Abstract
Acute onset of severe proteinuria during pregnancy obliges physicians to clinically discriminate between gestational proteinuria (GP) and new onset of nephritis. A multiparous woman developed severe proteinuria (5.8 g/day) without hypertension at 32 weeks of gestation. We measured the maternal level of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng), which were extremely high (41.3 and 54.8 ng/ml, respectively), leading us to consider this condition as GP rather than acute onset of nephritis. Thus, we did not perform a kidney biopsy and did not administer a steroid agent. Non-reassuring fetal status required emergency Cesarean section at 33 weeks. Proteinuria decreased to 0.36 g/day at 12 weeks after delivery, and finally disappeared 26 weeks postpartum. Measurement of sFlt-1 and sEng in a pregnant woman with severe proteinuria without hypertension may assist in differential diagnosis of GP from acute onset of nephritis, and thus help to decide whether to perform kidney biopsy during pregnancy.
Keywords: Gestational proteinuria, Nephritis, Pregnancy, Soluble fms-like tyrosine kinase 1, Soluble endoglin
Introduction
Acute onset of proteinuria without hypertension after 20 weeks of gestation suggests occurrence of gestational proteinuria (GP); however, acute onset of nephritis during pregnancy, although rare, should be differentiated from it, for which kidney needle biopsy is needed. Depending on the results, steroid administration may be considered; however, biopsy is sometimes dangerous during pregnancy, or is at least invasive [1].
One possible way to discriminate the two conditions may be to measure the levels of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng). While GP might show elevated levels, nephritis might not; however, this notion is not widely known, and few studies have focused on it [2, 3]. Here, we present a woman for whom their measurement may have contributed to deciding not to perform kidney biopsy, leading to nonadministration of steroids.
Case report
A 36-year-old Japanese woman developed GP repeatedly during her first and second pregnancies. In her first pregnancy, she underwent prenatal checks and delivery at a primary obstetric facility. She was normotensive without proteinuria throughout the first and second trimesters. After 32 weeks of gestation, proteinuria 2+ on dipstick urinalysis emerged and continued until 37 weeks, when she underwent Cesarean section due to breech presentation. Proteinuria improved after delivery and was negative at 16 months postpartum. In her recent second pregnancy, 6 years after her first pregnancy, she underwent initial prenatal care at our hospital at 8 weeks of gestation. She was normotensive (blood pressure, 105/66 mmHg) and did not show proteinuria. Proteinuria 2+ on dipstick urinalysis without hypertension first emerged at 26 weeks of gestation. We watched her carefully as an outpatient every 2 weeks. Since severe proteinuria (5.8 g/day) and fetal growth restriction (FGR) (<5th percentile) developed at 32+2 weeks gestation, we decided to admit her for close observation. Her blood pressure was 116/83 mmHg, and slight pretibial edema was noted. She had gained 2.8 kg in weight compared with her prepregnancy weight (from 52.5 to 55.3 kg gain) by 32 gestational weeks. There were no significant changes in laboratory data on admission except for an increased level of uric acid; white blood count (WBC) was 10.4 × 103/μl, red blood count (RBC) was 461 × 104/μl, hemoglobin was 14.2 g/dl, hematocrit was 41.4 %, platelet count was 22.4 × 104/μl, antithrombin activity was 100.6 %, albumin was 2.5 g/dl, creatinine was 0.64 mg/dl, and uric acid was 9.1 mg/dl. There were no urinary casts in urine.
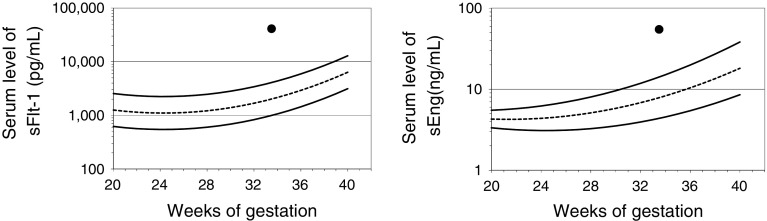
At that time, both GP and acute onset of nephritis were considered, for which different approaches must be employed. While the former requires close observation of maternal and fetal condition, the latter might require treatment, including steroid administration. Kidney biopsy could be needed and thus was scheduled. She was told to rest and eat a protein-restricted diet under a physician’s advice. One week later, we thought that measurement of serum levels of sFlt-1 and sEng might be useful to discriminate between GP and nephritis; both antiangiogenic factors were checked with informed consent at 33+3 weeks of gestation. Her blood sample was centrifuged at 4 °C at 2500 rpm for 15 min. Serum levels of sFlt-1 and sEng were immediately determined by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA), revealing that they were both significantly increased (41.3 and 54.8 ng/ml, respectively) according to the normal reference ranges (Fig. 1) [4, 5]. This suggested that her proteinuria had probably emerged by a similar mechanism to preeclampsia (PE), but not nephritis. This information assisted in our decision not to perform kidney biopsy and not to treat her with a steroid agent.
Fig. 1.
Serum levels of sFlt-1 and sEng in this case, plotted as quadratic curves representing the mean and 5th and 95th percentiles of serum level of sFlt-1 (a) and sEng (b) from 20 to 38 weeks of gestation. Solid curves represent the 5th and 95th percentiles of the reference values, and the dotted curve represents the mean. Filled circles represent the case in this study
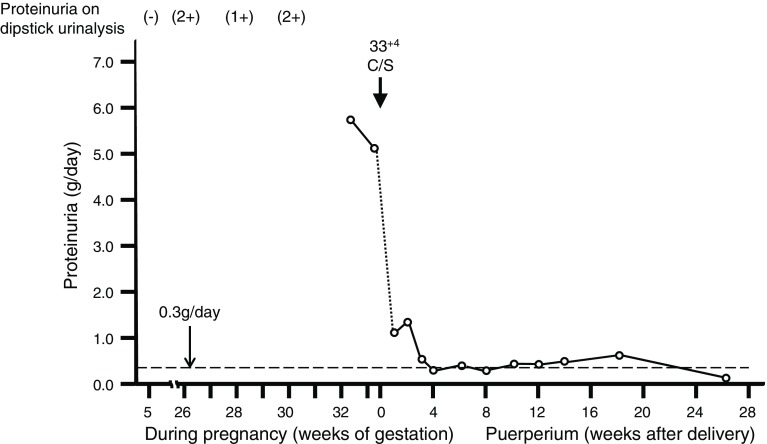
Her severe proteinuria did not improve and hypertension did not emerge, irrespective of the marked increases of uric acid, sFlt-1, and sEng. Non-reassuring fetal status emerged at 33+4 weeks of gestation and required emergency Cesarean section; a male infant was delivered weighing 1290 g with Apgar scores of 8 and 9 at 1 and 5 min, respectively. Proteinuria resolved to 1.3 g/day at 2 weeks after delivery, and decreased to 0.36 g/day at 12 weeks after delivery. We discussed the need for postpartum needle biopsy of the kidney for diagnosis, but decided to continue checking her proteinuria carefully without kidney biopsy, because her proteinuria gradually improved. Proteinuria finally disappeared 26 weeks postpartum. The detailed changes of 24-h proteinuria before and after delivery are shown in Fig. 2. In the pregnancy and postpartum period, this case did not have any microscopic hematuria.
Fig. 2.
Changes of proteinuria before and after delivery. Empty circles indicate proteinuria levels (g/day), and the changes of proteinuria levels before and after delivery are shown in a line chart. The proteinuria on dipstick urinalysis, time of Cesarean section (C/S), and a line of proteinuria of 0.3 g/day are indicated on the chart
Discussion
Kidney biopsy in a pregnant or puerperal woman with proteinuria may reveal whether she has underlying renal diseases; however, is kidney biopsy necessary in all women with severe proteinuria without hypertension? Is kidney biopsy necessary in all women with persistent proteinuria after 12 weeks postpartum? This case, at least partly, answers these questions.
First, during pregnancy, we considered that kidney biopsy was unnecessary in our case, because serum levels of sFlt-1 and sEng were markedly increased, similar to in GP and PE [2, 6–9]. This strongly indicated that her proteinuria had probably emerged by a similar mechanism to PE, but not acute onset of nephritis. We previously reported that sFlt-1 increased in 57 % and sEng increased in 86 % of 7 women with GP, including 3 cases of severe proteinuria [2]. We concluded that some women with GP have high risk for PE later; however, pregnancy is terminated during the GP stage before PE may become evident. Growing evidence indicates that sFlt-1 and sEng are frequently increased, and placental growth factor (PlGF) is frequently decreased, in women with PE [6–9]. In the present patient, we later measured the serum level of PlGF at 33 weeks using preserved material: it was markedly decreased (PlGF: 152 pg/ml; 5th percentile of PlGF at 33 weeks: 251 pg/ml). Thus, the change in not only sFlt-1 and sEng but also PlGF favors our assumption that the present patient was suffering from subclinical PE. Her proteinuria decreased to almost normal levels 12 weeks after delivery, and finally disappeared 26 weeks postpartum; disappearance of proteinuria after delivery is characteristic of GP or PE.
We must consider how serum levels of sFlt-1 and sEng change in pregnant women with chronic nephritis when they show deterioration of proteinuria during pregnancy. Masuyama et al. [3] indicated that the levels of sFlt-1 and sEng might not be increased in severe proteinuria due to chronic glomerulonephritis; five women with chronic glomerulonephritis showing severe proteinuria without hypertension had the same level of sFlt-1 and sEng as five women with chronic glomerulonephritis showing a normal clinical course. In short, while GP might show elevated sFlt-1 and sEng [2], glomerulonephritis, even with severe proteinuria during pregnancy, might not [3].
Second, during the postpartum period, we also considered that kidney biopsy was unnecessary in our case, because proteinuria gradually improved, although it remained slightly at 12 weeks postpartum. Unverdi et al. [10] reported that proteinuria and/or hematuria persisted after 3 months postpartum in 7.3 % (34/463) of women with PE; 14 patients without history of previous glomerulonephritis consented to kidney biopsy, and 29 % (4/14) were diagnosed with idiopathic PE. These results suggest that the period for disappearance of protein in PE could range widely. The same may hold true for GP: the period for disappearance of proteinuria in GP may also range widely. We believe that the fact that proteinuria did not disappear at 12 weeks postpartum does not preclude that this patient suffered from GP, or at least a disorder indistinguishable from GP, except that the disappearance of proteinuria required as long as 26 weeks postpartum.
Why did the increases of sFlt-1 and sEng bring about severe proteinuria before emergence of hypertension in this case? Serum levels of sFlt-1 and sEng were markedly increased, indicating that proteinuria had probably emerged by a similar mechanism to PE, but not acute onset of nephritis. Of 7 women with GP, 57 % showed sFlt-1 ≥95th percentile and 86 % showed sEng ≥95th percentile [2]. Morikawa et al. [11] reviewed 79 women with proteinuria and/or hypertension at and after 20 weeks of gestation; fifty-one percent (19/37) of women who exhibited new proteinuria in the absence of hypertension progressed to PE, thus suggesting that increases of sFlt-1 and sEng, which are common characteristics in women with PE, could emerge in women with imminent onset of PE, in whom only proteinuria appears before appearance of hypertension. In mice administered low and medium doses of adenovirus encoding the full-length mouse-sFlt-1 gene (Ad m-sFlt-1), only proteinuria, but not hypertension, occurred with increase of serum levels of sFlt-1, while in mice administered high doses of Ad m-sFlt-1, both proteinuria and hypertension occurred accompanied by the highest levels of sFlt-1 [12]. Thus, the increase of sFlt-1 could be the culprit of proteinuria without hypertension.
We must admit that this patient did not meet the current definition of GP, because diagnosing GP requires proteinuria disappearance by 12 weeks postpartum [13]; however, proteinuria finally disappeared 26 weeks postpartum in the current pregnancy, strongly suggesting that she was suffering from GP and not nephritis.
This case highlights the clinical usefulness of measuring sFlt-1 and sEng: increased levels of these substances may contribute to avoidance of kidney biopsy in severe proteinuria without hypertension in the second half of pregnancy. In addition, careful follow-up of proteinuria in women with persistent proteinuria beyond 12 weeks postpartum might also assist in deciding whether kidney biopsy is truly necessary. Although we discuss herein the possibility of kidney biopsy omission, its omission was a weakness of our study: we have no kidney biopsy data, and thus no definite data completely rejecting nephritis. However, since we believed that she was suffering from GP, or at least a condition indistinguishable from GP, performing a kidney biopsy only to obtain data would not have been ethical. Further investigation of the possible usefulness of measuring sFlt-1 and sEng in severe proteinuria without hypertension in the second half of pregnancy is warranted. In particular, clinical study comparing levels of sFlt-1, sEng, and PlGF among GP, nephritis with pregnancy, and nephritis occurring or aggravated during pregnancy will reveal the clinical meaning of measuring angiogenesis-related factors in women with proteinuria during pregnancy.
Acknowledgments
This work was supported by a grant-in-aid (24592482 to A.O.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Conflict of interest
All the authors have declared no competing interest.
References
- 1.Piccoli GB, Daidola G, Attini R, Parisi S, Fassio F, Naretto C, Deagostini MC, Castelluccia N, Ferraresi M, Roccatello D, Todros T. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG. 2013;120:412–427. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]
- 2.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Usui R, Suzuki M. Serum sFlt: PlGF ratio, PlGF, and soluble endoglin levels in gestational proteinuria. Hypertens Pregnancy. 2009;28:95–108. doi: 10.1080/10641950802419895. [DOI] [PubMed] [Google Scholar]
- 3.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–556. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Hirashima C, Ohkuchi A, Takahashi K, Suzuki H, Yoshida M, Ohmaru T, Eguchi K, Ariga H, Matsubara S, Suzuki M. Gestational hypertension as a subclinical preeclampsia in view of serum levels of angiogenesis-related factors. Hypertens Res. 2011;34:212–217. doi: 10.1038/hr.2010.212. [DOI] [PubMed] [Google Scholar]
- 5.Hirashima C, Ohkuchi A, Matsubara S, Suzuki H, Takahashi K, Usui R, Suzuki M. Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens Res. 2008;31:1541–1548. doi: 10.1291/hypres.31.1541. [DOI] [PubMed] [Google Scholar]
- 6.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group Soluble endoglin and other circulating angiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 8.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30:151–159. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 9.Ohkuchi A, Hirashima C, Matsubara S, Takahashi K, Matsuda Y, Suzuki M. Threshold of soluble fms-like tyrosine kinase 1/placental growth factor ratio for the imminent onset of preeclampsia. Hypertension. 2011;58:859–866. doi: 10.1161/HYPERTENSIONAHA.111.174417. [DOI] [PubMed] [Google Scholar]
- 10.Unverdi S, Ceri M, Unverdi H, Yilmaz R, Akcay A, Duranay M. Postpartum persistent proteinuria after preeclampsia: a single-center experience. Wien Klin Wochenschr. 2013;125:91–95. doi: 10.1007/s00508-013-0320-8. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa M, Yamada T, Yamada T, et al. Pregnancy outcome of women who developed proteinuria in the absence of hypertension after mid-gestation. J Perinat Med. 2008;36:419–424. doi: 10.1515/JPM.2008.062. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54:1129–1135. doi: 10.1161/HYPERTENSIONAHA.109.134668. [DOI] [PubMed] [Google Scholar]
- 13.Sato K. A proposal for a new definition and classification of “pregnancy induced hypertension (PIH)” (2004) In: Japan Society for the Study of Toxemia of Pregnancy, editor. Historical perspective of study of pregnancy-induced hypertension in Japan. Tokyo: Medical View Co; 2005. pp. 54–87. [Google Scholar]