Abstract
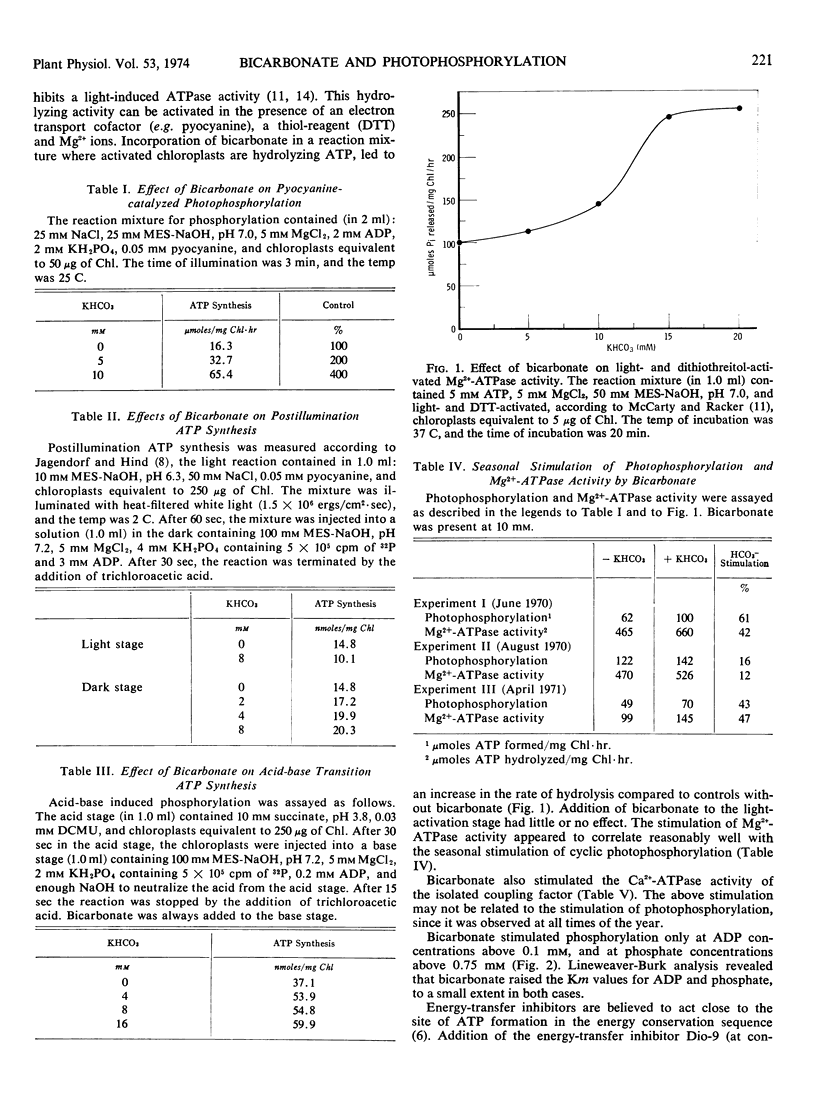
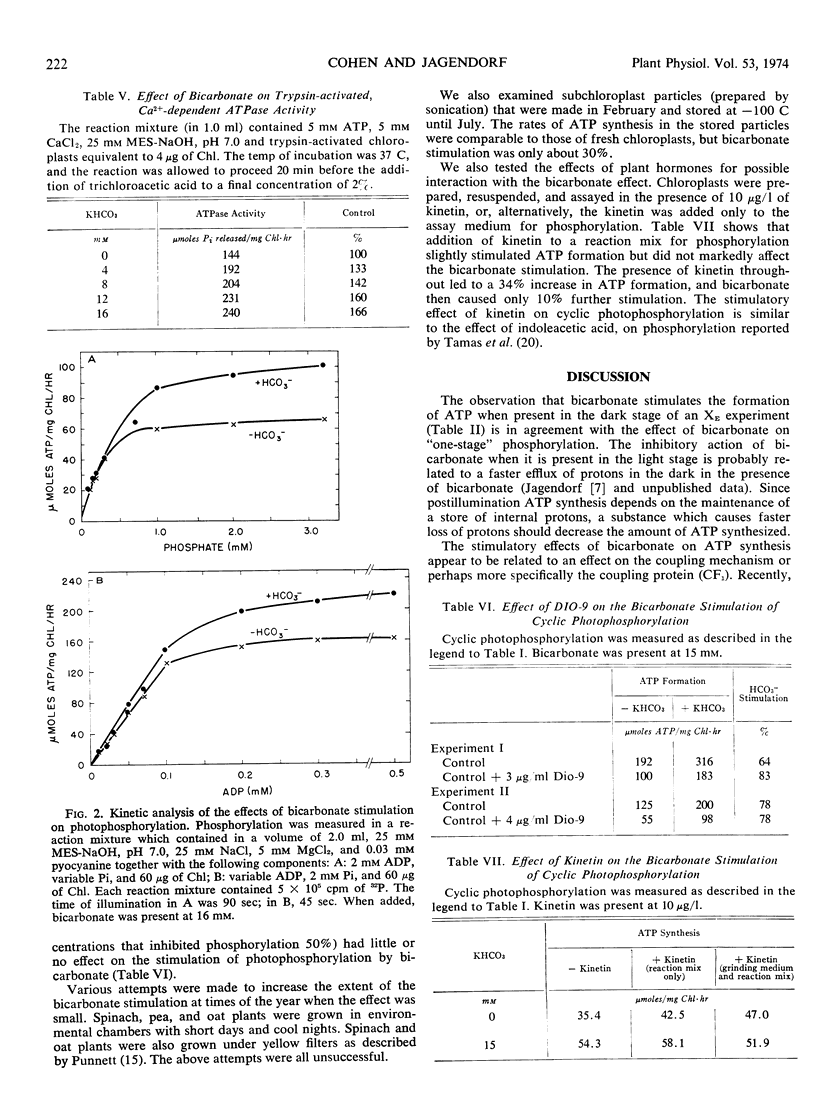
The bicarbonate effect in stimulating the rate of photophosphorylation by isolated spinach (Spinacia oleracea var. Virginia blight-resistant savoy) chloroplasts at a pH below the optimum has been re-examined. Its seasonal nature may be related to the hormonal status of the plants. Bicarbonate anions stimulate adenosine 5′-triphosphate synthesis if added in the final, adenosine 5′-triphosphate-forming stage of either a postillumination or an acid-base experiment. They also stimulate the membrane-bound, Mg2+-dependent adenosine 5′-triphosphatase of chloroplasts, and the Ca2+-dependent adenosine 5′-triphosphatase of detached coupling factor. These and other data point to the interaction between energized thylakoid membranes and the coupling factor as the probable site of action of bicarbonate anions when they stimulate photophosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P. P., Jagendorf A. T. Bicarbonate effects on the Hill reaction and photophosphorylation. Plant Physiol. 1965 Nov;40(6):1074–1079. doi: 10.1104/pp.40.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen W. S., Jagendorf A. T. Inhibition of energy-linked reactions in chloroplasts by polygalacturonate. Arch Biochem Biophys. 1972 May;150(1):235–243. doi: 10.1016/0003-9861(72)90031-8. [DOI] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Jagendorf A. T. Further studies on chloroplast adenosine triphosphatase activation by acid-base transition. J Biol Chem. 1968 Mar 10;243(5):972–979. [PubMed] [Google Scholar]
- McCarty R. E., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 3. Activation of adenosine triphosphatase and 32P-labeled orthophosphate -adeno-sine triphosphate exchange in chloroplasts. J Biol Chem. 1968 Jan 10;243(1):129–137. [PubMed] [Google Scholar]
- Murai T., Akazawa T. Biocarbonate Effect on the Photophosphorylation Catalyzed by Chromatophores Isolated from Chromatium Strain D: XII. Structure and Function of Chloroplast Proteins. Plant Physiol. 1972 Nov;50(5):568–571. doi: 10.1104/pp.50.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling factor I from chloroplasts. J Biol Chem. 1972 Oct 25;247(20):6506–6510. [PubMed] [Google Scholar]
- PUNNETT T., IYER R. V. THE ENHANCEMENT OF PHOTOPHOSPHORYLATION AND THE HILL REACTION BY CARBON DIOXIDE. J Biol Chem. 1964 Jul;239:2335–2339. [PubMed] [Google Scholar]
- Punnett T. Influence of Growth Conditions on the Enhancement of Photophosphorylation by Carbon Dioxide. Plant Physiol. 1965 Nov;40(6):1283–1284. doi: 10.1104/pp.40.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J., Jagendorf A. T. An energy-linked conformational change in the coupling factor in chloroplasts. Studies with hydrogen exchange. J Biol Chem. 1971 Jun 10;246(11):3771–3774. [PubMed] [Google Scholar]
- Ryrie I. J., Jagendorf A. T. Correlation between a conformational change in the coupling factor protein and the high energy state in chloroplasts. J Biol Chem. 1972 Jul 25;247(14):4453–4459. [PubMed] [Google Scholar]
- Ryrie I. J., Jagendorf A. T. Inhibition of photophosphorylation in spinach chloroplasts by inorganic sulfate. J Biol Chem. 1971 Feb 10;246(3):582–588. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]


