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
G. J. Scheffer and P. Pickkers share senior authorship.
Take-home message: Plasma levels of DAMPs are associated with immune suppression, which is apparent within minutes/hours of trauma. Furthermore, aggravated immune suppression during the initial phase following trauma is associated with increased susceptibility towards infections.
References
- 1.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma mortality in mature trauma systems: are we doing better? An analysis of trauma mortality patterns, 1997–2008. J Trauma. 2010;69:620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 2.Peden M, McGee K, Sharma G (2002) The injury chart book: a graphical overview of the global burden of injuries. World Health Organization, Geneva
- 3.Adminaite D, Allsop R, Jost G (2015) Ranking EU progress on road safety: 9th road safety performance index report. European Transport Safety Council, Brussels
- 4.Papia G, McLellan BA, El-Helou P, Louie M, Rachlis A, Szalai JP, Simor AE. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma. 1999;47:923–927. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG, Inflammation, Host Response to Injury Large-Scale Collaborative Research Program A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC., Jr Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 7.Asehnoune K, Seguin P, Allary J, Feuillet F, Lasocki S, Cook F, Floch H, Chabanne R, Geeraerts T, Roger C, Perrigault PF, Hanouz JL, Lukaszewicz AC, Biais M, Boucheix P, Dahyot-Fizelier C, Capdevila X, Mahe PJ, Le Maguet P, Paugam-Burtz C, Gergaud S, Plaud B, Constantin JM, Malledant Y, Flet L, Sebille V, Roquilly A, Corti-TC Study Group Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir Med. 2014;2:706–716. doi: 10.1016/S2213-2600(14)70144-4. [DOI] [PubMed] [Google Scholar]
- 8.Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443–450. doi: 10.1097/00024382-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38:1346–1357. doi: 10.1016/j.injury.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Gouel-Cheron A, Allaouchiche B, Floccard B, Rimmele T, Monneret G. Early daily mHLA-DR monitoring predicts forthcoming sepsis in severe trauma patients. Intensive Care Med. 2015;41:2229–2230. doi: 10.1007/s00134-015-4045-1. [DOI] [PubMed] [Google Scholar]
- 11.Flach R, Majetschak M, Heukamp T, Jennissen V, Flohe S, Borgermann J, Obertacke U, Schade FU. Relation of ex vivo stimulated blood cytokine synthesis to post-traumatic sepsis. Cytokine. 1999;11:173–178. doi: 10.1006/cyto.1998.0412. [DOI] [PubMed] [Google Scholar]
- 12.Vester H, Dargatz P, Huber-Wagner S, Biberthaler P, van Griensven M. HLA-DR expression on monocytes is decreased in polytraumatized patients. Eur J Med Res. 2015;20:84. doi: 10.1186/s40001-015-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West SD, Mold C. Monocyte deactivation correlates with injury severity score, but not with heme oxygenase-1 levels in trauma patients. J Surg Res. 2012;172:5–10. doi: 10.1016/j.jss.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile LF, Moldawer LL. DAMPs, PAMPs, and the origins of SIRS in bacterial sepsis. Shock. 2013;39:113–114. doi: 10.1097/SHK.0b013e318277109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366 [DOI] [PubMed]
- 16.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone H-J, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JZ, Liu Z, Liu J, Ren JX, Sun TS. Mitochondrial DNA induces inflammation and increases TLR9/NF-kappaB expression in lung tissue. Int J Mol Med. 2014;33:817–824. doi: 10.3892/ijmm.2014.1650. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atamaniuk J, Ruzicka K, Stuhlmeier KM, Karimi A, Eigner M, Mueller MM. Cell-free plasma DNA: a marker for apoptosis during hemodialysis. Clin Chem. 2006;52:523–526. doi: 10.1373/clinchem.2005.058883. [DOI] [PubMed] [Google Scholar]
- 20.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC (2002) Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 52:611–617 (discussion 617) [DOI] [PubMed]
- 21.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 22.Breitbach S, Tug S, Simon P. Circulating cell-free DNA: an up-coming molecular marker in exercise physiology. Sports Med. 2012;42:565–586. doi: 10.2165/11631380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Kox M, Timmermans K, Vaneker M, Scheffer GJ, Pickkers P. Immune paralysis in trauma patients; implications for prehospital intervention. Crit Care. 2013;17:S3–S4. doi: 10.1186/cc11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmermans K, Kox M, Vaneker M, Pickkers P, Scheffer GJ. Immune paralysis in trauma patients; implications for prehospital intervention. Intensive Care Med. 2014;40:S246. [Google Scholar]
- 25.Kox M, Vrouwenvelder MQ, Pompe JC, van der Hoeven JG, Pickkers P, Hoedemaekers CW. The effects of brain injury on heart rate variability and the innate immune response in critically ill patients. J Neurotrauma. 2012;29:747–755. doi: 10.1089/neu.2011.2035. [DOI] [PubMed] [Google Scholar]
- 26.Pachot A, Blond JL, Mougin B, Miossec P. Peptidylpropyl isomerase B (PPIB): a suitable reference gene for mRNA quantification in peripheral whole blood. J Biotechnol. 2004;114:121–124. doi: 10.1016/j.jbiotec.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Cajander S, Backman A, Tina E, Stralin K, Soderquist B, Kallman J. Preliminary results in quantitation of HLA-DRA by real-time PCR: a promising approach to identify immunosuppression in sepsis. Crit Care. 2013;17:R223. doi: 10.1186/cc13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C, Camus C, Fauchet R, Thomas R, Drenou B. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004;169:1144–1151. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 29.Pachot A, Monneret G, Brion A, Venet F, Bohe J, Bienvenu J, Mougin B, Lepape A (2005) Messenger RNA expression of major histocompatibility complex class II genes in whole blood from septic shock patients. Crit Care Med 33:31–38 (discussion 236–237) [DOI] [PubMed]
- 30.Tabachnick BG, Fidell LS (2007) Using multivariate statistics, 5th edn, Allyn & Bacon, Needham Heights, p 635
- 31.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 32.Lam NY, Rainer TH, Chiu RW, Joynt GM, Lo YM. Plasma mitochondrial DNA concentrations after trauma. Clin Chem. 2004;50:213–216. doi: 10.1373/clinchem.2003.025783. [DOI] [PubMed] [Google Scholar]
- 33.Chiu RW, Chan LY, Lam NY, Tsui NB, Ng EK, Rainer TH, Lo YM. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin Chem. 2003;49:719–726. doi: 10.1373/49.5.719. [DOI] [PubMed] [Google Scholar]
- 34.Nakos G, Malamou-Mitsi VD, Lachana A, Karassavoglou A, Kitsiouli E, Agnandi N, Lekka ME. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30:1488–1494. doi: 10.1097/00003246-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Howard M, O’Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 36.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 37.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med. 2013;187:1287–1293. doi: 10.1164/rccm.201301-0036CP. [DOI] [PubMed] [Google Scholar]
- 39.Cazalis MA, Friggeri A, Cave L, Demaret J, Barbalat V, Cerrato E, Lepape A, Pachot A, Monneret G, Venet F. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care. 2013;17:R287. doi: 10.1186/cc13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Curr Opin Immunol. 2014;29:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma levels of TNF-α in trauma patients and healthy controls. The number of patients/controls included at each time-point is indicated below each time-point. The number of patients/controls included at each time-point is indicated below each time-point. * indicates p < 0.05 compared with healthy controls. pre-hosp prehospitally, Er emergency room (TIFF 550 kb)
Correlation between injury severity and the first principal component (PC1), to which IL-10, IL-6, IL-8, TNF-α, nDNA, and HSP70 contributed more than the expected average (TIFF 831 kb)
Supplementary material 5 (DOCX 17 kb)Additional file 5: Table. S3.