Abstract
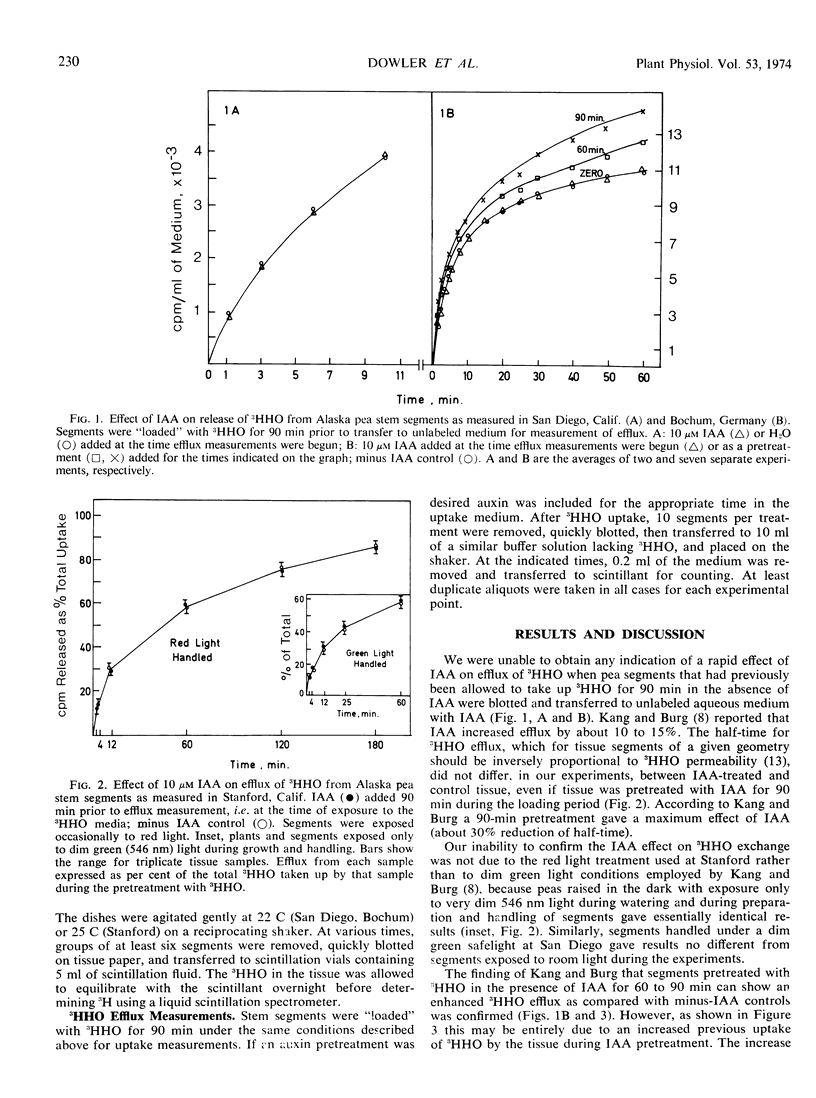
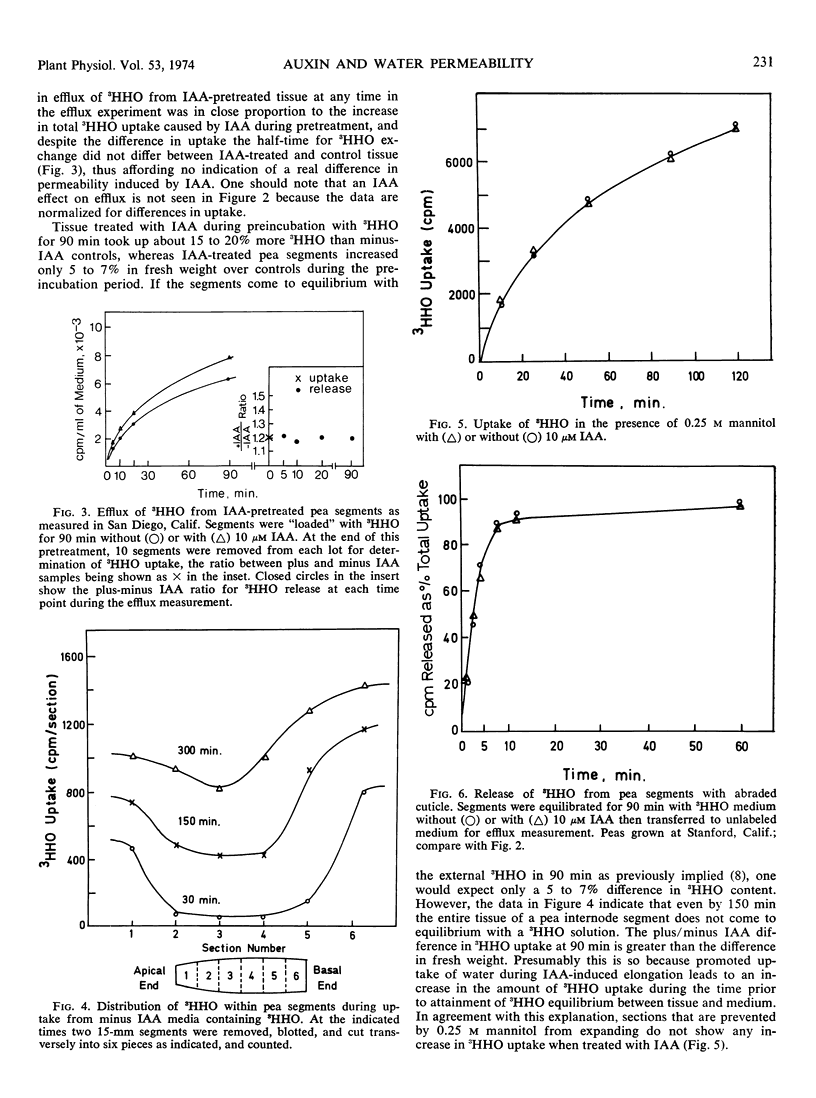
The possibility of an auxin effect on the permeability of pea (Pisum sativum L. ev. Alaska) segments to tritium-labeled water has been investigated by three separate laboratories, and the combined results are presented. We were unable to obtain any indication of a rapid effect of indoleacetic acid on the efflux of 3HHO when pea segments previously “loaded” for 90 minutes with 3HHO were transferred to unlabeled aqueous medium with indoleacetic acid. We were able to confirm that segments pretreated with 3HHO plus indoleacetic acid for 60 to 90 minutes can show an enhanced 3HHO release as compared with minus indoleacetic acid controls. However, this phenomenon appears to be due to an increased uptake of 3HHO during the prolonged indoleacetic acid pretreatment, and therefore we conclude that auxin does not alter the permeability of pea segments to 3HHO in either short term or long term tests. We confirm previous reports that the uptake of 3HHO in pea segments proceeds largely through the cut surfaces, and that the cuticle is a potent barrier to 3HHO flux.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Rapid change in water flux induced by auxins. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1730–1733. doi: 10.1073/pnas.68.8.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Higinbotham N., Pallaghy C. K. Electrochemical evidence of specific action of indole acetic acid on membranes in Mnium leeaves. Z Naturforsch B. 1972 Oct;27(10):1239–1242. doi: 10.1515/znb-1972-1024. [DOI] [PubMed] [Google Scholar]
- Ordin L., Bonner J. Permeability of Avena Coleoptile Sections to Water Measured by Diffusion of Deuterium Hydroxide. Plant Physiol. 1956 Jan;31(1):53–57. doi: 10.1104/pp.31.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Kramer P. J. Permeability of Vicia Faba Root Segments to Water as Measured by Diffusion of Deuterium Hydroxide. Plant Physiol. 1956 Nov;31(6):468–471. doi: 10.1104/pp.31.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip J. R. Osmosis and Diffusion in Tissue: Half-times and Internal Gradients. Plant Physiol. 1958 Jul;33(4):275–278. doi: 10.1104/pp.33.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Evans M. L., Hertel R. Action of auxin on cell elongation. Proc Natl Acad Sci U S A. 1970 Jan;65(1):184–191. doi: 10.1073/pnas.65.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Samuel E. W. THE PERMEABILITY OF POTATO TISSUE TO WATER. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1029–1033. doi: 10.1073/pnas.41.12.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]


