Abstract
We experienced a case that was considered as gefitinib-associated membranous nephropathy (MGN) in treatment for pulmonary adenocarcinoma. A female patient aged 80 who had been treated for lung cancer was referred and hospitalized at our hospital, because of nephrotic syndrome. The patient had pulmonary adenocarcinoma (cT4N2M1a) with positive for epidermal growth factor receptor (EGFR) mutation. Gefitinib, EGFR tyrosine kinase inhibitor, was initiated from 1 year and 2 months ago. At that time, proteinuria was negative. The treatment effect on lung cancer was so favorable that partial response had been maintained. However, from 4 months ago, edema of legs appeared, leading to become nephrotic syndrome. Renal biopsy was performed, and secondary MGN was diagnosed, because of deposition of peripheral IgG, mesangial IgA, and C3, as well as the deposition of peripheral IgG4, IgG1, IgG2, and weak IgG3. We considered drug-induced MGN and discontinued the administration of gefitinib. Subsequently, the proteinuria tended to decrease gradually and became negative 10 months later. However, the lung cancer recurred 3 months after discontinuation of gefitinib and another molecular target drug, erlotinib, was administered. At present, 13 months after discontinuation of gefitinib, absence of proteinuria is maintained. It has been generally considered that secondary MGN can be induced by both malignant tumor and their treatment. In the present case, the clinical course and pathological characteristics showed the secondary MGN that might be associated with gefitinib during the treatment for pulmonary adenocarcinoma. The present case, to our knowledge, may be a first case of gefitinib-associated MGN.
Keywords: Gefitinib, Molecular target drug, Membranous nephropathy
Introduction
Membranous nephropathy (MGN) is one of the most common causes of nephrotic syndrome, and the percentage of MGN is 44–68 % in nephrotic syndrome in adults. The vast majority of cases of MN are idiopathic, with unknown underlying cause, while the remaining are secondary to known disease such as malignancy, autoimmune disease, infectious disease or drug toxicity.
MGN complicated with solid cancer accounts for 5.8–10.9 % of patients with MN.
In the world, the frequency of cases complicated with lung cancer is the highest, followed by cancers of digestive system and renal cancer [1]. On the other hand, in Japan, characteristically, the frequency of lung cancer cases has been relatively less and the cancers of digestive system have occurred more frequently. As for histologic types of lung cancer, frequently reported types include squamous cell carcinoma, small cell carcinoma, and adenocarcinoma [2].
Secondary MGN can also be induced by drugs, among which representative drugs include antirheumatic drugs such as gold drug, penicillamine and bucillamine, and non-steroid anti-inflammatory drugs such as diclofenac and antihypertensive drugs such as captopril. Besides those, various drugs have been reported [3–5].
We recently experienced a case of nephrotic syndrome induced by MGN during treatment for lung adenocarcinoma. Although the possibility of accidental complication of idiopathic MGN with lung adenocarcinoma or secondary MGN from lung cancer is not completely deniable, from the clinical course and pathological characteristics, we considered strongly the possibility of drug-induced MGN by gefitinib.
Case report
A female patient aged 80 who was receiving treatment for lung cancer was referred and hospitalized at our hospital because of hyposarca and increased body weight. From about 1 year and 9 months before hospitalization, cough persisted at night. She consulted at other hospital and underwent chest CT, which revealed a tumor of 5 cm at the right upper lobe and multiple nodes in both the lung fields. Thus, she was referred to our hospital, department of pulmonary medicine and oncology. Based on scrutinized examinations, lung cancer (adenocarcinoma) with clinical stage cT4N2M1a was diagnosed. The patient was positive for epidermal growth factor receptor (EGFR) mutation. Thus, administration of a molecular targeting drug, gefitinib 250 mg/day, was initiated 12 months before admission. At that time, proteinuria was negative. As for treatment effect, partial responses persisted and mild acne was present after its administration. There was no other critical harmful event. Because edema of legs appeared 4 months before admission, urinalysis was conducted after a long interval, which revealed proteinuria 3(+) and serum albumin level decreasing to 2.6 g/dL. Subsequently, in accordance with increase in proteinuria, the edema became worse and the body weight increased by 2 kg in 1 month. Consequently, the patient was referred and hospitalized at our department.
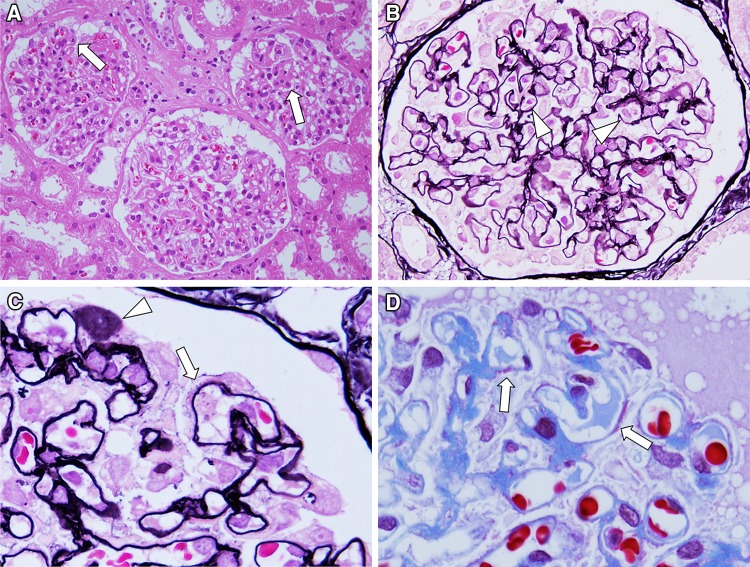
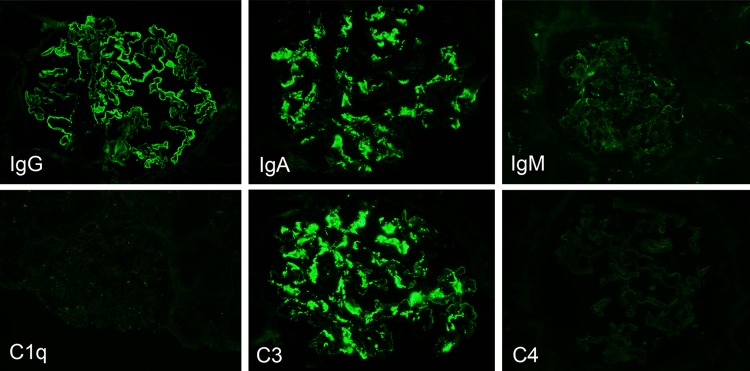
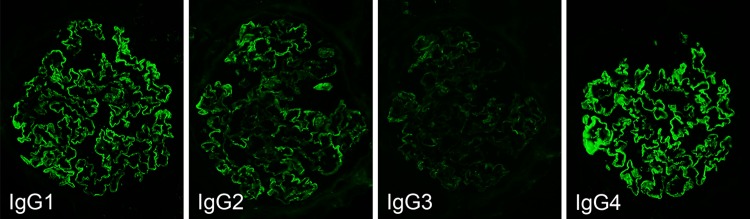
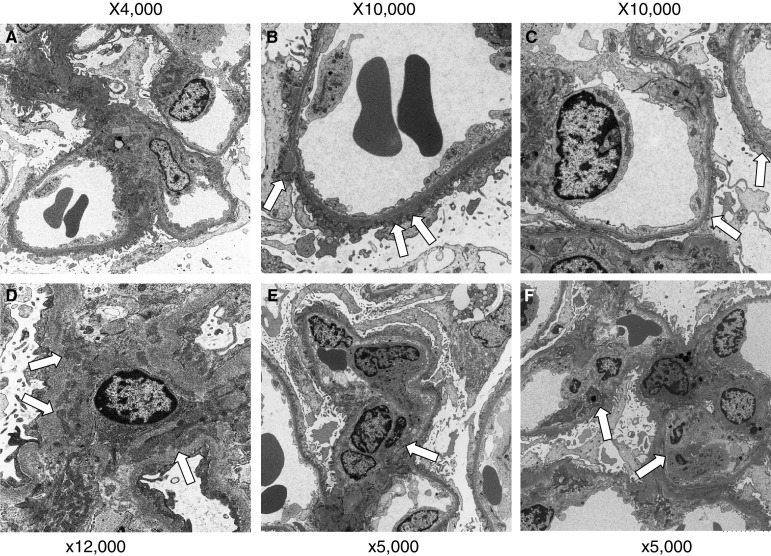
Table 1 shows findings from examinations at admission. The results indicated the conditions of nephrotic syndrome without urinary occult blood and normal renal function. The selectivity index was 0.13. As for results of renal biopsy, one glomerulus among a total of 40 was obsolescence and remaining glomeruli showed diffuse and irregularly thickening capillary walls with small granular deposits and spikes formation (Fig. 1). Mesangial proliferative lesions were not conspicuous. Immunofluorescence study showed the deposition of IgG and C3 on glomerular capilalries (Fig. 2). The subclass of IgG revealed predominant deposition of IgG4 and IgG1, and weak deposition of IgG2, and weak and segmental IgG3 (Fig. 3). The deposition of IgA and C3 was also found mainly in the mesangial areas. The expression of phospholipase A2 receptor (PLA2R), which is considered as the antigen of primary MGN [6, 7], did not enhance in glomeruli, although it was enhanced on glomerular capillaries in some cases of primary MGN (data not shown). As electron microscopic findings, granular electron dense deposit was found irregularly at the subepithelial region on the glomerular basement membrane (GBM) (Fig. 4). Mesangial electron dense deposits were noted with mild mesangial hypercellular lesions. From those findings in renal biopsy, MGN, stage I–II, and mesangial IgA deposits was diagnosed.
Table 1.
Laboratory data at admission
| Peripheral blood | Serological test | ||
|---|---|---|---|
| White blood cell count | 8800/μL | Immunoglobulin G | 445 mg/dL |
| Red blood cell count | 402 × 104/μL | Immunoglobulin A | 191 mg/dL |
| Hemoglobin | 9.6 g/dL | Immunoglobulin M | 94 mg/dL |
| Hematocrit | 32.2 % | Complement activity | >60 U/mL |
| Platelets | 34.9 × 104/μL | C3 complement | 147 mg/dL |
| Blood chemistry | C4 complement | 44 mg/dL | |
| Total protein | 4.8 g/dL | Antinuclear antibody | <40 |
| Albumin | 1.8 g/dL | Hepatitis B virus, surface antigen | (−) |
| Aspartate aminotransferase | 29 IU/L | Hepatitis C virus antibody | (−) |
| Alanine aminotransferase | 18 IU/L | Treponemal antibody test | (−) |
| Lactate dehydrogenase | 298 IU/L | Sialylated carbohydrate antigen KL-6 | 492 U/mL |
| Total cholesterol | 282 mg/dL | Carcinoembryonic antigen | 6.2 ng/mL |
| LDL-Cholesterol | 124 mg/dL | Urinalysis | |
| Triglycerides | 273 mg/dL | Gravity | 1.022 |
| Uric acid | 4.5 mg/dL | pH | 5.5 |
| Urea nitrogen | 24.0 mg/dL | Protein | 3+ |
| Creatinine | 0.54 mg/dL | 6.2 g/day | |
| Sodium | 141 mEq/L | Occult blood | (±) |
| Potassium | 4.0 mEq/L | Glucose | (−) |
| Chloride | 107 mEq/L | Ketone | (−) |
| C-reactive protein | 1.05 mg/dL | ||
Fig. 1.
Light microscopy findings. Renal biopsy samples included 44 glomeruli and 1 glomerulus underwent obsolescence. Remaining all glomeruli showed diffuse but irregular thickening glomerular capillary walls (arrow in a). No apparent mesangial hypercellularity was noted in glomeruli (HE stain, ×400). In glomeruli, infiltrating cells in capillary lumens were seen (arrowhead in b) (PAM stain, ×600). High magnification of glomeruli showed focal and segmental formation of spike (arrow in c) and stippling (arrowhead in c) in glomerular basement membrane (PAM stain, ×1,000). Focal and segmental subepithelial side of deposits was also noted on glomerular capillary walls (arrow in d) (Masson stain, ×1,000)
Fig. 2.
Immunofluorescence findings. Immunofluorescence study showed that the granular deposition of IgG was noted as segmental peripheral pattern. The deposition of IgA was seen as mesangial granular pattern. The minimal deposition of IgM was observed in glomeruli. In addition, the granular deposition of C3 was noted as mesangial and segmental peripheral pattern
Fig. 3.
The findings of IgG subclass. Immunofluorescence study for IgG subclass showed that the deposition of IgG4 > IgG1 and IgG2 was noted as segmental peripheral granular pattern. Minimal deposition of IgG3 was also seen as segmental peripheral granular pattern
Fig. 4.
Electron microscopic findings. In glomeruli, irregular thickening glomerular capillary walls was noted with expansion of mesangial areas (a ×4,000). High magnification of glomerular capillary walls showed irregular size and irregular distribution of subepithelial deposits (arrow in b) was seen with effacement of foot processes of podocytes (b ×10,000). In some glomerular capillary walls, subepithelial deposits could not be detected (arrow in c), suggesting the segmental membranous nephropathy (c ×10,000). Electron dense deposits were noted in mesangial areas (arrow in d ×12,000) with segmental mesangial proliferative lesions (arrow in e ×5,000). Endocapillary proliferative lesions (arrow in f) were also noted with the infiltration of macrophages (f ×5,000)
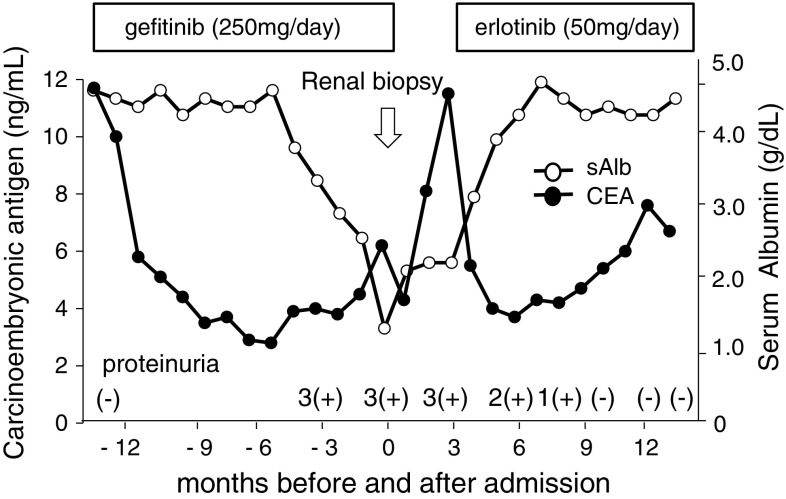
Considering from clinicopathological findings, we presumed the possibility of gefitinib-induced MGN and thus discontinued the administration of it. After discontinuation of gefitinib, the proteinuria tended to gradually decrease and disappeared 10 months later (Fig. 5). As for lung cancer, chest CT revealed its multiple metastases 3 months after discontinuation of gefitinib. Because increases in blood Sialylated carbohydrate antigen KL-6 and carcinoembryonic antigens (CEA) were also found, the administration of erlotinib 50 mg/day, another EGFR inhibitor, was started. By present, 13 months after discontinuation of gefitinib and 10 months after erlotinib initiation, complete remission of proteinuria continued, and the recurrence of proteinuria is absent.
Fig. 5.
Clinical course. From 8 months after gefitinib administration, proteinuria 3(+) occurred and continued during treatment with gradually decrease of serum albumin (sAlb) levels. After discontinuation of gefitinib treatment, proteinuria was gradually decreased with gradual recovering of sAlb levels. From 10 months after discontinuation of gefitinib treatment, proteinuria could not be detected. After gefitinib administration, serum CEA levels quickly decreased, but it increased again after discontinuation of treatment. Serum CEA levels decreased again after erlotinib administration
Discussion
MGN is a glomerular disease characterized by diffuse thickening of glomerular capillary walls, which is induced by deposition of subepithelial immune complex on GBM and activation of complements. Recently, membranous type of PLA2R has been identified as an endogenous antigen existing in the glomerular epithelial cells and has gained attention as an etiology of idiopathic MGN [8]. A recent study demonstrates enhanced staining of PLA2R in glomeruli in cases with idiopathic MGN compared with negative staining in secondary MGN [9]. On the other hand, secondary MGN accounts for about 10–20 % of MGN, and causative antigens reported were drugs, tumor associated antigens such as CEA and cytokeratin 19 fragment, Hepatitis B virus, envelope antigen, and the other antigens in autoimmune diseases [6, 7]. In the present case, three possible causes of MGN were considered; coincidence of idiopathic MGN, MGN associated with pulmonary adenocarcinoma or gefitinib treatment.
Between idiopathic and secondary MGN, it is reported that the subclass of IgG in subepithelial deposits differs in immunofluorescence technique [10]. It is considered that main subclass of IgG deposition in idiopathic MGN is IgG4. By contrast, in secondary MGN, various subclass pattern of IgG deposition have been reported [11]. There have been also reports that IgG1 and IgG2 are stained strongly in MGN induced by malignant tumor, and IgG3 is positive in drug-induced MGN induced by bucillamine [12, 13]. Qu et al. [14] noted that IgG4 was not stained in seven of eight cases of MGN complicated with malignant tumor and IgG2 was stained moderately or higher in seven cases. In the present case, the expression of PLA2R did not enhance in glomeruli in immunohistochemistry for biopsy sample, suggesting the secondary MGN. In addition, IgG4 and IgG1 deposited predominantly at the GBM, as similar as idiopathic MGN. However, the deposition of IgG2 and IgG3 was also present along GBM, although they were weak and segmental, suggesting the secondary MGN. The deposition of IgA on mesangial areas was also found. As for IgA deposits in the present case, it could not be clarified if its cause is a drug, is associated with mucosal immunity of lung cancer, or complication of IgA nephropathy. Although rare, both solid tumor such as renal cell cancer, lung cancer, pancreas cancer, and hematological malignancies such as Hodgkin’s and non-Hodgkin’s diseases, could induce IgA nephropathy, termed paraneoplastic IgA nephropathy [15, 16]. Interestingly, a case is recently reported that IgA nephropathy occurs in gefitinib treatment for lung cancer [17].
Overexpression of EGFR was found in many cases of malignant tumor including non-small cell lung cancer and its involvement in growth and maintaining of tumors has been clarified. Moreover, as for tumors with expression or overexpression of EGFR, it is reported that they have higher metastasis and poorer prognosis than those without its expression [18]. Gefitinib targets the EGFR tyrosine kinase and inhibits signal transmission under it causing growth of tumor cells. This drug is generally used for surgery impossible malignant tumor with genetic mutation of EGFR, or recurrent cases of non-small cell lung cancer. As for side effects of gefitinib, eruption and acne occurred with the highest frequency. The critical side effects were acute lung impairment and interstitial pneumonia occurring in 5.8 % in Japan. However, the reports of side effects of gefitinib on the kidney have been very rare. Renal dysfunction was occurred in about 1 %. Other side effects were post-renal acute renal failure due to urinary bleeding or retroperitoneal fibrosis [19]. A case of acute renal failure due to thrombotic thrombocytopenic purpura/hemolytic uremic syndrome was also reported [20]. Reports on the cases causing nephrotic syndrome have been so rare that only a report on minimal change nephrotic syndrome has been presented [21].
In the present study, secondary MGN might be associated with gefitinib for pulmonary adenocarcinoma. The secondary MGN induced by lung adenocarcinoma could not be completely ruled out, however, clinical course and laboratory findings did not show any correlation between nephrotic syndrome in MGN and course of lung carcinoma and serum CEA levels. It has been known that MGN induced by lung cancer was alleviated in accordance with reductions in the tumor and tumor markers, and the conditions got worse due to its recurrence. On the other hand, in MGN induced by drug, the diagnosis is likely to be made when nephrotic syndrome is alleviated by discontinuation of drug or recurs after its re-administration.
In the present case, the serum CEA levels did not correspond to the condition of nephrotic syndrome. Although tumor markers do not always reflect the pathologic condition [22, 23], the relationship between course of lung carcinoma and nephrotic syndrome in MGN was not also observed. Furthermore, nephrotic syndrome appeared 8 months after initiation of the administration of gefitinib, and proteinuria disappeared 10 months after its discontinuation, despite no special treatment for nephrotic syndrome such as administration of steroids or immunosuppresants was conducted. There have been many reports that MGN induced by the drugs, but not gefitinib, usually occurs within a half to 1 year after the initiation of the administration, and proteinuria disappears a half to 1 year after its discontinuation [24]. From these findings, it is unlikely that MGN in the present study could be associated with lung carcinoma, and we strongly considered that MGN might be induced by gefitinib. The mechanism by which gefitinib induced MGN is unclear. However, the possible mechanisms whereby gefitinib may be associated with MGN include: (a) The gefitinib can function as a hapten attached to some native protein, eliciting an antibody response. (b) The gefitinib can have immunomodulatory effects through and may develop autoimmune phenomena during prolonged use. Indeed, gefitinib might induce nephrotic syndrome due to minimal change nephrotic syndrome by an allergic or immune reaction [21]. (c) The gefitinib can bind locally to the increased phosphorylation of the EGFR on podocytes, alter its structure and induce antibodies, and results in subepithelial immune complexes leading to MGN. A recent study has established the paradigm of autoantibody action against a podocyte protein, such as PLA2R, in the pathogenesis of MGN [8].
The recent progress of molecular biologic studies related to cancers has been rapid, and the achievements have been actively applied to the field of drug discovery. The several drugs of molecular targeted therapy for the treatment of advanced carcinoma may induce various renal complications, including proteinuria, hematuria, nephrotic syndrome, thrombotic microangiopathy, acute kidney injury, IgA nephropathy, crescentic glomerulonephritis, and interstitial nephritis [25].
In bevacizumab, a monoclonal antibody drug to vascular endothelial growth factor (VEGF), (anti-VEGF agent) proteinuria is complicated in 21–63 % cases after its administration and the renal biopsies in most of the cases show features of thrombotic microangiopathy [26]. As for small-molecule chemicals, it has been reported that proteinuria has been detected in 3–20 % of patients who received the administration of multi-kinase inhibitors used for advancing renal cell cancer [27].
In the present study, MGN might be induced by gefitinib, but did not induced by erlotinib. Erlotinib is a small-molecule chemical targeting EGFR having similar Quinazoline skeleton to gefitinib. As for the frequency of pulmonary impairment, time of the occurrence, and pathologic conditions, although both the drugs are considered to be almost same, there has been a report that because hepatic dysfunction occurred with gefitinib, the treatment was changed to erlotinib, with which no problem occurred. It is speculated that as the cause, there may be metabolic differences between both the drugs [28]. From these findings, it is considered the take change from gefitinib to other drugs including erlotinib when abnormal conditions occur.
We report here, to our knowledge, the first case of MGN induced by gefitinib for pulmonary adenocarcinoma. Recently, it has been clarified that malignant tumors occur due to accumulated genetic abnormalities, and development of molecular targeted therapies for those abnormal genes have progressed. EGFR tyrosine kinase inhibitor such as gefitinib is considered to have rare renal impairment. However, it was considered that whenever gefitinib is administered, urinalysis should be conducted regularly, and when abnormalities appear, change to other drugs should be taken into consideration.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Burstein DM, Korbet SM, Schwartz MM. Membranous glomerulonephritis and malignancy. Am J Kidney Dis. 1993;22:5–10. doi: 10.1016/S0272-6386(12)70160-9. [DOI] [PubMed] [Google Scholar]
- 2.Wągrowska-Danilewicz M, Danilewicz M. Glomerulonephritis associated with malignant disease of non-renal origin. A report of three cases and a review of the literature. Pol J Pathol. 1995;46:195–198. [PubMed] [Google Scholar]
- 3.Ross JH, McGinty F, Brewer DG. Penicillamine nephropathy. Nephron. 1980;26:184–186. doi: 10.1159/000181984. [DOI] [PubMed] [Google Scholar]
- 4.Nagahama K, Matsushita H, Hara M, et al. Bucillamine induces membranous glomerulonephritis. Am J Kidney Dis. 2002;39:706–712. doi: 10.1053/ajkd.2002.31987. [DOI] [PubMed] [Google Scholar]
- 5.Sturgill BC, Shearlock KT. Membranous glomerulopathy and nephrotic syndrome after captopril therapy. JAMA. 1983;250:2343–2345. doi: 10.1001/jama.1983.03340170069032. [DOI] [PubMed] [Google Scholar]
- 6.Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol. 2005;16:1188–1194. doi: 10.1681/ASN.2005010028. [DOI] [PubMed] [Google Scholar]
- 7.Shiiki H, Saito T, Nishitani Y, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. 2004;65:1400–1407. doi: 10.1111/j.1523-1755.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 8.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoxha E, Kneißler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 10.Doi T, Mayumi M, Kanatsu K, et al. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58:57–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroki A, Iyoda M, Shibata T, et al. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68:302–310. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transpl. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira DB. Membranous nephropathy: an IgG4-mediated disease. Lancet. 1998;351:670–671. doi: 10.1016/S0140-6736(97)04122-6. [DOI] [PubMed] [Google Scholar]
- 14.Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transpl. 2012;27:1931–1937. doi: 10.1093/ndt/gfr534. [DOI] [PubMed] [Google Scholar]
- 15.Lien YH, Lai LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7:85–95. doi: 10.1038/nrneph.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhaveri KD, Shah HH, Calderon K, et al. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013;84:34–44. doi: 10.1038/ki.2012.484. [DOI] [PubMed] [Google Scholar]
- 17.Masutani K, Fujisaki K, Maeda H, et al. Tubulointerstitial nephritis and IgA nephropathy in a patient with advanced lung cancer treated with long-term gefitinib. Clin Exp Nephrol. 2008;12:398–402. doi: 10.1007/s10157-008-0066-1. [DOI] [PubMed] [Google Scholar]
- 18.Selvaggi G, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 19.Mori H, Hhno Y, Ito F, et al. Massive hematuria from the bilateral upper urinary tract in a patient treated for advanced lung cancer with gefitinib. Jpn J Clin Oncol. 2010;40:263–266. doi: 10.1093/jjco/hyp141. [DOI] [PubMed] [Google Scholar]
- 20.Wan HL, Yao NS. Acute renal failure associated with gefitinib therapy. Lung. 2006;184:249–250. doi: 10.1007/s00408-005-2581-0. [DOI] [PubMed] [Google Scholar]
- 21.Kumasaka R, Nakamura N, Shirato K, et al. Side effects of therapy: case 1. Nephrotic syndrome associated with gefitinib therapy. J Clin Oncol. 2004;22:2504–2505. doi: 10.1200/JCO.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 22.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–377. doi: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Jefferson JA, Couser WG. Therapy of membranous nephropathy associated with malignancy and secondary causes. Semin Nephrol. 2003;23:400–405. doi: 10.1016/S0270-9295(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 24.Nakano M, Ueno M, Nishi S, et al. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol. 1998;50:154–160. [PubMed] [Google Scholar]
- 25.Ito C, Fujii H, Ogura M, et al. Cetuximab-induced nephrotic syndrome in a case of metastatic rectal cancer. J Oncol Pharm Pract. 2013;19:265–268. doi: 10.1177/1078155212459668. [DOI] [PubMed] [Google Scholar]
- 26.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayman SR, Leung N, Grande JP, et al. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14:285–294. doi: 10.1007/s11912-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kijima T, Shimizu T, Nonen S, et al. Safe and successful treatment with erlotinib after gefitinib-induced hepatotoxicity: difference in metabolism as a possible mechanism. J Clin Oncol. 2011;29:588–590. doi: 10.1200/JCO.2010.34.3368. [DOI] [PubMed] [Google Scholar]