Abstract
A 69-year-old woman presented with periodic hypertension, edema, and hypokalemia that occurred within an interval of a few weeks. Her laboratory test values showed autonomously elevated plasma adrenocorticotropic hormone (ACTH) and cortisol concentrations. The patient’s Cushingoid features were not evident on first admission. Several weeks later, in spite of constant oral potassium supplementation, severe hypokalemia recurred with Cushingoid features and worsening symptoms of leg edema and pigmentation, which spontaneously disappeared within a few days. Her periodic symptoms occurred in parallel with fluctuations of plasma ACTH and cortisol concentrations. A series of endocrinological and pituitary imaging findings led to a tentative diagnosis of cyclic Cushing’s syndrome caused by ectopic ACTH secretion. However, chest and abdominal computed tomography did not reveal any candidate lesion. The patient’s periodic hypercortisolemia and symptoms were well controlled after treatment with metyrapone plus dexamethasone. This is a very rare case of periodic hypokalemia and hypertension caused by cyclic Cushing’s syndrome.
Keywords: Cyclic Cushing’s syndrome, Hypokalemia, Ectopic ACTH syndrome
Introduction
The differential diagnosis of hypertension associated with hypokalemia includes a variety of diseases: essential hypertension with diuretic use, primary aldosteronism, renal vascular disease, Cushing’s syndrome (CS), malignant hypertension, pheochromocytoma, and Liddle’s syndrome [1]. Although CS is a relatively rare disorder, accounting for <0.1 % of cases of hypertension [1], it should be considered in cases of hypertensive patients presenting with so-called Cushingoid features, which include moon face, central obesity, buffalo hump, skin atrophy, and striae.
Cyclic Cushing’s syndrome (CCS), a rare subtype of CS, is characterized by autonomous and excessive cortisol secretion in a periodic fashion, with intervals ranging from a few days to a few years (cyclic phase), interspaced with periods of normal cortisol secretion (intercyclic phase) [2, 3]. Due to the periodic fluctuation in cortisol secretion, the typical Cushingoid features of CS can be blurred and fluctuate in patients with CCS [2, 3]. In addition, the resultant fluctuating activity in the pituitary–adrenal axis could mislead the interpretation of endocrinological examinations, thus making the diagnosis of CCS challenging [2, 3].
We report herein a rare case in which the first episodes of periodic hypertension, edema, and hypokalemia all occurred within an interval of a few weeks. These symptoms were caused by CCS.
Case report
A 69-year-old woman with marked hypertension, hypokalemia, metabolic alkalosis, and malaise was admitted to Yokosuka Kyosai Hospital on 4 June 2012. Four months prior to admission, she had noticed episodic symptoms of faintness, mental depression, muscle weakness of all four limbs, and edema on both legs occurring within an interval of a few weeks. She had no significant past medical history other than appendicitis, and no smoking history. Her hypertension had been treated with methyldopa hydrate, beginning at 2 months prior to admission. On admission, her height and weight were 140 cm and 40 kg, respectively, and she presented with high blood pressure (177/100 mmHg), marked pitting edema on both lower extremities, and pigmentation of her nails, but without any Cushingoid features. Laboratory examination revealed leukocytosis with decreased lymphocyte and eosinophil counts, hypokalemia with increased transtubular potassium gradient (TTKG: 8.3), metabolic alkalosis, and diabetes mellitus (Table 1).
Table 1.
Laboratory data on admission
| Blood count | |
| White blood cells | 9700 /μL |
| Neutrophils | 82.9 % |
| Lymphocytes | 11.8 % |
| Monocytes | 3.7 % |
| Eosinophils | 0 % |
| Basophils | 0.2 % |
| Red blood cells | 424 × 104/μL |
| Hemoglobin | 12.4 g/dL |
| Platelets | 27.5 × 104/μL |
| Blood chemistry | |
| Sodium | 145 mEq/L |
| Potassium | 2.4 mEq/L |
| Chloride | 95 mEq/L |
| Urea nitrogen | 18 mg/dL |
| Creatinine | 0.45 mg/dL |
| Total protein | 6.5 g/dL |
| Albumin | 3.5 g/dL |
| AST | 37 IU/L |
| ALT | 55 IU/L |
| Glucose | 264 mg/dL |
| HbA1c (NGSP) | 7 % |
| Arterial blood gases | |
| pH | 7.527 |
| pCO2 | 45.2 torr |
| pO2 | 104 torr |
| HCO3 − | 37.3 mmol/L |
| BE | 12.9 mEq/L |
| Endocrinological findings | |
| Cortisol | 45.7 μg/dL |
| PRA | 0.3 ng/mL/h |
| PAC | 54.7 pg/mL |
| Free T3 | 2.06 pg/mL |
| Free T4 | 1.13 ng/dL |
| TSH | 0.68 μIU/mL |
| Urinalysis | |
| Specific gravity | ≥1.050 |
| Protein | (1+) |
| Sugar | (2+) |
| Blood | (1+) |
| Daily potassium excretion | 56.2 mEq/day |
AST aspartate aminotransferase, ALT alanine aminotransferase, BE base excess, PRA plasma renin activity, PAC plasma aldosterone concentration, TSH thyroid-stimulating hormone
We then conducted a series of examinations for the differential diagnosis of hypertension with hypokalemia and metabolic alkalosis. Her plasma renin activity (PRA: 0.3 ng/mL/h) and plasma aldosterone concentration (PAC: 54.7 pg/mL) were at subnormal levels. Kidney Doppler ultrasound did not show any signs of renovascular stenosis. The patient was found to have elevated plasma adrenocorticotropic hormone (ACTH) (66.2 pg/mL) and serum cortisol (28.5 μg/dL) concentrations and to be lacking a circadian rhythm of cortisol (18.8 μg/dL at 23:00) (Table 2). Meanwhile, her blood pressure, serum potassium concentrations, and blood glucose concentrations were well controlled by performing antihypertensive drug adjustment, oral potassium supplementation, and caloric restriction, respectively. She was discharged and scheduled for further examination 1 month later.
Table 2.
Time course of laboratory results and corresponding clinical conditions
| Date | 5 June | 14 June | 21 June | 18 July | 19 July | 24 July |
|---|---|---|---|---|---|---|
| Condition | 1st admission | 2nd admission | After DEX (1 mg) | After DEX (8 mg) | ||
| K (mEq/L) | 2.1 | 2.9 | 4.2 | 3.7 | ||
| ACTH (pg/mL) | 66.2 | 53 | 89.9 | 66.2 | ||
| Cortisol (μg/dL) | 45.7 | 28.5 | 39 | 40 | 24.3 | 13 |
| UFC (μg/day) | ||||||
| TTKG | 8.26 | |||||
| K suppl. (mEq/day) | 0 | 48 | 72 | 72 | 72 | 72 |
| Date | 31 July | 28 Aug. | 3 Sept. | 5 Sept. | 10 Oct. | 25 Oct. |
|---|---|---|---|---|---|---|
| Condition | CRH test | 3rd admission | CSS | CSS | CRH test | |
| K (mEq/L) | 4.6 | 2.1 | 4.4 | 3.4 | ||
| ACTH (pg/mL) | 34.1 | 119.4 | 40.3 | 36.2 | 75.7 | 60.1 |
| Cortisol (μg/dL) | 20.3 | 42.1 | 12 | 18.8 | 31.2 | 28.9 |
| UFC (μg/day) | 275 | >457.2 | 849.4 | |||
| TTKG | 12.9 | 9.89 | 7.4 | |||
| K suppl. (mEq/day) | 72 | 72 | 72 | 24 | 48 | 24 |
| Date | 31 Oct. | 2 Nov. | 9 Nov. | 14 Nov. | 25 Jan. |
|---|---|---|---|---|---|
| Condition | 2 days after metyrapone | ||||
| K (mEq/L) | 5.2 | 5.1 | 4.8 | 3.9 | |
| ACTH (pg/mL) | 18.4 | 42.3 | 52.6 | 26.6 | 18 |
| Cortisol (μg/dL) | 9.1 | 29.5 | 31.3 | 5.6 | 0.9 |
| UFC (μg/day) | 41.3 | ||||
| TTKG | 6.39 | ||||
| K suppl. (mEq/day) | 24 | 24 | 24 | 0 | 0 |
UFC urinary free cortisol of 24 h, DEX dexamethasone test, suppl. supplementation, TTKG transtubular potassium gradient
At her second hospital admission on 17 July 2012 (Table 2), her symptoms (edema and pigmentation) were improved compared with the first admission. Her cortisol level was not suppressed by the overnight dexamethasone challenge test at low dose (0.5 mg: 24.3 μg/dL), but tended to decrease at high dose (8 mg: 13 μg/dL) (Table 2). Her plasma ACTH concentrations in response to corticotropin-releasing hormone (CRH) stimulation were equivocal (basal/peak plasma ACTH concentrations: 27.9/41.4 pg/mL). No pituitary lesion was detected by magnetic resonance imaging (MRI) (Fig. 1). These results strongly suggested a diagnosis of ACTH-dependent CS, although her Cushingoid features were unremarkable and the results of endocrine examinations were equivocal in differentiating between Cushing’s disease (CD) and ectopic ACTH syndrome (EAS). She was discharged pending cavernous sinus sampling to determine the localization of the ACTH-secreting lesion.
Fig. 1.
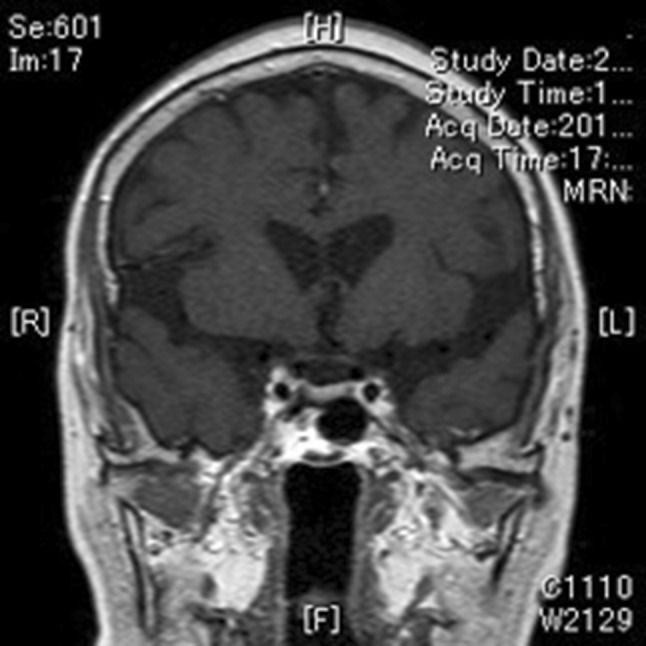
Magnetic resonance imaging (MRI) scan of the patient’s pituitary gland. T1-weighted, gadolinium-contrast-enhanced coronal view. No candidate lesion was detectable in her pituitary
On 28 August 2012 (third admission), she presented with severe hypokalemia (2.1 mEq/L) with elevated concentrations of serum cortisol (42.1 μg/dL) and plasma ACTH (119.4 pg/mL), despite continuing the same doses of oral potassium supplementation (Table 2). Physical examination on admission revealed some Cushingoid features (moon face, buffalo hump, and purpura) with worsening symptoms of leg edema and pigmentation. However, her serum potassium concentrations as well as her cortisol concentrations spontaneously normalized at 6 days after admission (serum K: 4.4 mEq/L, serum cortisol: 12.0 μg/dL). This episode, taken together with the symptoms of intermittent muscle weakness and leg edema prior to the first admission, led us to suspect a diagnosis of CCS. Cavernous sinus sampling without ACTH stimulation was conducted at 8 days after admission, which revealed no central-to-peripheral (C/P) ACTH elevation (C/P ratio <1) from both the right and left cavernous sinuses (Table 3).
Table 3.
Results of bilateral cavernous sinus sampling without CRH stimulation just after the third admission
| LCS | RCS | Peripheral | |
|---|---|---|---|
| ACTH level (pg/mL) | 46.4 | 51 | 63.6 |
| C/P ratio | 0.7 | 0.8 |
CRH corticotropin-releasing hormone, ACTH adrenocorticotropic hormone, C/P ratio central-to-peripheral ratio, LCS left cavernous sinus, RCS right cavernous sinus
Because the results of cavernous sinus sampling could not be accurately interpreted in the absence of hypercortisolemia, a second cavernous sinus sampling procedure with CRH stimulation was conducted 1 month later, when elevated concentrations of plasma ACTH (75.5 pg/mL) and serum cortisol (31.2 μg/dL) were confirmed (Table 2). Although catheterization to both cavernous sinuses was successful, no central-to-peripheral ACTH elevation (C/P ratio <3) after CRH stimulation was observed from either cavernous sinus (Table 4). Considering all of the findings above, we made a tentative diagnosis of CCS caused by ectopic ACTH secretion. However, chest and abdominal computed tomography (CT) to explore the source of the ectopic ACTH secretion did not reveal any candidate lesion.
Table 4.
Results of second bilateral cavernous sinus sampling with CRH stimulation
| LCS | RCS | Peripheral | |
|---|---|---|---|
| ACTH level before stimulation (pg/mL) | 67.9 | 69.8 | 77.4 |
| C/P ratio | 0.9 | 0.9 | |
| ACTH level 5 min after stimulation (pg/mL) | 67.3 | 80.3 | 91.5 |
| C/P ratio | 0.7 | 0.9 | |
| ACTH level 10 min after stimulation (pg/mL) | 88.9 | 82.9 | 75 |
| C/P ratio | 1.2 | 1.1 | |
| ACTH level 15 min after stimulation (pg/mL) | 93.7 | 85.8 | 78.7 |
| C/P ratio | 1.2 | 1.1 |
CRH corticotropin-releasing hormone, ACTH adrenocorticotropic hormone, C/P ratio central-to-peripheral ratio, LCS left cavernous sinus, RCS right cavernous sinus
The patient was referred and admitted to Tokyo Medical and Dental University Hospital for re-evaluation of the diagnosis and the treatment of CCS on 23 October 2012. Repeated hormonal measurement during that admission demonstrated distinct periodic fluctuations in serum cortisol and plasma ACTH concentrations (Table 2). Re-evaluation of hypothalamic–pituitary–adrenal (HPA) axis function was performed at the period of hypercortisolemia. Plasma ACTH concentrations did not respond to CRH stimulation (basal/peak ACTH levels: 83.6/75.4 pg/mL), while the response of plasma ACTH concentrations to desmopressin was equivocal (basal/peak ACTH levels: 52.6/77.1 pg/mL). Imaging analysis by pituitary MRI, neck, chest, and abdominal CT and thyroid ultrasonography revealed no abnormal mass except for bilateral adrenal enlargement. In order to control the periodic fluctuation in hypercortisolemia, metyrapone (2 g) was introduced.
At 4 months after the initiation of metyrapone treatment (as of the time of this writing), the patient’s blood pressure, serum potassium concentrations, and blood glucose concentrations were normalized without any antihypertensive drug and potassium supplementation. Serum cortisol concentrations remained low (<5 μg/dL) with dexamethasone (0.5 mg) replacement, and her Cushingoid features gradually disappeared.
Discussion
CCS is a rare subtype of CS that is characterized by periodic repetition of a cortisol hypersecretion phase (cyclic phase) ranging from 12 h to 86 days and a period of normal cortisol secretion (intercyclic phase) ranging from days to years [2, 3]. The confirmation of alternations of at least three cyclic phases and two intercyclic phases is required for a diagnosis of CCS [4]. In the present case, periodic ACTH-dependent hypercortisolemia with Cushingoid features, which were later unmasked during the patient’s clinical course, verified the diagnosis of CCS.
The diagnosis of CCS is often difficult because typical Cushingoid features can be mild or can fluctuate due to intermittent hypercortisolemia [2, 3]. The present patient initially had hypertension, metabolic alkalosis, and hypokalemia with elevated urinary TTKG values. In particular, TTKG is used as a semiquantitative index of the activity of the potassium secretory process, monitoring daily urinary potassium excretion [5]. In hypokalemia, a TTKG value >2 indicates an inappropriate renal response and increased mineralocorticoid activity [6]. Therefore, the signs and symptoms of the present case were compatible with those of mineralocorticoid excess. In addition, subnormal levels of PRA and PAC accompanied by ACTH-dependent autonomous hypercortisolemia led us to consider that a series of these clinical manifestations in this patient could be caused by ACTH-dependent CS, although the patient’s Cushingoid features were initially not evident. Indeed, the fact that these periodic symptoms were well associated with the hypercortisolemia at the cyclic phase, together with the resolution of these symptoms after the initiation of metyrapone treatment, proved that a series of periodic symptoms such as hypokalemia and hypertension in our patient were caused by CCS.
A recent clinical review documented that the underlying cause of CCS is CD in 54 % of cases, EAS in 26 % of cases, and adrenal CS in 11 % of cases [2]. In ACTH-dependent CCS cases, however, it is extremely challenging to differentiate between CD and EAS, for two reasons. First, periodic fluctuation in ACTH and cortisol secretion can lead to erroneous interpretation of results from endocrine tests for the differential diagnosis of ACTH-dependent CS [2, 3]. Second, it has been shown that each endocrine test on its own has a relatively limited diagnostic accuracy to differentiate between CD and EAS [7, 8]. Therefore, in addition to the combination of multiple endocrine tests and imaging analyses, cavernous sinus sampling, which has been regarded as the gold standard for differential diagnosis between CD and EAS [9–11], is often required for definitive diagnosis.
In the present case, considering the time course changes of plasma ACTH and cortisol concentrations, a high-dose (8 mg) dexamethasone suppression test (DST) on the second admission appeared to be performed during the declining part of the cyclic phase, which made its interpretation difficult. Similarly, the results of the CRH stimulation test conducted on the second admission were not consistent with the results on the last admission; the former test was conducted during the declining part of the cyclic phase and the latter test during the inclining or peak part of the cyclic phase. The result of the DDAVP (1-deamino-8-d-arginine vasopression) test conducted at the peak part of the cyclic phase, which has been shown to be useful for the differential diagnosis of ACTH-dependent CS [12], was also equivocal. Thus, even a combination of multiple endocrine tests could not differentiate between CD and EAS in the present case.
Cavernous sinus sampling is the most accurate examination for distinguishing patients with CD from those with EAS [9–11]. In the present patient, cavernous sinus sampling was conducted twice. The result of the first sampling revealed no central-to-peripheral ACTH elevation (C/P ratio <1). However, the sampling was conducted at the intercyclic phase, so we considered that the result might be a false negative due to a decrease in ACTH secretion from the pituitary (lesion). Nevertheless, the second sampling with CRH stimulation at the cyclic phase revealed no central-to-peripheral ACTH elevation. Taken together with the results of endocrine tests, imaging analyses, and cavernous sinus sampling, we tentatively diagnosed the present case as CCS caused by ectopic ACTH secretion.
Localization of an ectopic ACTH-secreting lesion is also extremely challenging, since no lesion is found in about 15 % of patients with EAS [13, 14]. So far, we have not been able to find any candidate lesion for ectopic ACTH secretion in this patient. Several epidemiologic studies have shown that nearly 50 % of ectopic ACTH-secreting tumors are localized in the chest, including small cell lung carcinoma and carcinoid tumors [8, 15]. In the present case, continuous effort should be made to explore the candidate lesion through the combination of anatomical and functional imaging modalities, including CT, MRI, and somatostatin receptor scintigraphy.
The most effective treatment for ACTH-dependent CS is surgical resection of the source of the ACTH-secreting lesion. However, if extensive imaging analysis fails to localize the source of the ACTH-secreting lesion, medical therapy is warranted to control hypercortisolemia [16]. In Japan, three drugs (metyrapone, mitotane, trilostane) are approved for this purpose. Among them, metyrapone blocks cortisol biosynthesis through the selective inhibition of adrenal 11β-hydroxylase without major adverse effects during long-term use [8, 16]. As shown in the present case, complete blockade of cortisol synthesis by metyrapone, with glucocorticoid replacement such as dexamethasone, could be a useful therapeutic option to control hypercortisolemia, even under the condition of periodic fluctuation of serum cortisol concentrations due to CCS.
In summary, we have reported a rare case of periodic hypokalemia caused by CCS. A series of endocrinological and imaging findings led us to the tentative diagnosis of CCS caused by unidentified ectopic ACTH secretion. The patient’s periodic hypercortisolemia and symptoms were well controlled after treatment with metyrapone plus dexamethasone.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Handler J. Cushing’s syndrome with uncontrolled hypertension, occasional hypokalemia, and two pregnancies. J Clin Hypertens (Greenwich) 2010;12:516–521. doi: 10.1111/j.1751-7176.2010.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol. 2007;157:245–254. doi: 10.1530/EJE-07-0262. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson AB, Kennedy AL, Carson DJ, Hadden DR, Weaver JA, Sheridan B. Five cases of cyclical Cushing’s syndrome. Br Med J (Clin Res Ed) 1985;291:1453–1457. doi: 10.1136/bmj.291.6507.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RD, Van Loon GR, Orth DN, Liddle GW. Cushing’s disease with periodic hormonogenesis: one explanation for paradoxical response to dexamethasone. J Clin Endocrinol Metab. 1973;36:445–451. doi: 10.1210/jcem-36-3-445. [DOI] [PubMed] [Google Scholar]
- 5.Ethier JH, Kamel KS, Magner PO, Lemann J, Jr, Halperin ML. The transtubular potassium concentration in patients with hypokalemia and hyperkalemia. Am J Kidney Dis. 1990;15:309–315. doi: 10.1016/s0272-6386(12)80076-x. [DOI] [PubMed] [Google Scholar]
- 6.Choi MJ, Ziyadeh FN. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol. 2008;19:424–426. doi: 10.1681/ASN.2007091017. [DOI] [PubMed] [Google Scholar]
- 7.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 8.Doi M, Sugiyama T, Izumiyama H, Yoshimoto T, Hirata Y. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J. 2010;57:1061–1069. doi: 10.1507/endocrj.K10E-265. [DOI] [PubMed] [Google Scholar]
- 9.Kaltsas GA, Giannulis MG, Newell-Price JD, Dacie JE, Thakkar C, Afshar F, Monson JP, et al. A critical analysis of the value of simultaneous inferior petrosal sinus sampling in Cushing’s disease and the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1999;84:487–492. doi: 10.1210/jcem.84.2.5437. [DOI] [PubMed] [Google Scholar]
- 10.Graham KE, Samuels MH, Nesbit GM, Cook DM, O’Neill OR, Barnwell SL, Loriaux DL. Cavernous sinus sampling is highly accurate in distinguishing Cushing’s disease from the ectopic adrenocorticotropin syndrome and in predicting intrapituitary tumor location. J Clin Endocrinol Metab. 1999;84:1602–1610. doi: 10.1210/jcem.84.5.5654. [DOI] [PubMed] [Google Scholar]
- 11.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 12.Sakai Y, Horiba N, Tozawa F, Sakai K, Kuwayama A, Demura H, Suda T. Desmopressin stimulation test for diagnosis of ACTH-dependent Cushing’s syndrome. Endocr J. 1997;44:687–695. doi: 10.1507/endocrj.44.687. [DOI] [PubMed] [Google Scholar]
- 13.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: 20 years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 14.Wajchenberg BL, Mendonca BB, Liberman B, Pereira MA, Carneiro PC, Wakamatsu A, Kirschner MA. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994;15:752–787. doi: 10.1210/edrv-15-6-752. [DOI] [PubMed] [Google Scholar]
- 15.Imura H. Ectopic hormone syndromes. Clin Endocrinol Metab. 1980;9:235–260. doi: 10.1016/S0300-595X(80)80032-6. [DOI] [PubMed] [Google Scholar]
- 16.Nieman LK. Medical therapy of Cushing’s disease. Pituitary. 2002;5:77–82. doi: 10.1023/A:1022308429992. [DOI] [PubMed] [Google Scholar]


