Abstract
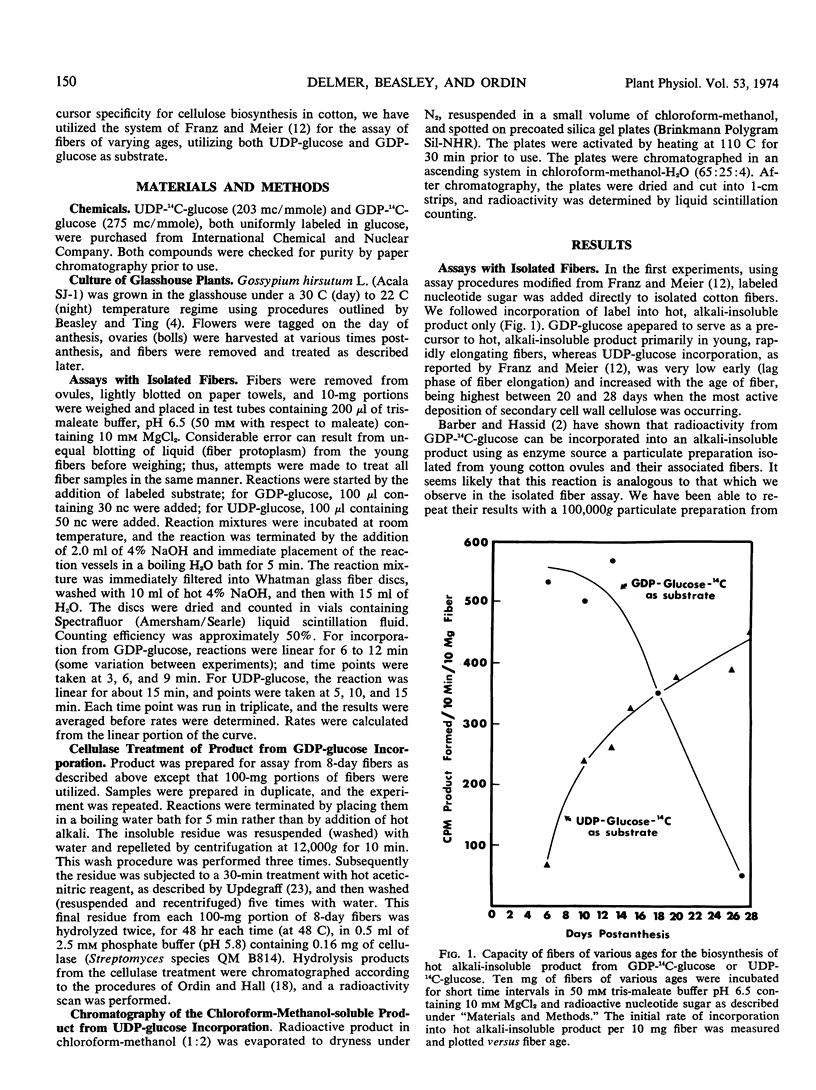
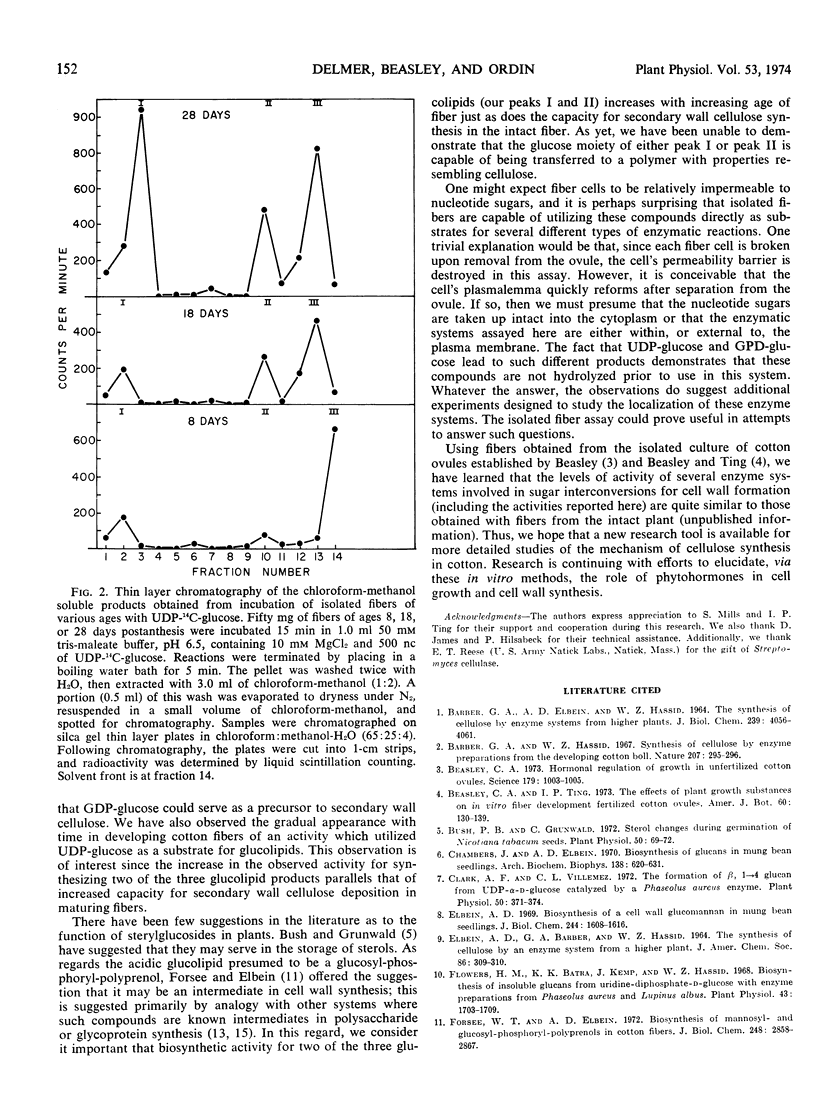
The capacity for biosynthesis of hot alkali-insoluble products using uridine diphosphate (UDP)-glucose and guanosine diphosphate (GDP)-glucose as substrate has been studied in isolated cotton fibers harvested at various stages of development following anthesis. During the period of rapid elongation and primary wall synthesis (7-14 days postanthesis), incorporation of radioactivity from GDP-14C-glucose into hot alkali-insoluble product is high. This activity gradually declines and is not demonstrated in older fibers undergoing active deposition of secondary wall. With respect to all characteristics examined, the product from GDP-glucose resembles cellulose. Incorporation of UDP-14C-glucose into hot alkali-insoluble product was low in young fibers but increased to high levels in older fibers. This product was shown to be soluble in chloroform-methanol, and when chromatographed in lipid solvents it was separated into three components. Activity for the production of two of these three presumed glucolipids increased with increasing age of fibers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER H. A., ELBEIN A. D., HASSID W. Z. THE SYNTHESIS OF CELLULOSE BY ENZYME SYSTEMS FROM HIGHER PLANTS. J Biol Chem. 1964 Dec;239:4056–4061. [PubMed] [Google Scholar]
- Barber G. A., Hassid W. Z. Synthesis of cellulose by enzyme preparations from the developing cotton boll. Nature. 1965 Jul 17;207(994):295–296. doi: 10.1038/207295b0. [DOI] [PubMed] [Google Scholar]
- Beasley C. A. Hormonal regulation of growth in unfertilized cotton ovules. Science. 1973 Mar 9;179(4077):1003–1005. doi: 10.1126/science.179.4077.1003. [DOI] [PubMed] [Google Scholar]
- Bush P. B., Grunwald C. Sterol Changes during Germination of Nicotiana tabacum Seeds. Plant Physiol. 1972 Jul;50(1):69–72. doi: 10.1104/pp.50.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J., Elbein A. D. Biosynthesis of glucans in mung bean seedlings. Formation of beta-(1,4)-glucans from GDP-glucose and beta-(1,3)-glucans from UDP-glucose. Arch Biochem Biophys. 1970 Jun;138(2):620–631. doi: 10.1016/0003-9861(70)90389-9. [DOI] [PubMed] [Google Scholar]
- Clark A. F., Villemez C. L. The Formation of beta, 1 --> 4 Glucan from UDP-alpha-d-Glucose Catalyzed by a Phaseolus aureus Enzyme. Plant Physiol. 1972 Sep;50(3):371–374. doi: 10.1104/pp.50.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Biosynthesis of a cell wall glucomannan in mung bean seedlings. J Biol Chem. 1969 Mar 25;244(6):1608–1616. [PubMed] [Google Scholar]
- Flowers H. M., Batra K. K., Kemp J., Hassid W. Z. Biosynthesis of Insoluble Glucans From Uridine-Diphosphate-d-Glucose With Enzyme Preparations From Phaseolus aureus and Lupinus albus. Plant Physiol. 1968 Oct;43(10):1703–1709. doi: 10.1104/pp.43.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Elbein A. D. Biosynthesis of mannosyl- and glucosyl-phosphoryl-polyprenols in cotton fibers. J Biol Chem. 1973 Apr 25;248(8):2858–2867. [PubMed] [Google Scholar]
- Heath E. C. Complex polysaccharides. Annu Rev Biochem. 1971;40:29–56. doi: 10.1146/annurev.bi.40.070171.000333. [DOI] [PubMed] [Google Scholar]
- Laine R. A., Elbein A. D. Steryl glucosides in Phaseolus aureus. Use of gas-liquid chromatography and mass spectrometry for structural identification. Biochemistry. 1971 Jun 22;10(13):2547–2553. doi: 10.1021/bi00789a020. [DOI] [PubMed] [Google Scholar]
- Leloir L. F. Two decades of research on the biosynthesis of saccharides. Science. 1971 Jun 25;172(3990):1299–1303. doi: 10.1126/science.172.3990.1299. [DOI] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Cellulose synthesis in higher plants from UDP glucose. Plant Physiol. 1968 Mar;43(3):473–476. doi: 10.1104/pp.43.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Studies on Cellulose Synthesis by a Cell-free Oat Coleoptile Enzyme System: Inactivation by Airborne Oxidants. Plant Physiol. 1967 Feb;42(2):205–212. doi: 10.1104/pp.42.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F. S., Ziola B., Maclachlan G. A. Particulate glucan synthetase activity: dependence on acceptor, activator, and plant growth hormone. Can J Biochem. 1971 Dec;49(12):1326–1332. doi: 10.1139/o71-192. [DOI] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Solubilization and Separation of Uridine Diphospho-d-glucose: beta-(1 --> 4) Glucan and Uridine Diphospho-d-glucose:beta-(1 --> 3) Glucan Glucosyltransferases from Coleoptiles of Avena sativa. Plant Physiol. 1971 Jun;47(6):740–744. doi: 10.1104/pp.47.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Villemez C. L., Heller J. S. Is guanosine diphosphate-D-glucose a precursor of cellulose? Nature. 1970 Jul 4;227(5253):80–81. doi: 10.1038/227080a0. [DOI] [PubMed] [Google Scholar]


