Abstract
Nephrotic syndrome due to renovascular hypertension is uncommon. We herein report a case of nephrotic syndrome associated with unilateral atherosclerotic renal artery stenosis. A 76-year-old woman who had been taking antihypertensive medication for more than 15 years was referred to our hospital for treatment of uncontrolled hypertension and massive proteinuria in the nephrotic range. An abdominal bruit was heard, and laboratory findings showed high plasma renin activity and hypokalemia. Renal computed tomography angiography showed severe stenosis of the ostium of the right renal artery and an atrophic right kidney. The left renal artery was normal and the left kidney was compensatorily enlarged. After admission, we started treatment with an angiotensin II receptor blocker and subsequently performed percutaneous transluminal renal angioplasty with renal artery stent placement. As a result, her blood pressure became well controlled and the massive proteinuria disappeared. In addition, her stenotic-side renal atrophy was resolved, concomitant with an improvement in her renal function. The contralateral renal hypertrophy was also resolved.
Keywords: Nephrotic syndrome, Hypertension, Renal artery stenosis
Introduction
Nephrotic syndrome in patients with renovascular hypertension (RVHT) is rare. The relationship between these conditions is unclear, but nephrotic syndrome may be based on compensatory glomerular hyperfiltration in the nonstenotic-side kidney caused by RVHT with unilateral renal artery stenosis. Previous reports have shown that nephrectomy and the administration of a renin–angiotensin system (RAS) inhibitor, such as an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker, decreases massive proteinuria associated with RVHT [1–5]. We herein report a case of nephrotic syndrome with unilateral atherosclerotic renal artery stenosis that was successfully treated with percutaneous renal angioplasty (PTRA) and stenting. Endovascular treatment of the renal artery stenosis remarkably reduced the patient’s proteinuria, concomitant with resolution of the contralateral renal hypertrophy.
Case report
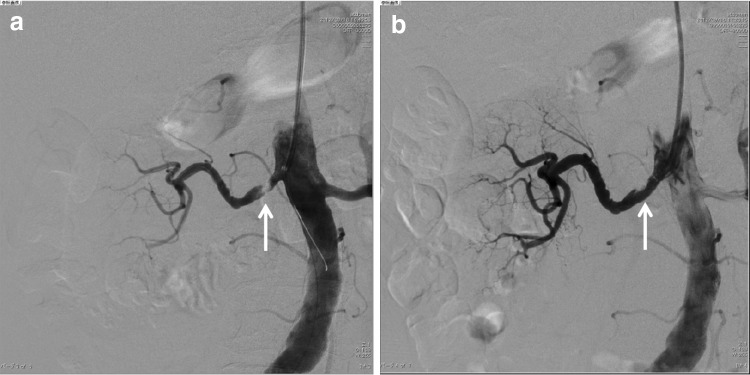
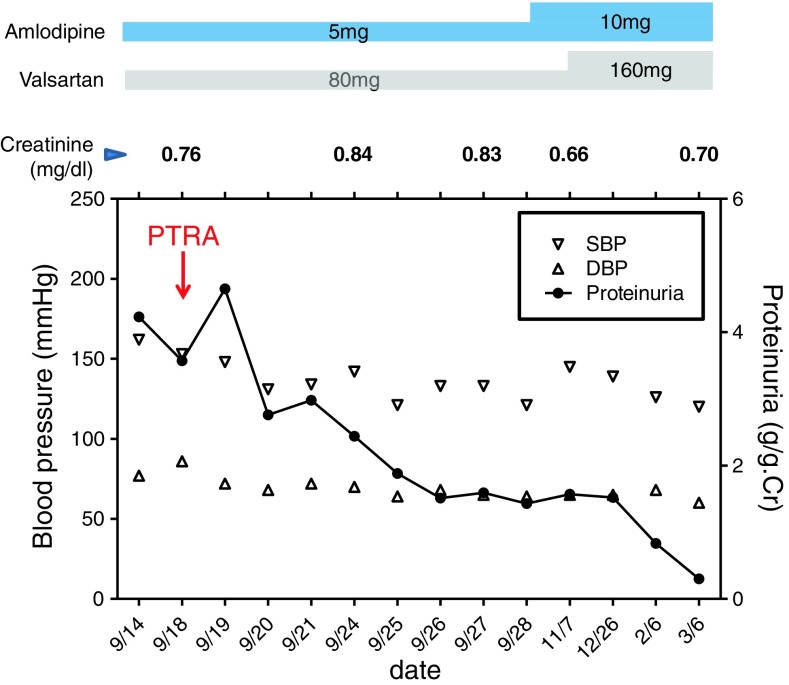
A 76-year-old woman had been treated for hypertension for more than 15 years at a nearby medical clinic. Her blood pressure (BP) had been controlled within 120–145/60–90 mmHg using amlodipine (5 mg/day). Although proteinuria had not been observed before February 2012, urine examination showed 1+ proteinuria in May 2012 and 3+ in August 2012. Her BP was also elevated, at 170/110 mmHg. In September 2012, she was referred to our hospital and admitted for intensive examination and treatment. On admission, her BP was 192/96 mmHg. She had pitting edema in her lower legs. An abdominal systolic bruit was heard. Urine examination on admission showed massive proteinuria (4+, 8.9 g/24 h) without hematuria. Her serum total protein level was 5.9 g/dl, albumin was 3.0 g/dl, cholesterol was 292 mg/dl, and triglycerides were 446 mg/dl. Her plasma renin activity was very high (>20 ng/ml/h), and her potassium level was low (2.6 mEq/l). Abdominal ultrasound examination revealed asymmetrical kidneys: the right was 84.7 × 39.4 mm, and the left was 120.0 × 55.3 mm. Renal computed tomography angiography showed severe stenosis of the ostium of the right renal artery and an atrophic right kidney. The left renal artery was normal, and the left kidney was enlarged. Because she was elderly and the stenotic lesion was located at the ostium of the renal artery, we diagnosed atherosclerotic right renal artery stenosis. A captopril-stimulated diethylenetriamine pentaacetic acid (DTPA) renogram showed that the right kidney was nonfunctional [glomerular filtration rate (GFR): right side, 9.4 ml/min; left side, 36.9 ml/min]. After admission, the patient was treated with valsartan at a dose of 80 mg/day. The patient’s BP fell to 150/90 mmHg, concomitant with a decrease in her proteinuria, but it remained in the nephrotic range (about 4 g/gCr). PTRA and stenting were subsequently performed in the right kidney on 18th September (Fig. 1). Her BP then decreased to 130/70 mmHg, and her proteinuria markedly decreased. Six days after PTRA, abdominal ultrasound examination showed symmetrical kidneys: the right was 104.2 × 50.3 mm, and the left was 104.4 × 44.5 mm. In addition, 3 months after PTRA, her right renal function, as measured by a captopril-stimulated DTPA renogram, was significantly improved (GFR: right side, 20.7 ml/min; left side, 31.1 ml/min). Six months after PTRA, our patient achieved complete remission of proteinuria (0.3 g/gCr) (Fig. 2).
Fig. 1.
Renal angiography in the patient. a Before and b after revascularization with percutaneous renal angioplasty (PTRA) and stenting. The arrows indicate the location of the stenosis before and after PTRA
Fig. 2.
The clinical course of the patient. Follow-up of proteinuria, serum creatinine, and blood pressure values (SBP systolic blood pressure, DBP diastolic blood pressure)
Discussion
In general, RVHT is rarely complicated with massive proteinuria, but there are several reported cases of nephrotic syndrome associated with RVHT. As a possible mechanism involved in the pathogenesis of massive proteinuria associated with RVHT, it seems likely that increased RAS activity plays an important role. Angiotensin II regulates the vascular tone of the afferent and efferent arterioles through the angiotensin type 1 receptor. In RVHT, excessive stimulation of angiotensin II constricts the efferent arterioles more than the afferent arterioles, resulting in an increase in the intraglomerular pressure. Angiotensin II also stimulates mesangial cell proliferation and extracellular matrix synthesis, and directly provokes widening of the intracellular junctions in vascular endothelial cells to promote glomerular capillary permeability. These multiple synergistic effects of angiotensin II would contribute to the pathogenesis and progression of proteinuria associated with RVHT. In this regard, several case reports have described the resolution of RVHT-associated nephrotic syndrome after nephrectomy [1–3], the administration of an RAS inhibitor [4, 5], aortorenal bypass surgery [6], and PTRA [7].
Combination therapy comprising angiotensin II receptor blocker administration and PTRA with stenting helped to control our patient’s BP and decrease her proteinuria. Patients with atherosclerotic renal artery stenosis reportedly have an increased risk of adverse cardiovascular events and mortality. Results from several randomized controlled trials did not prove a clinical benefit, such as preservation of renal function or blood pressure control, of PTRA compared with medical therapy [8, 9]. However, these trials involved patients with stable clinical conditions and, in many cases, only moderate renal artery lesions. Our patient exhibited rapidly progressive proteinuria and hypertension, along with severe stenosis of the renal artery. Therefore, we performed PTRA and stenting in addition to the administration of an angiotensin II receptor blocker.
Generally, the kidneys do not restore their size after having shrunk. Intriguingly, however, our patient’s stenotic-side renal atrophy was resolved, concomitant with an improvement in her renal function. This may have occurred because her clinical course was rapid and followed by a reversible ischemic condition that was probably not associated with chronic interstitial fibrosis or tubular atrophy.
With respect to histological assessment, there are a few case reports of biopsy-proven focal segmental glomerulosclerosis (FSGS) in the contralateral kidney in patients with unilateral renal artery stenosis and nephrotic syndrome [1, 6]. On the other hand, it has been reported that the post-stenotic kidney may show normal glomeruli with hyperplasia of the juxtaglomerular apparatus, a so-called protected kidney. Thus, severe stenosis of the unilateral renal artery leads to renal ischemia, subsequently activating renin excretion and, thus, provoking compensatory glomerular hyperfiltration in the contralateral kidney in an RAS-dependent manner, as described above. This hyperfiltration can cause secondary FSGS-like lesions, such as obesity-related glomerulopathy [10, 11]. Despite the lack of abnormal histological findings in the contralateral kidney of our patient, it is possible that our patient had a renal disease such as hyperfiltration-related FSGS, because the decrease in proteinuria after PTRA was accompanied by resolution of the contralateral renal hypertrophy, as estimated by abdominal ultrasound examination only 6 days after PTRA [proteinuria was reduced by 40 % between before PTRA (4.2 g/gCr) and 6 days after PTRA (2.4 g/gCr)]. However, there is a discrepancy in the timing between the complete remission of the proteinuria and the resolution of the contralateral renal hypertrophy. The reason for this is unclear, and further pathophysiological investigation is needed.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Alchi B, Shirasaki A, Narita I, Nishi S, Ueno M, Saeki T, et al. Renovascular hypertension: a unique cause of unilateral focal segmental glomerulosclerosis. Hypertens Res. 2006;29:203–207. doi: 10.1291/hypres.29.203. [DOI] [PubMed] [Google Scholar]
- 2.Ogata H, Ishiyama N, Hamabe K, Tabata T, Mitsuhasi K, Miki T, et al. Renovascular hypertension with massive proteinuria. Inter Med. 1996;35:569–573. doi: 10.2169/internalmedicine.35.569. [DOI] [PubMed] [Google Scholar]
- 3.Sekkarie M, Olutade B, Peterson P. Multiple manifestations of renovascular hypertension. Am J Kidney Dis. 1994;23:866–868. doi: 10.1016/s0272-6386(12)80142-9. [DOI] [PubMed] [Google Scholar]
- 4.Martinez Vea A, García Ruiz C, Carrera M, Oliver JA, Richart C. Effect of captopril in nephrotic-range proteinuria due to renovascular hypertension. Nephron. 1987;45:162–163. doi: 10.1159/000184103. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi F, Hasebe N, Chinda J, Okada M, Takeuchi T, Hirayama T, et al. A case of nephrotic syndrome associated with renovascular hypertension successfully treated with candesartan. Hypertens Res. 2003;26:123–127. doi: 10.1291/hypres.26.123. [DOI] [PubMed] [Google Scholar]
- 6.Alkhunaizi AM, Chapman A. Renal artery stenosis and unilateral focal and segmental glomerulosclerosis. Am J Kidney Dis. 1997;29:936–941. doi: 10.1016/S0272-6386(97)90469-8. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Chitrit S, Korzets Z, Podjarny E, Bernheim J. Reversal of the nephrotic syndrome due to renovascular hypertension by successful percutaneous angioplasty and stenting. Nephrol Dial Transplant. 1995;10:1460–1461. [PubMed] [Google Scholar]
- 8.ASTRAL Investigators. Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 9.Meier P. Atherosclerotic renal artery stenosis: update on management strategies. Curr Opin Cardiol. 2011;26:463–471. doi: 10.1097/HCO.0b013e32834a6fe8. [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/NEJM198207223070403. [DOI] [PubMed] [Google Scholar]
- 11.Praga M, Morales E. Obesity, proteinuria and progression of renal failure. Curr Opin Nephrol Hypertens. 2006;15:481–486. doi: 10.1097/01.mnh.0000242172.06459.7c. [DOI] [PubMed] [Google Scholar]