Abstract
A 66-year-old man presented with a penile ulcer, an acute clinical onset of nephrotic syndrome and hepatitis. Secondary syphilis was diagnosed on the basis of the history of rash and the result of strongly positive serological test for syphilis. A renal biopsy demonstrated membranous glomerulonephritis with subepithelial electron-dense deposits. After treatment with amoxicillin for 2 weeks, he achieved clinical recovery. It is important to recognize syphilis as a reversible cause of nephrotic syndrome and acute hepatitis because antibiotic therapy can result in complete remission.
Keywords: Nephrotic syndrome, Hepatitis, Membranous glomerulonephritis, Syphilis
Introduction
Syphilis has been called “the great imitator” because it is associated with various clinical presentations. Despite the existence of inexpensive and effective antibiotic treatment regimens, more than 10 million new syphilis cases occur annually throughout the world. Nephrotic syndrome and acute hepatitis are known as complications of secondary syphilis [1], but they rarely occur simultaneously. In this case, we describe a patient with syphilitic nephrotic syndrome and hepatitis who achieved complete remission after treatment with amoxicillin.
Case report
A 66-year-old man with no prior history of systemic disease was admitted to our hospital because of peripheral edema lasting for 1 week and increasing general fatigue for 1 month.
The patient noticed penile swelling and induration 2 months before admission, followed by a sore throat and general fatigue. A non-pruritic eruption gradually spread over the arms and back. 2 weeks before admission, he went to our hospital for sore throat and decreased appetite and was administered cefcapene pivoxil hydrochloride for 5 days; however, he developed bilateral lower extremity edema. He had no fever, chills, nausea, vomiting, arthralgia, history of renal disease, alcohol abuse, or illicit drug use. His sexual history included sexual contact 4 months before with a female other than his wife.
On physical examination, his height was 158.9 cm, body weight was 55.2 kg, blood pressure was 149/76 mmHg, pulse was 63 beats per minute and regular, and body temperature was 36.2 °C. There was pitting edema of lower extremities bilaterally. External genitalia revealed penile swelling and an almost resolved penile ulcer with erythematous macules. There was a minimal residual rash in the upper extremities. His liver and spleen were not palpable. There was no sign of meningeal irritation or any other neurological abnormalities. Examination of the head and neck, lungs, heart, and abdomen was normal. Uveitis was not evident on ophthalmic examination.
Laboratory findings showed white cell count of 6450/μl, serum protein of 5.3 g/dl, albumin of 2.0 g/dl, cholesterol of 243 mg/dl, triglyceride of 250 mg/dl, blood urea nitrogen of 32.8 mg/dl, serum creatinine of 2.15 mg/dl, alanine aminotransferase of 76 IU/l, aspartate amino transferase of 51 IU/l, total bilirubin of 0.5 mg/dl, and alkaline phosphatase of 3793 IU/l. A work up including antimitochondrial antibody, antinucleolar antibody, and hepatitis A, B, C, CMV, and EBV serology were unremarkable. The initial spot urine protein-to-creatinine ratio increased to 19 g/gCre. The rapid plasma reagent (RPR) test was markedly reactive at 1:128 and a treponema pallidum hemagglutination (TPHA) test was positive at 1:41.2. Human immunodeficiency virus antibody test was negative. Serum complement C3 level was 137 mg/dl (normal range 65–135 mg/dl), and C4 was 28 mg/dl (normal range 13–35 mg/dl). Serum immunoglobulin (Ig) G level was 1646 mg/dl (870–1700 mg/dl), IgA was 314 mg/dl (110–410 mg/dl), and IgM was 335 mg/dl (33–190 mg/dl). Ultrasonography indicated a dull liver edge, kidneys of normal size, and a celiac lymph node swelling (11.0 × 6.8 mm).
Nephrotic syndrome was diagnosed and secondary syphilis was strongly suspected. Besides he might have hepatitis.
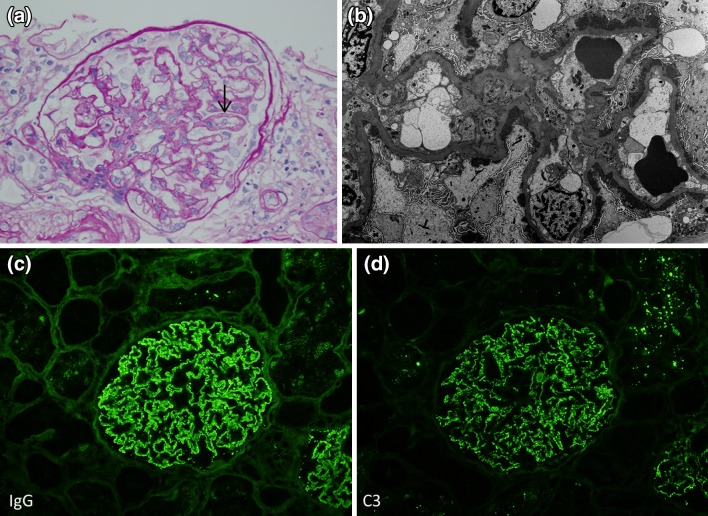
A kidney biopsy was performed on the eighth day of hospital stay, which revealed renal cortex and medulla with a total of 18 well-perfused glomeruli and five with glomerulosclerosis. Glomeruli were essentially normal except a double contour of the glomerular basement membrane in some glomeruli on staining with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and Jones methenamine silver (Fig. 1a). There was evidence of mild interstitial fibrosis and atrophy and severe tubular injury. Direct immunofluorescence (DIF) showed bright diffuse granular glomerular capillary wall staining for IgG, C1q, and C3 (Fig. 1c, d). DIF staining with antibodies to IgG subtypes showed moderate IgG1, IgG2, and IgG3; however, IgG4 was trace to negative. Electron microscopy showed subepithelial and intramembranous electron-dense immune-type deposits varying in size and density (Ehrenreich and Churg glomerular stage I; Fig. 1b).
Fig. 1.
a Hematoxylin and eosin (H&E)-stained slides of kidney biopsy: essentially normal except a double contour of glomerular basement membrane (black arrow). b Subepithelial and intramembranous electron-dense immune-type deposits with differences in size and density (Ehrenreich and Churg glomerular stage I). c, d Direct immunofluorescence (DIF) showed bright diffuse granular glomerular capillary wall staining for IgG and C3
On the basis of the history of rash and the results of serology and kidney biopsy, the patient was diagnosed as secondary syphilis with syphilitic membranous nephritis and hepatitis.
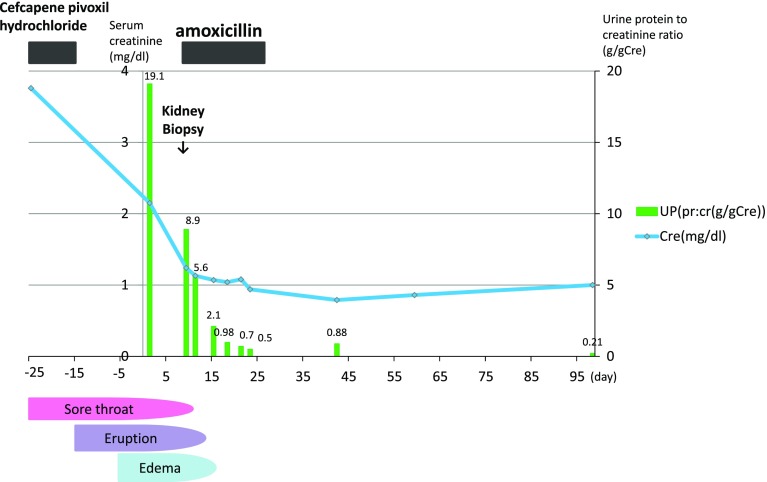
He received amoxicillin for 14 days and a low-protein diet for edema, and was hydrated with intravenous fluids. He was responsive to the treatment. It seems urinary protein levels and serum creatinine began to decrease and liver and biliary enzymes returned to normal soon after amoxicillin treatment. Nephrotic syndrome had completely resolved with normalization of urinary protein excretion (Fig. 2). He has remained in complete remission for 1 year after treatment.
Fig. 2.
Clinical course
Discussion
Nephrotic syndrome is a well-recognized but rare complication of secondary syphilis. The association between syphilis and renal disease was first reported in the 1900s. The prevalence of nephrotic syndrome in early syphilis was reported to be 0.28 % [2].
The manifestations of syphilitic renal disease include albuminuria, nephrotic syndrome, acute nephritis, and rapidly progressive nephritis (Table 1). Membranous glomerulonephritis (MGN), as seen in this case, is the most commonly observed histopathological lesion, and is thought to be mediated by immune complex deposition within the capillary walls [3–9]. Renal function is normal in most cases, but this case is reported to involve acute kidney injury (AKI). Volume depletion due to anorexia and tubular injury was associated with infection, and cephalosporin may cause AKI. Syphilitic nephritis can be resolved with penicillin in most cases, but hemodialysis, plasmapheresis, and methylprednisolone are required in some cases with rapidly progressive glomerulonephritis [20].
Table 1.
Renal biopsy and treatment of syphilitic nephropathy
| Year | Author | Renal biopsy | Treatment |
|---|---|---|---|
| 1991 | Balikocioglu | MGN | Unknown |
| 1991 | David J. Kusner | No biopsy | Penicillin |
| 1992 | Hruby | MGN | No treatment |
| 1992 | Hruby | MGN, vasculitis | Penicillin |
| 1992 | Hruby | RPGN | Penicillin, hemodialysis |
| 1992 | Magarian G. J. | No biopsy | Penicillin |
| 1993 | Hunte | MGN, mesangial proliferation | Penicillin |
| 2005 | Yung-Chih Chen | Interstitial nephritis | Penicillin |
| 2006 | Z. Soehardy | MGN | Penicillin |
| 2008 | Y.-C. Tsai | Mesangial proliferation | Penicillin |
| 2010 | K. Boslooper | MGN | Penicillin |
| 2010 | Anjali A. Satoskar | MGN | Penicillin |
| 2011 | John P. Havil | MGN | Penicillin |
| 2011 | M. T. Mora Mora | MGN | Penicillin |
| 2012 | Lisa M. Scheid | MGN, mesangial proliferation | Unknown |
| 2013 | M. Louis Handoko | MGN | Penicillin |
MGN membranous glomerulonephritis, RPGN rapidly progressive glomerulonephritis
It is interesting that in our patient, the proteinuria seemed to have essentially abated before specific treatment for syphilis with amoxicillin. Cephalosporin administered early in the course for presumptive upper respiratory infection might be partially efficacious against syphilis.
To our knowledge, there have been six reports in the literature describing a similar case where nephrotic syndrome and acute hepatitis occurred simultaneously (Table 2) [1, 10–14]. Clinically apparent syphilitic hepatitis has a similar incidence as nephrotic syndrome (0.24 %) as reported by a large retrospective study [15]. Although the exact pathogenesis of the liver and kidney damage is uncertain in syphilis infection, glomerular injury via deposition of antigen–antibody complexes might be a contributory factor.
Table 2.
Simultaneous nephrotic syndrome and hepatitis in six patients with syphilis
| Ref No. | Presentations | Clinical features | Pathology | ||
|---|---|---|---|---|---|
| Kidney | Liver | Kidney | Liver | ||
| [1] | Edema, rash | Nephrotic syndrome | Elevated ALP, AST and ALT | MGN, IgA nephropathy | No biopsy |
| [10] | Edema | Nephrotic syndrome | None | No biopsy | Active hepatitis |
| [11] | Fever, rash, edema | Nephrotic syndrome | Hepatosplenomegaly, elevated ALP | MGN | Granulomatous hepatitis |
| [12] | Jaundice, rash, dyspnea | Nephrotic syndrome | Elevated ALP and bilirubin | MGN | No biopsy |
| [13] | Edema, general fatigue | Nephrotic syndrome | Elevated AST and ALT | Mild increase of mesangial matrix | No biopsy |
| [14] | Rash, edema | Nephrotic syndrome | Elevated AST and ALT | MGN | Granulomatous hepatitis |
ALP alkaline phosphatase, AST aspartate transaminase, ALT alanine transaminase, MGN membranous glomerulonephritis
Most reports agree that in syphilitic hepatitis, alkaline phosphatase is disproportionately elevated in comparison with serum bilirubin and transaminases. Key historical findings include a nonspecific periportal hepatocyte necrosis and pericholangiolar inflammation [16–19]. Some reports have attributed cholestasis and elevation of serum alkaline phosphatase levels to a pericholangiolar inflammation. We did not perform liver biopsy and cannot exclude the possibility of coincidental viral hepatitis or liver dysfunction due to cefcapene pivoxil hydrochloride, but the clinical course and time of occurrence would not contradict syphilitic hepatitis.
Our case illustrates that it is important to recognize syphilis as an underlying cause of MGN, because appropriate antibiotic treatment can lead to complete remission [3, 20–23]. In addition, syphilis should always be considered in the differential diagnosis of nephrotic syndrome.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Tang S, Chan KW, Chan TM, Lai KN. Skin lesions, hepatitis, and nephropathy in a 30-year-old man. Am J Kidney Dis. 1999;34:380–383. doi: 10.1016/S0272-6386(99)70374-4. [DOI] [PubMed] [Google Scholar]
- 2.Hermann G, Marr WL. Clinical syphilitic nephropathies. A study of new cases and survey of reported cases. Am J Syph Neurol. 1935;19:1–29. [Google Scholar]
- 3.Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal anantibody from glomerular immune-complex deposits. N Engl J Med. 1975;292:449–454. doi: 10.1056/NEJM197502272920903. [DOI] [PubMed] [Google Scholar]
- 4.Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3:1351–1355. doi: 10.1681/ASN.V371351. [DOI] [PubMed] [Google Scholar]
- 5.Solling J, Solling K, Jacobsen KU, Olsen S, From E. Circulating immune complexes in syphilitic nephropathy. A case report. Br J Vener Dis. 1978;54:53–56. doi: 10.1136/sti.54.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643–648. doi: 10.1016/0002-9343(70)90016-1. [DOI] [PubMed] [Google Scholar]
- 7.Yuceoglu AM, Sagel I, Tresser G, Wasserman E, Lange K. The glomerulopathy of congenital syphilis: a curable immune-deposit disease. JAMA. 1974;229:1085–1089. doi: 10.1001/jama.1974.03230460035018. [DOI] [PubMed] [Google Scholar]
- 8.Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216:1159–1166. doi: 10.1001/jama.1971.03180330035006. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan BS, Wiglesworth FW, Marks MI, Drummond KN. The glomerulopathy of congenital syphilis—an immune deposit disease. J Pediatr. 1972;81:1154–1156. doi: 10.1016/S0022-3476(72)80251-8. [DOI] [PubMed] [Google Scholar]
- 10.McCracken JD, Hall WH, Pierce HI. Nephrotic syndrome and acute hepatitis in secondary syphilis. Milit Med. 1969;134:682–686. [PubMed] [Google Scholar]
- 11.Bansal RC, Cohn H, Fani K, Lynfield YL. Nephrotic syndrome and granulomatous hepatitis in secondary syphilis. Arch Dermatol. 1978;114:1228–1229. doi: 10.1001/archderm.1978.01640200080025. [DOI] [PubMed] [Google Scholar]
- 12.Tang AL, Thin RNT, Croft DN. Nephrotic syndrome and hepatitis in early syphilis. Postgrad Med J. 1989;65:14–15. doi: 10.1136/pgmj.65.759.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai YC, Chen LI, Chen HC. Simultaneous acute nephrosis and hepatitis in secondary syphilis. Clin Nephrol. 2008;70(6):532–536. doi: 10.5414/CNP70532. [DOI] [PubMed] [Google Scholar]
- 14.Morrison EB, Norman DA, Wingo CS, Henrich WL. Simultaneous hepatic and renal involvement in acute syphilis. Case report and review of the literature. Dig Dis Sci. 1980;25:875–878. doi: 10.1007/BF01338531. [DOI] [PubMed] [Google Scholar]
- 15.Hahn RD. Syphilis of the liver. Am J Syph Gonor Ven Dis. 1943;27:529–562. [Google Scholar]
- 16.Baker AL, Kaplan MM, Wolfe HJ, McGowan JA. Liver disease associated with early syphilis. N Engl J Med. 1971;284:1422–1423. doi: 10.1056/NEJM197106242842507. [DOI] [PubMed] [Google Scholar]
- 17.Lee RV, Thornton GF, Conn HO. Liver disease associated with secondary syphilis. N Engl J Med. 1971;284:1423–1425. doi: 10.1056/NEJM197106242842508. [DOI] [PubMed] [Google Scholar]
- 18.Sobel HJ, Wolf EH. Liver involvement in early syphilis. Arch Pathol. 1972;93:565–568. [PubMed] [Google Scholar]
- 19.Feher J, Somogyi T, Timmer M, Joza L. Early syphilitic hepatitis. Lancet. 1975;2:896–899. doi: 10.1016/S0140-6736(75)92129-7. [DOI] [PubMed] [Google Scholar]
- 20.Hruby Z, Kuüniar J, Rabczyński J, et al. The variety of clinical and histopathological presentations of glomerulonephritis associated with latent syphilis. Int Urol Nephrol. 1992;24:541–547. doi: 10.1007/BF02550123. [DOI] [PubMed] [Google Scholar]
- 21.Losito A, Bucciarelli E, Massi-Benedetti F, et al. Membranous glomerulonephritis in congenital syphilis. Clin Nephrol. 1979;12:32–37. [PubMed] [Google Scholar]
- 22.O’Reagan S, Fong JS, de Chadarévian JP, et al. Treponemal antigens in congenital and acquired syphilis nephritis: demonstration by immunofluorescence studies. Ann Intern Med. 1976;85:325–327. doi: 10.7326/0003-4819-85-3-325. [DOI] [PubMed] [Google Scholar]
- 23.Inam N, Izhar A, Gonzales Y, et al. Membranous glomerulonephritis secondary to syphilis. Am J Kidney Dis. 2009;53:B43. [Google Scholar]