Abstract
The patient was a 76-year-old male who developed nephrotic syndrome. Idiopathic membranous nephropathy was diagnosed by renal biopsy and clinical findings. The patient had been refractory to predonisolone and cyclosporine A therapies, and overhydration recurred repeatedly during the clinical course. One year after an initial hospitalization, he had to be hospitalized a second time because of overhydration. During the hospitalization, he underwent fluid removal by the extracorporeal ultrafiltration method (ECUM), as his response to diuretics was too weak to permit the control of cardiac insufficiency. The ECUM alleviated his overhydration, but no remission of nephrotic syndrome was achieved. The patient was then discharged temporarily, but overhydration developed again 2 months later. Peritoneal dialysis (PD) using an overnight dwell of a single dose of icodextrin was initiated to obtain stable fluid removal. This promptly alleviated the refractory subcutaneous edema, and type I incomplete remission of nephrotic syndrome was achieved about 2 weeks after the start of PD. The patient could be withdrawn from the PD therapy 4 months later. Subsequently, the urinary volume was maintained and the serum creatinine level was stabilized at about 2 mg/dl. In our patient, the protein leakage into the drainage was small enough to permit remission of the nephrotic syndrome with stable fluid removal. On this basis, we believe that PD using icodextrin is considered as one of the options for the treatment of refractory nephrotic syndrome with poor water control.
Keywords: Refractory nephrotic syndrome, Membranous nephropathy, Peritoneal dialysis, Icodextrin
Introduction
Membranous nephropathy has a long and variable clinical course. In some cases, the condition remits naturally, and in others, renal insufficiency develops. For this reason, no standard treatment has yet been established. If patients present with refractory nephrotic syndrome, poor prognosis is generally expected and active treatment, such as steroid therapy, is required. Peritoneal dialysis (PD) therapy for overhydration in nephrotic syndrome is usually avoided, as this therapy is not considered to be an active indication. In this study, we performed an icodextrin-single PD therapy for refractory nephrotic syndrome induced by an idiopathic membranous nephropathy that had become refractory to various treatments, including predonisolone (PSL) and cyclosporine A (CyA), and induced poor water control. The nephrotic syndrome remitted (type I incomplete remission) after about 2 weeks of PD therapy, and the therapy was withdrawn. We believe that PD using icodextrin may be considered as one of the options for the treatment of refractory nephrotic syndrome.
Case report
A 76-year-old Japanese male with edema of the lower thigh was admitted to our hospital. The patient had a past history of bronchial ectasia at the age of 60 years and had no family history. He was diagnosed with diabetes mellitus and had been treated with oral drugs (metformin) at a local clinic for 4 years. His hemoglobin A1c (HbA1c) level ranged from 6.5 to 7.1%. Proteinuria was detected about 4 months before the first hospitalization, and edema of the lower thigh appeared about 3 months after the proteinuria. A physician at a local clinic suspected nephrotic syndrome and referred the patient to our hospital for detailed examination and treatment.
On admission, a physical examination demonstrated a clear consciousness, a blood pressure of 130/94 mmHg, and a pulse of 80/min. His height was 161.5 cm and his body weight was 68.0 kg. He had no abnormality of the heart sounds, respiratory sounds, or abdominal findings. Pitting edema was observed in bilateral lower thighs. The laboratory and urinary data were as follows, as also shown in Table 1. The urinary protein excretion value was 6.0 g/day. The serum total protein, albumin (Alb), and total cholesterol value was 5.3, 2.6 g/dl, and 325 mg/dl, respectively. Mildly reduced renal function was found. The HbA1c value was 7.1%. The abdominal computed tomography (CT) scan and ultrasonography revealed no atrophic change of bilateral kidneys. In addition, there were no symptoms of diabetic retinopathy and neuropathy.
Table 1.
Laboratory and urinary data of the patient
| Urinalysis | Peripheral blood | Blood chemistry | Serological examination | ||||
|---|---|---|---|---|---|---|---|
| Protein | (3+) | WBC | 5,500/μl | TP | 5.3 g/dl | CRP | <0.05 mg/dl |
| 5.5 g/day | RBC | 472 × 104/μl | Alb | 2.6 g/dl | IgG | 595 mg/dl | |
| Occult blood | (−) | Hb | 15.7 g/dl | AST | 28 IU/l | IgA | 335 mg/dl |
| Glucose | (−) | Hct | 49.10% | ALT | 20 IU/l | IgM | 63 mg/dl |
| Sediment | Plt | 20.4 × 104 μg/dl | LDH | 201 IU/l | C3 | 137 mg/dl | |
| WBC | 1–4/HPF | CK | 97 IU/l | C4 | 37 mg/dl | ||
| RBC | 0–1/HPF | T-cho | 325 mg/dl | ANA | <×40 | ||
| Hyaline cast | 1–2/HPF | TG | 127 mg/dl | RA | 23 IU/ml | ||
| Granular cast | 0–1/HPF | BUN | 18.2 mg/dl | HCV-Ab | (−) | ||
| Cr | 0.92 mg/dl | HBs-Ag | (−) | ||||
| Ccr | 66.4 ml/min | eGFR | 59.3 ml/min/1.73 m2 | TPHA | (−) | ||
| Urine volume | 1,100 ml/day | Cystatin C | 1.05 mg/l | STS | (−) | ||
| Selectivity index | 0.076 | UA | 5.5 mg/dl | CEA | 5.1 ng/ml | ||
| Na | 140 mEq/l | ||||||
| K | 4.5 mEq/l | ||||||
| Cl | 109 mEq/l | ||||||
| Ca | 8.3 mg/dl | ||||||
| P | 2.5 mg/dl | ||||||
| Glu | 126 mg/dl | ||||||
| HbA1c | 7.1% | ||||||
After admission, the first biopsy was performed. Membranous nephropathy (stage I–II) was diagnosed (Fig. 1). A secondary form of membranous nephropathy, associated with malignant tumor, infection, autoimmune disease, and drugs, was ruled out. Oral administration of furosemide during the hospitalization alleviated the edema and reduced the body weight by 7 kg. A natural remission was expected and the patient was discharged temporarily. He was prescribed diuretics and angiotensin II receptor-antagonist (ARB) and followed up in our outpatient clinic. No decrease of urinary protein levels was noted in the ensuing months.
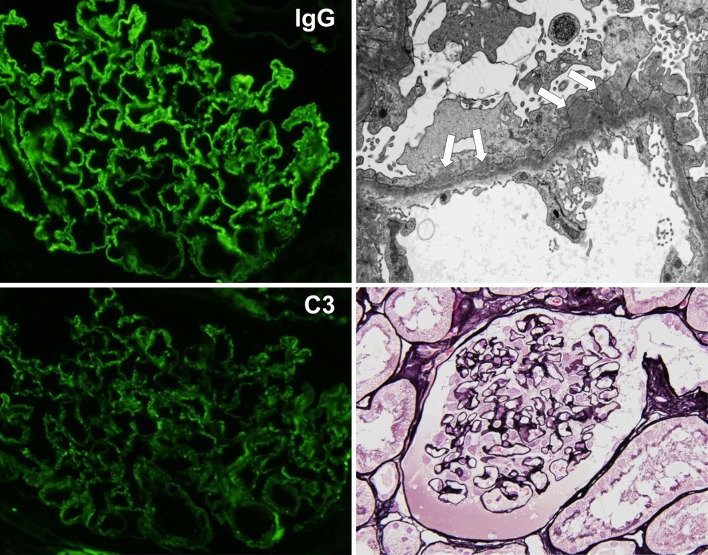
Fig. 1.
Findings of renal biopsy (first biopsy). The biopsy specimen showed 23 glomeruli, and 5 of them were deteriorated. Periodic acid silver-methenamine (PAM) staining of the non-deteriorated glomeruli revealed the partial formation of stippling and spike at the capillary. The immunofluorescence (IF) method revealed positive granular patterns of IgG and C3 along the glomerular capillary wall, and electron microscopy showed subepithelial electron-dense deposits. There were no findings suggestive of diabetic nephropathy, such as mesangial matrix accumulation, nodular glomerulosclerosis, and arteriolar hyalinosis. Membranous nephropathy (stage I–II) was diagnosed on the basis of these findings
Then, from about 7 months after his discharge, his edema worsened and his serum Alb value fell to 1.3 g/dl. PSL was initiated at about 9 months after the hospital discharge (Fig. 2). Congestion and stagnation of pleural effusion appeared, and the patient was re-hospitalized at about 12 months. He received a therapy of CyA combined with PSL. His systemic edema and congestion continued to worsen, and intravenous diuretics elicited no response. The extracorporeal ultrafiltration method (ECUM) was commenced. Fluid removal of 20 kg alleviated his overhydration, but not his refractory subcutaneous edema. Worse still, no remission of nephrotic syndrome was achieved. Low-density lipoprotein apheresis was repeated five times, whereupon his daily urinary protein level fell to about 1 g/day and his serum Alb rose to 2.2 g/dl, indicating a temporal improvement. But then, in the expectation that further fluid removal by ECUM would be required in the future was considered. Yet, the systemic subcutaneous edema was so severe, that the epidermal detachment of the skin was considered to be likely. This was likely to hinder the use of an internal shunt, hence, fluid removal by PD was considered instead. A peritoneal catheter was inserted by the stepwise initiation of PD using the Moncrief and Popovich technique (SMAP), and the patient was then discharged temporarily.
Fig. 2.
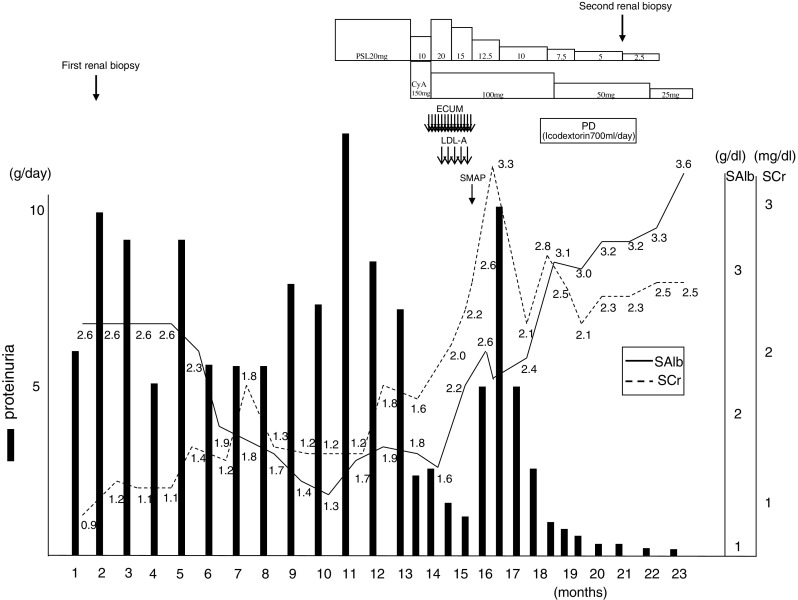
Clinical course of our patient between the first visit and remission of nephrosis. ECUM extracorporeal ultrafiltration method, LDL-A low-density lipoprotein apheresis, SMAP stepwise initiation of peritoneal dialysis using the Moncrief and Popovich technique, PD peritoneal dialysis, SAlb serum albumin, SCr serum creatinine
At about 2 months after the second discharge, he was hospitalized again and placed on PD. PD therapy with an overnight dwell of icodextrin 700 ml at each session promptly alleviated the systemic edema and pleural effusion. The refractory subcutaneous edema, a condition that persisted even after abundant water loss, disappeared completely. Two weeks after initiation of the PD therapy, the urinary protein level decreased to 1 g/day and the serum Alb level rose to 3 g/dl or higher, indicating the remission of nephrotic syndrome (incomplete remission type I). Subsequently, the urinary volume was maintained and the serum creatinine level remained stable at about 2 mg/dl. Consequently, the PD therapy was completed in 4 months (Fig. 3). The protein leakage into the PD drainage was examined several times during the course, and at each examination, the level of leakage was less than expected, ranging from about 2 to 4 g per examination.
Fig. 3.
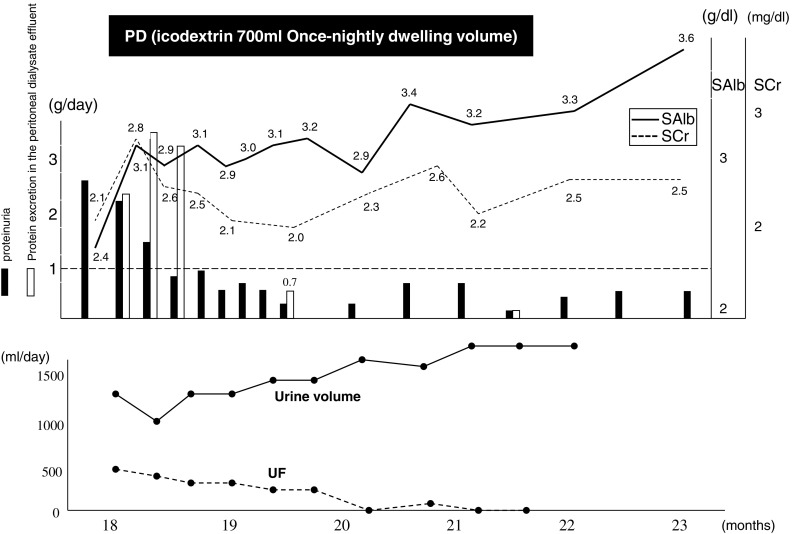
Clinical course of our patient after the initiation of peritoneal dialysis. PD peritoneal dialysis, SMAP stepwise initiation of PD using the Moncrief and Popovich technique, SAlb serum albumin, SCr serum creatinine, UF ultrafiltration
After partial remission, the second biopsy was performed. Membranous nephropathy (stage II–III) was diagnosed (Fig. 4). The immunofluorescence study showed that the expression of podocyte makers, such as nephrin and podocin, along the glomerular capillary wall was ameliorated as compared to the first biopsy findings (Fig. 5).
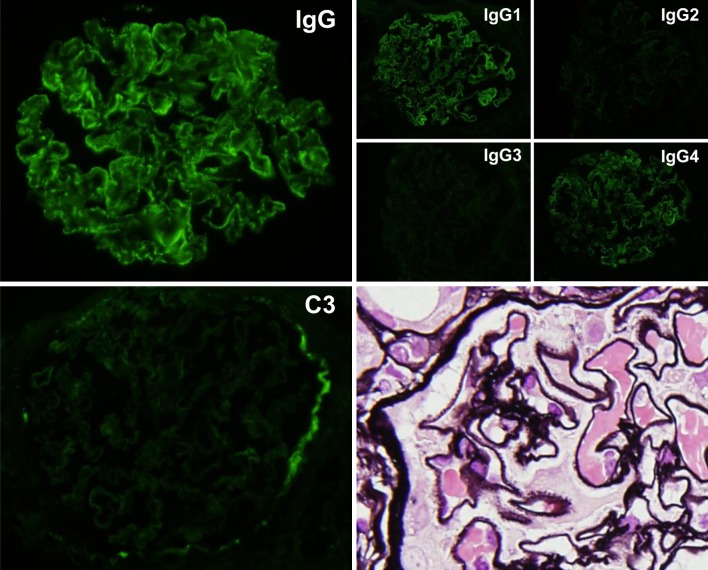
Fig. 4.
Findings of renal biopsy (second biopsy). Seventeen glomeruli were counted, and 6 of them were deteriorated. PAM staining of non-deteriorated glomeruli revealed notable spike formation, and IF showed deposits of IgG but no deposits of C3. In the determination of the IgG subclass, the results were positive for IgG1 and IgG4. No glomeruli were present in the electron microscopic specimens, but a diagnosis of membranous nephropathy (stage II–III) was determined from optical microscopic findings
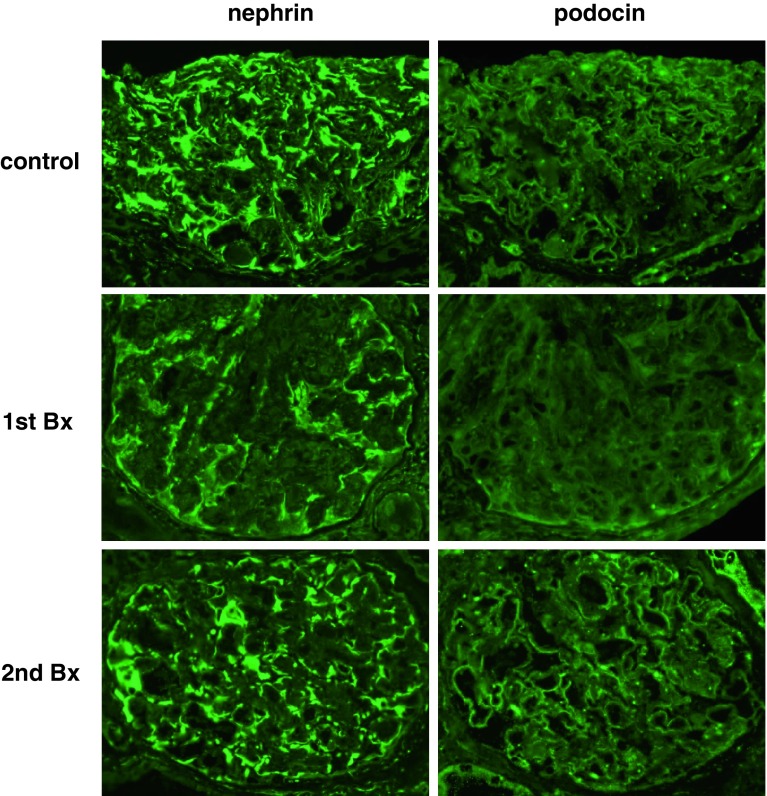
Fig. 5.
Alteration of podocyte markers’ (nephrin and podocin) expression. The expression of nephrin and podocin in control glomeruli were observed clearly on glomerular epithelial cells. However, in the first biopsy, both podocyte markers’ expression in glomeruli were weak and discontinuous. On the other hand, in the second biopsy, both podocyte markers’ expression in glomeruli were markedly ameliorated
Discussion
Steroid therapy in combination with immunosuppressants is the recommended treatment for idiopathic membranous nephropathy [1–3]. Yet, the absence of an established treatment for membranous nephropathy and the presence of natural remission in some cases make it difficult to determine whether an active treatment is recommendable or how long such a treatment should be continued. Treatment with steroids or other active agents may worsen prognosis by aggravating underlying diseases such as diabetes or leaving patients susceptible to infection. Often, preservative treatments such as ARB and diuretics may be selected as alternatives to steroids and immunosuppressants. Our patient also received these approaches to therapy. However, because of the poor control of cardiac insufficiency due to overhydration, PD therapy had to be introduced and then the nephrotic syndrome surprisingly remitted.
Many reports have stated that PD therapy is superior to hemodialysis in stabilizing hemodynamics, preventing intravascular dehydration, and preserving residual renal function by means of slow fluid removal [4–7]. When fluid removal is required for patients whose prospects for residual renal function are favorable, such as in this case, one can easily infer that slow fluid removal by PD is far more desirable than ECUM or hemodialysis.
Icodextrin, a glucose polymer with a basic glucose skeleton, has a large molecule that reduces the chance of membrane permeation. The choroidal osmotic action of the non-permeable large molecules induces water transportation through cell fine pores, which enables persistent fluid removal, compared with glucose [8]. Compared with PD using a glucose solution, a dialysis using icodextrin reportedly improves diabetes, inhibits body weight increase, and improves fat accumulation [9–11]. Icodextrin is also reported to help preserve residual renal function and urinary volume, and to reduce urinary protein [9, 12–15]. These actions may have facilitated the remission of nephrotic syndrome in this case.
For pathologic conditions presenting nephrotic syndrome, physicians tend to avoid PD therapy, as the protein leakage from the peritoneum to the drainage may worsen the condition [16]. Yet, in our case, only 2–4 g of protein, far less than that expected, leaked into the drainage. While the levels of protein leaking into the peritoneal dialysis drainage have yet to be established in patients with nephrotic syndrome, daily levels of about 3.5–13.2 g have been reported [17]. Earlier papers have reported on the effective use of PD for nephrotic syndrome, but there are only a few [18, 19]. Bertoli et al. [20] found that icodextrin administered once a day alleviated cardiac insufficiency and decreased serum Cr in 3 of 4 patients with chronic renal insufficiency complicated with refractory cardiac insufficiency. Morimoto et al. [18] reported a case in which PD with icodextrin alone, with no immunosuppressant therapy, led to a remission of nephrotic syndrome and could eventually be withdrawn. Given that renal function is likely to recover after a remission from glomerulonephritis-induced nephrotic syndrome, one can assume, logically, that PD therapy may be more useful for retaining renal function in refractory cases requiring forced fluid removal by ECUM. PD for cases with overhydration induced by diabetic nephropathy is considered to be more effective in preserving the residual renal function and prolonging the period up to the initiation of hemodialysis.
The mechanism of remission of nephrotic syndrome in our case may have had something to do with the effects of PD with icodextrin in enabling fluid removal without worsening the renal function. The improvement of fluid over volume would be reasonable to suppose that the treatment effects of steroids and immunosuppressants finally appeared at that time. It was remarkable that the refractory subcutaneous edema, a condition refractory to the ECUM, was alleviated promptly after the PD was introduced. This response may have been a specific effect of icodextrin: this agent is absorbed from the lymph ducts and moves into the blood, and its metabolites increase the plasma osmotic pressure [21]. If this is the case, we can infer that the effect in increasing the plasma osmotic pressure may facilitate the shift of water from the third-space to the blood vessels, resulting in an elimination of water from the tissues [13]. Accordingly, icodextrin might bring out an effect which was more prominent than the fluid removal effect itself in the course of the remission of nephrotic syndrome. The remission of nephrotic syndrome was achieved soon after the PD. The PD can be presumed to have had a direct effect on the remission of nephrotic syndrome.
We can also speculate that several effects are conferred when the icodextrin moves into the blood and the metabolites pass through the glomerular basement membrane (GBM). First, the circulating immune complex or in situ immune complex trapped at the GBM may be removed. This seems unlikely, however, as the nephrotic syndrome remitted only 2 weeks after the PD was introduced. Next, in order to elucidate the causes of the remarkable remission of nephrotic syndrome after the introduction of PD, we conducted a second renal biopsy. The comparison between biopsies before and after the remission revealed a pathologic change of the C3 finding from positive to negative, and an advance in the stage of membranous nephropathy. Moreover, the expression of podocyte markers (nephrin and podocin) were markedly restored (Fig. 5) [22]. We can infer that PD using icodextrin produced the protective effect of the glomerular podocyte. Third, because icodextrin metabolites are assumed to be negatively charged when passing through the GBM, we can surmise that some positively charged glomerular permeable factor may be neutralized, and that albumin leakage may then be suppressed. While the direct effect of icodextrin on the kidney still is unknown, PD therapy offers reasonable benefits in preserving the residual renal function and enabling the slow fluid removal. We believe that these actions favorably affected our patient and, ultimately, led to the remission of nephrotic syndrome.
Conclusion
Peritoneal dialysis (PD) therapy, especially that using icodextrin, can be expected to alleviate water stagnation while preserving residual renal function. We, therefore, recommend that this therapy be considered as a treatment option for refractory nephrotic syndrome with poor water control.
References
- 1.A report by the Investigation Research Group on Progressive Nephropathy specified by the Health, Labour and Welfare Ministry Diagnostic guideline for refractory nephrotic syndrome (adult cases)—from investigations and researches up to 2001. Jpn Kidney Assoc J. 2002;44:751–61. [Google Scholar]
- 2.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3:905–919. doi: 10.2215/CJN.04321007. [DOI] [PubMed] [Google Scholar]
- 3.Section of Refractory Nephrotic Syndrome, Investigation and Research Group about Progressive Nephropathy. Research project for conquest of refractory diseases by the Health, Labor and Welfare Ministry (Guidelines for the treatment of nephrotic syndrome). Jpn Kidney Assoc J. 2011;53:78–122.
- 4.Lang SM, Bergner A, Töpfer M, Schiffl H. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Perit Dial Int. 2001;21(1):52–57. [PubMed] [Google Scholar]
- 5.Tam P. Peritoneal dialysis and preservation of residual renal function. Perit Dial Int. 2009;29:S108–S110. [PubMed] [Google Scholar]
- 6.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, NECOSAD Study Group Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 7.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 8.Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 2000;20:S22–S42. [PubMed] [Google Scholar]
- 9.Cho KH, Do JY, Park JW, Yoon KW. Effect of icodextrin dialysis solution on body weight and fat accumulation over time in CAPD patients. Nephrol Dial Transplant. 2010;25:593–599. doi: 10.1093/ndt/gfp473. [DOI] [PubMed] [Google Scholar]
- 10.Babazono T, Nakamoto H, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, et al. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol. 2007;27:409–415. doi: 10.1159/000105123. [DOI] [PubMed] [Google Scholar]
- 11.Paniagua R, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009;24:422–432. [PubMed] [Google Scholar]
- 12.Adachi Y, Nakagawa Y, Nishio A. Icodextrin preserves residual renal function in patients treated with automated peritoneal dialysis. Perit Dial Int. 2006;26:405–407. [PubMed] [Google Scholar]
- 13.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003;14:2338–2344. doi: 10.1097/01.ASN.0000083904.12234.27. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Ookusa H, Tsuchida M, Takauchi Y, Yoshida I, Fujita T, et al. A dialysis re-introduction case after renal transplantation that icodextrin peritoneal dialysis solution was used preservation of transplanted residual renal function and improved QOL were found. Jpn Dial Assoc J. 2007;40:195–201. doi: 10.4009/jsdt.40.195. [DOI] [Google Scholar]
- 15.Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant. 2008;23:2982–2988. doi: 10.1093/ndt/gfn176. [DOI] [PubMed] [Google Scholar]
- 16.Blumenkrantz MJ, Gahl GM, Kopple JD, Kamdar AV, Jones MR, Kessel M, et al. Protein losses during peritoneal dialysis. Kidney Int. 1981;19:593–602. doi: 10.1038/ki.1981.57. [DOI] [PubMed] [Google Scholar]
- 17.Cooper S, Iliescu EA, Morton AR. The relationship between dialysate protein loss and membrane transport status in peritoneal dialysis patients. Adv Perit Dial. 2001;17:244–247. [PubMed] [Google Scholar]
- 18.Morimoto S, Takahashi N, Someya K, Morita T, Jo F, Toyoda N, et al. A patient with refractory nephrotic syndrome withdrawn from peritoneal dialysis. Clin Exp Nephrol. 2010;14:363–366. doi: 10.1007/s10157-010-0271-6. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto H, Masuko H, Takayama S, Nakayama M, Hosoya T. Effectiveness of E-APD on patients with chronic renal insufficiency presenting nephrotic syndrome. Kidneys Dial. 2006;61:174–177. [Google Scholar]
- 20.Bertoli SV, Ciurlino D, Maccario M, Martino S, Bigatti G, Traversi L, et al. Home peritoneal ultrafiltration in patients with severe congestive heart failure without end-stage renal disease. Adv Perit Dial. 2005;21:123–127. [PubMed] [Google Scholar]
- 21.Moberly JB, Mujais S, Gehr T, Hamburger R, Sprague S, Kucharski A, et al. Pharmacokinetics of icodextrin in peritoneal dialysis patients. Kidney Int Suppl. 2002;81:S23–S33. doi: 10.1046/j.1523-1755.62.s81.5.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, et al. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int. 2005;67:2239–2253. doi: 10.1111/j.1523-1755.2005.00328.x. [DOI] [PubMed] [Google Scholar]