Abstract
Autosomal dominant distal renal tubular acidosis (dRTA) is a rare disorder caused by a mutation in the AE1 gene encoding the chloride–bicarbonate (Cl−/HCO3 −) anion exchanger 1 (AE1). Most patients with this disorder present with clinical symptoms in adulthood and their phenotype is milder than that of those with autosomal recessive dRTA. In this report, we describe a Japanese family with autosomal dominant dRTA in which the mother and her daughter presented with severe symptoms caused by hypokalemia at 2 years of age. The heterozygous AE1 mutation G609R, which is a known causative mutation of dRTA, was identified in both patients. To our knowledge, this is the first report of a Japanese family with autosomal dominant type dRTA caused by an AE1 mutation. We, therefore, propose that alterations of AE1 should be considered causative of autosomal dominant dRTA even if typical symptoms appear during early childhood and the clinical features are severe.
Keyword: Anion exchanger 1, Autosomal dominant, Distal renal tubular acidosis, Dominant negative, Hypokalemia, SLC4A1
Introduction
Patients with renal tubular acidosis have impaired acid secretion or bicarbonate reabsorption, resulting in hyperchloremic metabolic acidosis [1, 2]. Distal renal tubular acidosis (dRTA) is caused by the defective acidification of urine in the distal nephron following the impaired excretion of hydrogen ions, and affected patients show hypokalemia, hypercalciuria, metabolic bone disease, nephrocalcinosis, and/or nephrolithiasis [3, 4]. dRTA is often a secondary development to autoimmune diseases, systemic diseases, or drug administration. Familial dRTA is etiologically rare and is inherited as an autosomal dominant or recessive trait [1]. Mutations in the anion exchanger 1 (AE1) protein encoded by AE1 (SLC4A1) (MIM109270) have been reported as the sole genetic cause of autosomal dominant dRTA (MIM#179800) [1–4].
Most patients with autosomal dominant dRTA present with nephrocalcinosis during adulthood, and show no clinical symptoms in their childhood [1, 3]. Causative mutations in AE1 were previously shown to have a dominant negative (DN) effect in a study of genetically modified cells [5–7]. Such DN mutations are often observed in Caucasian patients [6–8], whereas defective-type AE1 mutations are more commonly seen in East Asian patients with autosomal recessive dRTA [9]. To the best of our knowledge, there has been no report of Japanese patients with dRTA caused by DN-type mutations in AE1. Here, we report the first known Japanese family in which a mother and her daughter diagnosed with autosomal dominant dRTA caused by a DN-type mutations in AE1 presented with symptoms in their early childhood.
Case report
A 2-year-old Japanese girl was referred and admitted to her local hospital because of muscle weakness and gait disturbance following a mild upper respiratory tract infection. She was born at full term by normal vaginal delivery with a birth weight of 3,021 g, and showed normal development.
On admission, her height was 87 cm (−1.4 SD) and her weight was 13 kg (0.0 SD). Physical examination showed paralysis and extremely decreased deep tendon reflex in the extremities. Consciousness, cranial nerves, respiratory muscles, and peripheral sensations were all intact. Laboratory evaluation of the patient is shown in Table 1. She showed hyperchloremic metabolic acidosis with a normal serum anion gap and high urine pH in spite of acidemia associated with severe hypokalemia, hypercalcinuria, and hyperammonemia. A complete blood count, and hepatic and renal function were within normal ranges. Abdominal ultrasound showed normal kidneys without nephrocalcinosis. She showed no evidence of failure to thrive or rickets. Her red cell morphology on a blood smear was normal without ovalocytosis or spherocytosis. Based on clinical symptoms and laboratory data, we diagnosed dRTA. After starting oral alkali therapy by sodium citrate and potassium citrate for dRTA, metabolic acidosis and hypokalemia were soon corrected and her growth rate improved, with her height catching up to −0.4 SD in a year.
Table 1.
Laboratory evaluation of the patient on admission
| Blood | Urine | ||
|---|---|---|---|
| Venous blood gas | Urinalysis | ||
| pH | 7.244 | pH | 7.0 |
| pCO2 | 27.7 mmHg | Specific gravity | 1.009 |
| HCO3 − | 13.8 mmol/L | Protein | (−) |
| Base excess | −14 mmol/L | Glucose | (−) |
| Complete blood count | Blood | (−) | |
| White blood cell | 6900/µL | Urinary sediments | |
| Red blood cell | 479 × 104/µL | Red blood cell | 1–4/HPF |
| Hemoglobin | 13.1 g/dL | White blood cell | 1–4/HPF |
| Hematocrit | 39.8 % | Cast | (−) |
| Platelet count | 28.4 × 104/µL | Urine chemistry | |
| Serum chemistry | Sodium | 39.0 mmol/L | |
| Total protein | 6.4 g/dL | Potassium | 11.0 mmol/L |
| Albumin | 4.7 g/dL | Chloride | 34.0 mmol/L |
| Total bilirubin | 0.6 mg/dL | Anion gap | 16.0 mmol/L |
| Asparate amino transferase | 43 U/L | Calcium | 15.7 mg/dL |
| Alanine transaminase | 25 U/L | Creatinine | 15 mg/dL |
| Lactate dehydrogenase | 191 U/L | β2-microglobulin | 92,000 µg/L |
| Alkaline phosphatase | 707 U/L | ||
| Creatine kinase | 27 U/L | ||
| Blood urea nitrogen | 18 mg/dL | ||
| Creatinine | 0.3 mg/dL | ||
| eGFR | 95 mL/min/1.73 m2 | ||
| Uric acid | 3.6 mg/dL | ||
| Sodium | 135 mmol/L | ||
| Potassium | 1.7 mmol/L | ||
| Chloride | 110 mmol/L | ||
| Anion gap | 11.2 mmol/L | ||
| Calcium | 9.5 mg/dL | ||
| Inorganic phosphorus | 5.4 mg/dL | ||
| Ammonia | 116 µg/dL | ||
eGFR estimated glomerular filtration rate
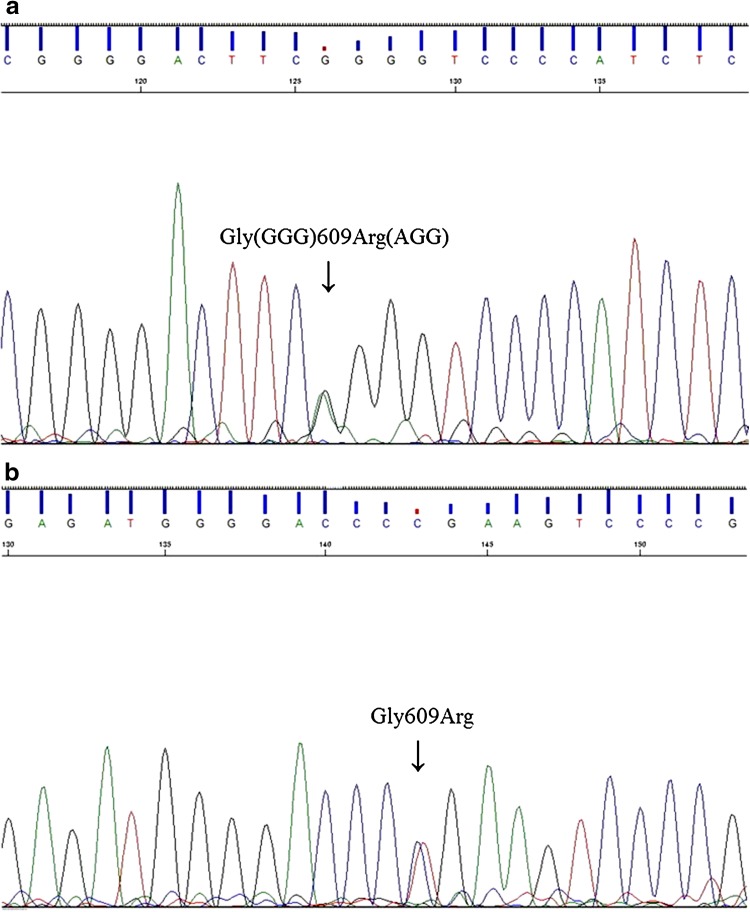
Her mother shared the same clinical history of polydipsia, polyuria, and periodic paralysis caused by hypokalemia at 2 years of age. She had been diagnosed with dRTA at 7 years of age, presenting with hyperchloremic metabolic acidosis, rickets, a severe failure to thrive (−2.8 SD in height), and bilateral nephrocalcinosis, and had received alkali therapy since then. Based on the clinical symptoms and laboratory data of the patient and her mother, we diagnosed them both as having autosomal dominant dRTA. After obtaining informed consent, we conducted genetic analysis of the patient and her mother under the approval of the Institutional Review Board (IRB)/Ethics Committee of Kyushu University (approval number 434-00). Both individuals were shown to carry the heterozygous missense mutation G609R in exon 15 of AE1 (Fig. 1). Because this mutation has been described as causative of dRTA [7] and since it was not identified in 50 Japanese normal control individuals, the mother and daughter were diagnosed as having familial dRTA with an autosomal dominant trait caused by an AE1 mutation.
Fig. 1.
AE1 mutation analysis of the patient (a, sense) and her mother (b, antisense). Both individuals were shown to carry the heterozygous missense mutation G609R (Gly609Arg) in AE1 exon 15
Discussion
The strict control of the acid–base balance within a narrow range of around pH 7.4 is vital for our survival and the optimal function of physiological process. Kidneys are key regulators of this through their secretion of protons (H+) from the distal nephron and reabsorption of bicarbonate (HCO3 −) in the proximal tubule. RTA is characterized by the presence of hyperchloremic metabolic acidosis with a normal anion gap in the setting of a preserved renal function [1, 2]. Inherited forms of RTA are rare and acquired forms such as Sjögren’s syndrome are more common in clinical practice [1].
dRTA represents a failure of α-intercalated cells (α-ICs) in the collecting tubules to acidify the urine [4]. α-ICs secrete protons into the tubular lumen through the apical H+-ATPase functionally coupled to the basolateral Cl−/HCO3 − exchanger AE1 [10]. AE1 mutations are causative of both autosomal dominant and recessive dRTA [6–9], whereas mutations in ATP6V1B1 or ATP6V0A4, encoding the subunits of H+-ATPase, are responsible for autosomal recessive dRTA, which is often associated with sensorineural hearing loss [11, 12]. AE1 belongs to the SLC4 family of genes located on chromosome 17q21–22. In mammals, AE1 is mainly expressed at the basolateral surface of α-ICs in the collecting duct of the kidney (kAE1) and erythrocyte cell membrane (eAE1). The eAE1 isoform is 65 amino acids longer at its NH2 terminus than the kAE1 isoform, and enables the erythrocyte to maintain its cytoskeletal structure [13]. Although most autosomal recessive dRTA patients in Southeast Asia present with hemolytic anemia as a result of ovalocytosis or hereditary spherocytosis, dominant mutant proteins were mainly shown to retain their normal Cl−/HCO3 − exchange function in erythrocytes, and hemolytic anemia was rarely observed [6].
Several studies have shown that a trafficking defect in the mutant protein rather than a lack of function was the major mechanism underlying the pathogenesis of dRTA caused by AE1 mutations [6, 7]. Rungroj et al. [7] previously identified the G609R (Gly609Arg) AE1 mutation, detected in our present cases, in a large Caucasian pedigree of patients with autosomal dominant distal RTA. This earlier study demonstrated that Gly609 in the seventh transmembrane domain played an important role in targeting kAE1 to the correct cell surface compartment, and that the G609R mutation had DN effects that impaired tracking and expression of AE1 on the basolateral membrane.
Although the epidemiology of this rare disorder remains unknown, most cases of autosomal dominant dRTA caused by AE1 mutations have been reported in Caucasians [6–8, 14], with only a few seen in Asian countries [15, 16]; no previous reports have been made of Japanese familial cases. Autosomal dominant cases of dRTA are usually diagnosed at a later age and show a milder phenotype than autosomal recessive cases [1, 3]. However, the spectrum of phenotypic severity of this disorder is considerably broad, and some studies reported that patients with the autosomal dominant type of dRTA presented with a severe phenotype in their early childhood [6–8, 15]. To date, genotype–phenotype correlations of autosomal dominant dRTA have not been reported. We speculate that even a small difference in expression from each AE1 allele might significantly affect the balance of normal and DN AE1 molecules, which in turn would influence phenotype diversity. The previous reported case with G609R developed dRTA at 6 years of age as early as our case [7]. Therefore, this mutation might be associated with clinical phenotypes of dRTA.
In conclusion, we report the first known Japanese familial cases of autosomal dominant dRTA confirmed by mutation analysis of AE1. We propose clinicians should consider that patients with autosomal dominant dRTA may show clinical symptoms in their early childhood. Further, genetic and clinical investigations are needed for a better understanding of this disorder.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Fry AC, Karet FE. Inherited renal acidoses. Physiology (Bethesda) 2007;22:202–211. doi: 10.1152/physiol.00044.2006. [DOI] [PubMed] [Google Scholar]
- 2.Alper SL. Familial renal tubular acidosis. J Nephrol. 2010;23(Suppl 16):S57–S76. [PubMed] [Google Scholar]
- 3.Karet FE. Inherited distal renal tubular acidosis. J Am Soc Nephrol. 2002;13:2178–2184. doi: 10.1097/01.ASN.0000023433.08833.88. [DOI] [PubMed] [Google Scholar]
- 4.Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transpl. 2012;27:3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 5.Quilty JA, Li J, Reithmeier RA. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol. 2002;282:F810–F820. doi: 10.1152/ajprenal.00216.2001. [DOI] [PubMed] [Google Scholar]
- 6.Bruce LJ, Cope DL, Jones GK, et al. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest. 1997;100:1693–1707. doi: 10.1172/JCI119694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rungroj N, Devonald MA, Cuthbert AW, et al. A novel missense mutation in AE1 causing autosomal dominant distal renal tubular acidosis retains normal transport function but is mistargeted in polarized epithelial cells. J Biol Chem. 2004;279:13833–13838. doi: 10.1074/jbc.M400188200. [DOI] [PubMed] [Google Scholar]
- 8.Karet FE, Gainza FJ, Gyory AZ, et al. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA. 1998;95(6337–6342):8. doi: 10.1073/pnas.95.11.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khositseth S, Bruce LJ, Walsh SB, et al. Tropical distal renal tubular acidosis: clinical and epidemiological studies in 78 patients. QJM. 2012;105:861–877. doi: 10.1093/qjmed/hcs139. [DOI] [PubMed] [Google Scholar]
- 10.Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physio. 1993;55:267–288. doi: 10.1146/annurev.ph.55.030193.001411. [DOI] [PubMed] [Google Scholar]
- 11.Karet FE, Finberg KE, Nelson RD, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84–90. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- 12.Karet FE, Finberg KE, Nayir A, et al. Localization of a gene for autosomal recessive distal renal tubular acidosis with normal hearing (rdRTA2) to 7q33-34. Am J Hum Genet. 1999;65:1656–1665. doi: 10.1086/302679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarolim P, Shayakul C, Prabakaran D, et al. Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl−/HCO3− exchanger. J Biol Chem. 1998;273:6380–6388. doi: 10.1074/jbc.273.11.6380. [DOI] [PubMed] [Google Scholar]
- 15.Shao L, Xu Y, Dong Q, et al. A novel SLC4A1 variant in an autosomal dominant distal renal tubular acidosis family with a severe phenotype. Endocrine. 2010;37:473–478. doi: 10.1007/s12020-010-9340-6. [DOI] [PubMed] [Google Scholar]
- 16.Bruce LJ, Wrong O, Toye AM, et al. Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: loss of up to 95% band 3 transport in red cells. Biochem J. 2000;350(Pt 1):41–51. doi: 10.1042/bj3500041. [DOI] [PMC free article] [PubMed] [Google Scholar]