Abstract
Over the recent years the non-invasive techniques for the evaluation of the small bowel have been playing a major role in the management of chronic intestinal diseases, such as inflammatory bowel diseases (IBD). The diagnostic performances of magnetic resonance imaging, computed tomography and ultrasound in the field of small bowel disorders, have been assessed and established for more than two decades. Newer sonographic techniques, such as strain elastography and shear wave elastography, have been put forward for the assessment of disease activity and characterization of IBD-related damage in the setting of Crohn’s disease and other gastrointestinal disorders. The data from the preliminary research and clinical studies have shown promising results as regards the ability of elastographic techniques to differentiate inflammatory from fibrotic tissue. The distinction between IBD activity (inflammation) and IBD-related damage (fibrosis) is currently considered crucial for the assessment and management of patients. Moreover, all the elastographic techniques are currently being considered in the setting of other intestinal disorders (e.g., rectal tumors, appendicitis). The aim of this paper is to offer both a comprehensive narrative review of the non-invasive techniques available for the assessment of small-bowel disorders, with particular emphasis on inflammatory bowel diseases, and a summary of the current evidence on the use of elastographic techniques in this setting.
Keywords: Elastography, Fibrosis, Inflammatory bowel disease, Bowel wall, Stricture
Core tip: Elastographic techniques, such as strain elastography and shear wave elastography, have shown promising results as regards their ability to differentiate inflammatory from fibrotic tissue, particularly in the setting of inflammatory bowel diseases where these techniques have been tested.
INTRODUCTION
Over the recent years the non-invasive techniques for the evaluation of the small bowel have been playing a major role in the management of chronic intestinal diseases, such as inflammatory bowel diseases (IBD). Computed tomography and magnetic resonance enterography/enteroclysis (CT-E, MR-E) are cross-sectional imaging techniques that show excellent performance in the diagnosis of intestinal disorders and promising results in the characterization of IBD lesions and prediction of their response to treatment[1,2]. In particular, the data from MR-E application have shown accuracy in the assessment of Crohn’s disease severity and stage[3-5]. MR techniques without contrast enhancement are being tested for the same purposes[6,7]. The diagnostic performance of ultrasound (US), both without and with intravenous contrast medium, has been evaluated for more than two decades. Technology advances[8,9] extending implementation have also occurred. More recently, new sonographic techniques, such as elastography, have been put forward for the assessment of disease activity and characterization of IBD-related damage in the setting of Crohn’s disease (CD)[10,11]. Noteworthy, from a clinical point of view particular attention is currently paid to the distinction between IBD activity (inflammation) and IBD-related damage (fibrosis). All the techniques are currently tested also against other intestinal disorders (e.g., rectal tumors, appendicitis)[12-14]. However, only scattered data is available regarding the performance of elastographic techniques in the assessment and characterization of small-bowel disorders. Therefore, the aim of this paper is: (1) to give a comprehensive narrative review of the non-invasive techniques available to date for the assessment of small-bowel disorders, with particular emphasis on IBD; and (2) to summarize the evidence currently available on the use of elastographic techniques in this setting.
LITERATURE SEARCH
A comprehensive MEDLINE search was performed in August 2016, combining the MeSH keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis, bowel, diagnosis, fibrosis, inflammation, elastography over the period 2010-2016. After the exclusion of non-pertinent articles, all the original papers covering the use of elastography in the setting of small-bowel disorders were selected for the purposes of this review. Select relevant articles concerning the use of other non-invasive techniques have been included in the discussion.
SMALL-BOWEL DISORDERS, INFLAMMATION AND FIBROSIS
In the setting of small-bowel diseases the evaluation of the small-bowel mucosa has long been considered essential for the diagnosis and management of patients during follow-up, especially as regards the monitoring of treatment response. To date the diagnosis of CD is based upon the combination of clinical signs, laboratory tests and the endoscopic evidence of specific lesions in the small bowel and/or other segments of the gastrointestinal system[1]. Biopsy specimens from the ileum and colon assist in the diagnostic process[1]. Endoscopic improvement and/or mucosal healing are considered endpoints for the response-to-treatment assessment along with clinical improvement[15]. Moreover, the clinical course of IBD is often characterized by frequent recurrence/relapse of symptoms, which prompts further investigations and possible changes of the therapeutic strategy[16]. However, the direct visualization of the small-bowel mucosa by means of endoscopic techniques and the sampling of biopsy specimens are often cumbersome and not always technically feasible. Different non-invasive techniques such CT-E, MR-E and ultrasonographic techniques have been validated for the diagnosis and follow-up of small-bowel disorders and are now widely used in clinical practice as an alternative or complement to traditional endoscopy[1,17,18].
It is widely accepted that the development of fibrosis has a major influence on the morbidity of IBD. In CD patients the presence of fibrotic strictures is associated with high rates of hospitalization and surgery[19-21]. The pathogenetic mechanisms responsible for the development of intestinal fibrosis have not been fully elucidated yet[22], but it is widely accepted that bowel wall fibrosis develops as a consequence of a chronic inflammatory process within the tissue, with the subsequent local release of fibrogenic mediators and cytokines[23-25]. The chronic inflammatory activation, typical of CD, is therefore responsible for the increase in extracellular matrix deposition and mesenchymal cell activation[26-28]. The close connection between chronic inflammation and fibrosis in the setting of CD enforces the concept that the different CD phenotypes are the expression of a progressively accumulating bowel damage, rather than the result of different pathogenetic pathways[19,21,29].
In the setting of CD-related lesions, for patient management purposes it helps to estimate the relative proportion of inflammatory vs fibrotic tissue, by considering the different prognosis and therapeutic options associated with the presence of a fibrotic stricture as compared to an inflammatory process[19]. The balance between fibrotic and inflammatory processes within the bowel tissue of patients with IBD has been extensively studied in pathological series, which have showed the coexistence of acute/chronic inflammatory infiltrate and bowel wall fibrosis in CD surgical specimens[8,10,30,31]. The opportunity of assessing the relative proportion of inflammatory vs fibrotic tissue within an affected bowel wall represents a major step towards the non-invasive evaluation of CD, on consideration that the presence of fibrosis in the affected tissue negatively influences the response to biological treatment and, therefore, leads to endoscopic or surgical treatment[16,19,32].
Some of the non-invasive techniques already available for the diagnosis of intestinal disorders (US, contrast-enhanced US, CT, MR) have been tested for their ability to discriminate between inflammatory and fibrotic tissue in the small-bowel wall of patients with IBD, with promising results especially as concerns MR techniques[2-8,31,33]. In this scenario, the development of new techniques, such as elastography, for the evaluation of inflammatory vs fibrotic tissue changes in small-bowel segments, has been welcomed as a potentially major breakthrough.
PRINCIPLES OF ELASTOGRAPHY
Elastography is a non-invasive method developed to assess the mechanical properties of a tissue, in particular its stiffness[34,35]. To date, a range of elastographic technologies is available and marketed (Table 1). Strain elastography is able to provide the quantitative imaging of strain and elastic modulus distributions in soft tissues: by exerting tissue compression, the strain profile along the transducer axis is calculated and then converted to an elastic modulus profile based on the measurement of the stress applied[35]. Usually, strain imaging or ultrasound elasticity imaging (UEI) defines the technique whereby the tissue deformation is produced by pressing on the tissue with an ultrasound transducer (external force) and then recorded along with real-time US images[35]. Different techniques applying a combination of static or dynamic deformations to internal or external forces, have been explored[34,36] ending with the current application of strain elastography - with pressure being applied manually[37] or by cardiovascular pulsation[38] - to several fields of clinical medicine, including breast lesions characterization[39]. A recently developed elastographic method is Acoustic Radiation Force Impulse (ARFI) imaging, which targets ultrasound beam pulses to deform a chosen tissue area[40]. Its application has been validated mainly in the liver setting[41,42], with promising results for kidney[43] and thyroid diseases as well. If the disturbance/deformation induced by the mechanical excitation of the tissue is measured as the velocity of an induced shear wave, the technique is labelled shear wave elastography (SWE, which uses ARFI technology) or transient elastography, the latter being a technology not developed for imaging[40,44]. The successful application of elastography in various clinical scenarios is consistent with the concept that normal and pathologic tissues have different mechanical properties, mainly due to the altered balance of normal, fibrotic and/or inflammatory tissue components. Because of this, the elastographic techniques, particularly UEI and shear wave elastography, have been put forward as useful tools for the assessment of intestinal fibrosis in CD: their use has been evaluated in preliminary studies on animal models (Table 2) and some feasibility studies on human subjects (Table 3)[10,11,45-49].
Table 1.
Summary of the technical features of the various elastographic techinques
| Technique | Physical characteristic measured | Excitation method |
| Strain elastography/UEI | Strain | Manual compression (operator pressure, cardiovascular pulse, breathing movements) |
| ARFI imaging | Strain | Acoustic radiation force impulse |
| Shear wave elastography | Shear wave speed | Acoustic radiation force impulse |
| Point-shear wave speed measurement | ||
| Shear wave speed imaging | ||
| Transient elastography | Shear wave speed | Controlled external vibration |
UEI: Ultrasound elasticity imaging; ARFI: Acoustic radiation force impulse.
Table 2.
Summary of the feasibility studies on elastography in animal models
| Ref. | Subjects | Results | |
| Kim et al[11], 2008 | Animal model (rats) | Technique | Strain difference of normal colon vs affected left colon P < 0.0002 |
| 6 Left-sided chronic colitides (from TNBS) | Strain elastography (UEI) | ||
| 5 controls | Comparison | Significant correlation between Young’s modulus and strain r = 0.67, P < 0.0005 | |
| Direct mechanical measurement, Histology | |||
| Stidham et al[10], 2011 | Animal model (TNBS rats) | Technique | Strain values: |
| 5 acute colitides | Strain elastography (UEI) | controls vs acute inflammation P = 0.015, | |
| 5 chronic fibrosis | Comparison | controls vs chronic fibrosis P = 0.001, | |
| 5 controls | Histology | acute inflammation vs chronic fibrosis P = 0.037 | |
| Strain ratio: | |||
| acute inflammation vs chronic fibrosis P = 0.030 | |||
| Dillman et al[45], 2013 | Animal model | Technique | SW velocity ratio: |
| 6 acute colitides | Shear wave elastography (Shear wave speed imaging) | AUROC curve for differentiating fibrosis from inflammation 0.971 | |
| 8 chronic colitides/fibrosis | |||
| 3 controls | Comparison | ||
| Histology |
TNBS: Trinitrobenzenesulfonic acid; UEI: Ultrasound elasticity imaging; CD: Crohn’s disease; AUROC: Area under receiver operating characteristic; SW: Shear wave; VAS: Visual analogue scale.
Table 3.
Studies assessing the utility of elastography in intestinal diseases
| Ref. | Subjects | Outcomes | |
| Stidham et al[10], 2011 | 7 CD patients with stricturing disease | Technique | Strain values: |
| Strain elastography (UEI) | Correlation with Young’s modulus r = -0.81 | ||
| Comparison | Significant difference between stenotic tissue and unaffected proximal tissue P = 0.0008 | ||
| Direct mechanical measurement, Histology | |||
| Dillman et al[46], 2014 | 17 Human intestinal surgical specimens (from subjects with known or suspected IBD) | Technique | Difference in SW speed between low and high fibrosis |
| Shear wave elastography (point SW speed measurement and SW speed imaging) | With point SW speed measurement, P = 0.004, AUROC = 0.91 | ||
| Comparison | With SW speed imaging P = 0.049 AUROC = 0.77 | ||
| Histology | No difference in mean SW speed between low and high inflammation | ||
| Havre et al[50], 2014 | Human intestinal surgical specimens | Technique | UEI able to discriminate between adenoma and adenocarcinoma/CD, not between adenocarcinoma and CD |
| Strain elastography (UEI) | |||
| 16 CD | Comparison | Reproducibility: | |
| 18 adenocarcinoma | Histology | SR: intraobserver correlation rho = 0.47-0.82 | |
| 4 adenomas | Visual categorical score: interobserver agreement k = 0.38 | ||
| VAS: interobserver correlation Pearson’s r = 0.55, P = 0.002 | |||
| Baumgart et al[47], 2015 | 10 CD patients elected for surgery | Technique | Higher strain values in affected vs unaffected bowel (P < 0.001) |
| Strain elastography (UEI) | |||
| Comparison | Good ICC among pre-, intra- and post-operative strain measurements (0.830 in affected segments) | ||
| Direct mechanical measurement, | |||
| Histology | Association between strain measurements and | ||
| internal muscularis mucosae and muscularis propria width (P = 0.044 and 0.012) | |||
| histologic fibrosis score (P < 0.001) | |||
| Fraquelli et al[49], 2015 | 23 CD patients elected for surgery | Technique | Interrater agreement: color scale (ICC 0.90) and SR (ICC 0.78) |
| Strain elastography (UEI) | |||
| 20 CD controls | Comparison | Correlation between SR and severity of bowel fibrosis P < 0.0001 but not with inflammation scores | |
| Histology | |||
| AUROC curve for prediction of severe fibrosis 0.917 (95%CI: 0.788-1.000) | |||
| Fufezan et al[48], 2015 | 14 pediatric CD patients (48 bowel segments) | Technique | Correlation between: |
| Strain elastography (UEI) | UEI “inflammatory type” and complications | ||
| Comparison | UEI “inflammatory” and “fibrotic” type and CRP | ||
| US signs and Clinical Data | UEI “inflammatory type” and ESR | ||
| UEI and bowel wall thickness and stratification |
TNBS: Trinitrobenzenesulfonic acid; UEI: Ultrasound elasticity imaging; CD: Crohn’s disease; AUROC: Area under receiver operating characteristic; SW: Shear wave; VAS: Visual analogue scale; ICC: Intraclass correlation coefficient; SR: Strain ratio; CRP: C reactive protein; ESR: Erythrocyte sedimentation rate; US: Ultrasound.
ELASTOGRAPHY FOR THE ASSESSMENT OF INTESTINAL FIBROSIS
Strain elastography
Kim et al[11] provided the first data on the performance of strain elastography/UEI in the setting of intestinal disorders in an animal model. In this preliminary study, 6 rats intrarectally administered with 2,4,6-trinitrobenzenesulfonic acid (TNBS) were used as an animal model of left-sided colitis and fibrosis. Strain measurement was performed in vivo by UEI with the aid of a laboratory-designed deformation device fixed to the transducer in order to exercise a uniform displacement. After euthanasia the mechanical properties of the bowel wall were measured directly by means of a specific elastometer device[11]. Interestingly, the data showed a significant difference between the strain measurements of the healthy colons (5 controls and 6 proximal colons of TNBS rats) and the affected left colons and between the correspondent values of Young’s modulus computed with direct elastometry, this confirming the hypothesis of an association between the degree of involvement in a chronic inflammatory/fibrotic process and tissue stiffness. Overall, the correlation coefficient between Young’s modulus and strain was highly significant, suggesting the good accuracy of UEI in the indirect assessment of tissue stiffness. Not long after these preliminary data, a similar study by the same group confirmed a significant correlation between UEI results and the direct measurement of bowel wall stiffness after surgical resection in a small group of patients with stricturing CD. This proved the ability of UEI to discriminate between healthy bowel and fibrotic tissue[10]. For the first time, the data from an animal model generated the hypothesis that UEI would be able to discriminate between inflammatory and fibrotic tissue[10]. In another study, Havre et al[50] performed UEI on a series of resected surgical specimens (16 CD, 18 adenocarcinomas and 4 adenomas). Tissue stiffness was assessed through 3 different methods: (1) a visual categorical score already described for pancreatic lesions[51]; (2) a visual analog scale (VAS) ranging from 0 to 100 (with 50 representing equal stiffness to the surrounding tissue, < 50 lower and > 50 higher stiffness); and (3) the strain ratio (SR), calculated as the ratio between the mean strain in reference tissue surrounding the lesions and the mean strain in the lesion. The results of the strain assessment were then compared to the final diagnosis by histology, showing the significant difference in stiffness of adenoma vs adenocarcinoma and CD, with visual categorical scores, VAS and SR, but not so strong results as concerns the differentiation of adenocarcinoma vs CD, probably because of the relevant distribution of fibrosis in both lesions, which affected tissue stiffness in the same way[50]. Nonetheless, this study provided important information about the reproducibility of strain elastography, considering that two independent operators showed a fair inter-observer agreement for the categorical score (Kappa value 0.38) and a good correlation between the operators for the VAS evaluation (Pearson’s r = 0.55, P = 0.002), while a moderate-to-good correlation was found between the separate intra-observer SR measurements (Spearman’s Rho 0.47 to 0.82).
Baumgart et al[47] performed UEI strain assessment in 10 patients with CD and elected for surgery, and compared the strain values with direct tensiometry and histology after surgery. The strain measurement was performed with the aid of a built-in press guide function assessing the compression adequacy of the probe in order to allow for the same amount of compression and to obtain values of the strain per se, the same way as in the previous studies. The results confirmed the concept that the bowel segments affected by CD show a significantly higher strain than those unaffected (mean ± SD, 169.0 ± 27.9 vs 43.0, 6, 25.9 and P = 0.001). This was also confirmed by the direct measurement of tissue stiffness[47]. Moreover, the comparison with histologic features showed the correlation between strain differences and histologic signs of fibrosis, such as collagen deposits or muscular fibers. However, the possible influence of inflammatory tissue changes was not fully explored in these patients[47].
A study carried out by our group[49] aimed to assess the in-vivo performance of strain elastography in predicting bowel fibrosis and discriminating between inflammation and fibrosis (Figure 1). UEI was performed on 23 CD patients with ileal or ileocolonic disease, elected for surgical resection, and on 20 controls (uncomplicated CD patients). Strain assessment was performed with 2 methods: (1) a semi-quantitative visual color scale; and (2) SR measurement. The SR was calculated in the same way as in the Havre study[50], with the mesenchimal tissue surrounding the affected bowel wall chosen as the reference tissue. The results showed that the SR values, but not the color scale values, were significantly correlated with the severity of fibrosis at both semiquantitative and quantitative histologic image analysis: SR showed excellent ability to discriminate severe bowel fibrosis (AUROC curve: 0.917; 95%CI: 0.788-1.000), with evidence of no influence of the histologic degree of inflammation on SR at multivariate analysis[49].
Figure 1.
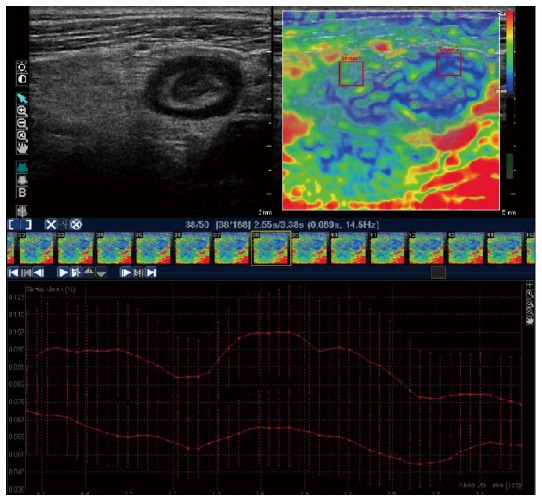
Upper left quadrant: Ultrasound image of the thickened terminal ileum of a Crohn’s disease patient, with a narrowed lumen and surrounding mesenteric tissue. Upper right quadrant: Appearance of the same ileal segment with strain elastography. Regions of interest (ROIs) can be selected for the quantitative assessment of the strain ratio (see text). Lower quadrant: Strain values of ROI 1 (mesenteric tissue) and ROI 2 (ileal wall) plotted over time for the software to calculate the mean strain ratio (adapted from Fraquelli et al[49]).
Later, Fufezan et al[48] tried to evaluate the role of strain elastography in the setting of pediatric Crohn’s disease, with the aim of widening the range of non-invasive methods to avail. In their study these authors examined 48 bowel segments of 14 pediatric CD patients, and attempted to develop a visual classification of bowel wall appearance at UEI (remission bowel, inflammatory wall and fibrotic wall) and to correlate it with clinical and US features. The results suggested some correlation between different UEI visual patterns and US and clinical signs of disease activity or complications. However, these preliminary results were not compared to histology or stratified for types of current therapy, thus requiring validation in a large prospective study[48].
Shear wave elastography
As regards shear wave (SW) speed techniques, Dillman et al[45] focused on SW speed imaging. The data from an animal model of acute and chronic colitis showed that shear wave speed measurement was significantly higher in the fibrosis cohort than in the acute inflammation one at different amounts of strain applied (0% and 30%, P = 0.047 and 0.02 respectively). The AUROC curve of the SW speed ratio (mean SW speed/applied strain) for differentiating fibrotic from inflamed bowel was 0.971, which is an excellent result (derived though from small numbers) suggesting the successful application of this technique in the clinical setting[45]. In another study the same group applied shear wave elastographic techniques (both point shear wave speed measurement and shear wave speed imaging) on 17 human resected bowel specimens and showed that these techniques were able to discriminate tissue with high vs low degree of fibrosis as histologically assessed, while no significant correlation was observed between the SW speed and the degree of inflammation[46]. Moreover, the data from this study could offer no information on the ability of SW elastographic techniques to discriminate between inflammation and fibrosis[46]. To date, no other studies are available on the applicability of SW elastography in the setting of IBD.
Role of elastography in other intestinal disorders
Elastography has been proposed as a diagnostic tool for various intestinal disorders. As far as the endoscopic use of elastography is concerned, only strain elastography is currently available for use with endoscopic devices. A pilot study on trans-rectal endoscopic UEI in IBD showed that the SR values of the rectal bowel wall were significantly different not only between CD patients and controls, but also between ulcerative colitis and CD patients, thus pointing to the possible role of elastography in differentiating between colonic CD and ulcerative colitis[52].
Some studies in the field of colonic diseases have shown that elastography is feasible and reproducible[53] especially as regards the differentiation between rectal adenomas and adenocarcinomas, where it has showed a level of accuracy superior to endoscopic ultrasound and MR[13,14,54]. However, not only further evidence but also further technological advancements are needed before elastography can be routinely applied to the diagnosis of malignant intestinal lesions, and also preliminary data on the application of this technique for the diagnosis of gastrointestinal stromal tumors seem to suggest the same[55].
A single study has recently evaluated the performance of SW elastography towards the diagnosis of appendicitis: the data from 30 patients with appendicitis and 11 controls showed that SW can differentiate between a normal and inflamed appendix (Sn 93%, Sp 100% considering a 12.5 kPA cut-off), but not better than CT[12].
Possible limitations of the application of elastography in small-bowel diseases
As some authors[49,56] have already pointed out, the performance of bowel elastography in routine clinical practice shows some limitations. First of all, due to the technical characteristics of this technology, which was developed as an add-on to routine ultrasonography devices, it is not possible to obtain a complete view of all the bowel segments, as opposed to cross-sectional imaging techniques. This feature limits the application field of elastography to selected bowel segments (i.e., terminal ileum, caecum). Moreover, the transabdominal strain imaging techniques have to deal with potential errors owed to peristaltic bowel movements, which are not present in the other anatomical sites where these techniques are widely validated (i.e., thyroid, breast), even though some software available on the market offers movement correction filters to minimize such an interference[49].
On the other hand, the non-invasivity of these techniques and the low cost of their application in clinical practice (limited to the installation of specific software in the already deployed US equipment) are likely to exceed the possible limitations.
CONCLUSION
Despite the small sample sizes of the available studies, elastography has showed extremely promising results in the field of intestinal diseases, especially as concerns IBD. The newest European recommendations for gastrointestinal US have stated that elastography “can be used to evaluate the stiffness of a patient’s pathological thickened bowel”[57]. Validating the strain elastography technique in larger cohorts is needed in order to confirm its very promising results, especially as regards its prognostic value and possible role as a predictor of the response-to-treatment of CD patients. The SR calculation task seems to be easy to approach in clinical practice against the consideration that direct strain measurements can be performed only if the external pressure exerted on the tissue is known and stable, which is per se technically difficult and has requested the use of mechanical devices or probe stabilizers in previous studies[10,11,47]. As regards SW elastography, the technical characteristics of this method would theoretically overcome some of the limitations typical of strain elastography, making it a simpler and more reproducible tool. However, the currently available data on its usefulness for bowel diseases is scattered and further validation is needed before SW elastography can be considered a reliable method for the assessment of disease severity in IBD patients. To date, there are no studies that directly compare the performance of elastography with MR imaging, which is the cross-sectional technique that is so far the most accurate in detecting bowel wall fibrosis. It should be pointed out that MR techniques have shown excellent accuracy in this field but are surely less available and more time-consuming than an elastographic evaluation in the context of a routine US examination. MR techniques also present contraindications and side effects when there is use of intravenous contrast medium.
The overall simplicity and feasibility of elastography lead to expect further developments including the broadening of the fields of application for these techniques.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All the authors declare no conflict of interest.
Peer-review started: October 8, 2016
First decision: December 29, 2016
Article in press: March 30, 2017
P- Reviewer: Caboclo JLF, Tarnawski AS, Meshikhes AW, Xu G S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Quencer KB, Nimkin K, Mino-Kenudson M, Gee MS. Detecting active inflammation and fibrosis in pediatric Crohn’s disease: prospective evaluation of MR-E and CT-E. Abdom Imaging. 2013;38:705–713. doi: 10.1007/s00261-013-9981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fornasa F, Benassuti C, Benazzato L. Role of Magnetic Resonance Enterography in Differentiating between Fibrotic and Active Inflammatory Small Bowel Stenosis in Patients with Crohn’s Disease. J Clin Imaging Sci. 2011;1:35. doi: 10.4103/2156-7514.82339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkmeier DT, Dillman JR, Al-Hawary M, Heider A, Davenport MS, Smith EA, Adler J. MR enterography-histology comparison in resected pediatric small bowel Crohn disease strictures: can imaging predict fibrosis? Pediatr Radiol. 2016;46:498–507. doi: 10.1007/s00247-015-3506-6. [DOI] [PubMed] [Google Scholar]
- 5.Quaia E, Cabibbo B, Sozzi M, Gennari AG, Pontello M, Degrassi F, Cova MA. Biochemical markers and MR imaging findings as predictors of crohn disease activity in patients scanned by contrast-enhanced MR enterography. Acad Radiol. 2014;21:1225–1232. doi: 10.1016/j.acra.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Dillman JR, Swanson SD, Johnson LA, Moons DS, Adler J, Stidham RW, Higgins PD. Comparison of noncontrast MRI magnetization transfer and T2 -Weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging. 2015;42:801–810. doi: 10.1002/jmri.24815. [DOI] [PubMed] [Google Scholar]
- 7.Rimola J, Planell N, Rodríguez S, Delgado S, Ordás I, Ramírez-Morros A, Ayuso C, Aceituno M, Ricart E, Jauregui-Amezaga A, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–440. doi: 10.1038/ajg.2014.424. [DOI] [PubMed] [Google Scholar]
- 8.Maconi G, Carsana L, Fociani P, Sampietro GM, Ardizzone S, Cristaldi M, Parente F, Vago GL, Taschieri AM, Bianchi Porro G. Small bowel stenosis in Crohn’s disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther. 2003;18:749–756. doi: 10.1046/j.1365-2036.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- 9.Nylund K, Jirik R, Mezl M, Leh S, Hausken T, Pfeffer F, Ødegaard S, Taxt T, Gilja OH. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med Biol. 2013;39:1197–1206. doi: 10.1016/j.ultrasmedbio.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Stidham RW, Xu J, Johnson LA, Kim K, Moons DS, McKenna BJ, Rubin JM, Higgins PD. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011;141:819–826.e1. doi: 10.1053/j.gastro.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Johnson LA, Jia C, Joyce JC, Rangwalla S, Higgins PD, Rubin JM. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med Biol. 2008;34:902–912. doi: 10.1016/j.ultrasmedbio.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha SW, Kim IY, Kim YW. Quantitative measurement of elasticity of the appendix using shear wave elastography in patients with suspected acute appendicitis. PLoS One. 2014;9:e101292. doi: 10.1371/journal.pone.0101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafaelsen SR, Vagn-Hansen C, Sørensen T, Lindebjerg J, Pløen J, Jakobsen A. Elastography and diffusion-weighted MRI in patients with rectal cancer. Br J Radiol. 2015;88:20150294. doi: 10.1259/bjr.20150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waage JE, Leh S, Røsler C, Pfeffer F, Bach SP, Havre RF, Haldorsen IS, Ødegaard S, Baatrup G. Endorectal ultrasonography, strain elastography and MRI differentiation of rectal adenomas and adenocarcinomas. Colorectal Dis. 2015;17:124–131. doi: 10.1111/codi.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–851. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95–101. doi: 10.1148/radiol.2361040799. [DOI] [PubMed] [Google Scholar]
- 19.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 21.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Rieder F, Fiocchi C. Intestinal fibrosis in IBD--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 23.Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, Sime PJ, Gauldie J, Collins SM. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G116–G128. doi: 10.1152/ajpgi.00051.2005. [DOI] [PubMed] [Google Scholar]
- 24.Mahavadi S, Flynn RS, Grider JR, Qiao LY, Murthy KS, Hazelgrove KB, Kuemmerle JF. Amelioration of excess collagen IαI, fibrosis, and smooth muscle growth in TNBS-induced colitis in IGF-I(+/-) mice. Inflamm Bowel Dis. 2011;17:711–719. doi: 10.1002/ibd.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013, 2013.e1-7. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 26.Rieder F, Georgieva M, Schirbel A, Artinger M, Zügner A, Blank M, Brenmoehl J, Schölmerich J, Rogler G. Prostaglandin E2 inhibits migration of colonic lamina propria fibroblasts. Inflamm Bowel Dis. 2010;16:1505–1513. doi: 10.1002/ibd.21255. [DOI] [PubMed] [Google Scholar]
- 27.Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman HJ. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J Clin Gastroenterol. 2003;37:216–219. doi: 10.1097/00004836-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007;102:2541–2550. doi: 10.1111/j.1572-0241.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, Kunisaki R, Kinoshita H, Yamamoto H, Kimura H, Hanzawa A, Shibata N, Yonezawa H, Miyajima E, Sakamaki K, et al. Use of color Doppler ultrasonography for evaluating vascularity of small intestinal lesions in Crohn’s disease: correlation with endoscopic and surgical macroscopic findings. Scand J Gastroenterol. 2014;49:295–301. doi: 10.3109/00365521.2013.871744. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein GR, Olson A, Travers S, Diamond RH, Chen DM, Pritchard ML, Feagan BG, Cohen RD, Salzberg BA, Hanauer SB, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101:1030–1038. doi: 10.1111/j.1572-0241.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 33.Ripollés T, Rausell N, Paredes JM, Grau E, Martínez MJ, Vizuete J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7:120–128. doi: 10.1016/j.crohns.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Lerner RM, Huang SR, Parker KJ. “Sonoelasticity” images derived from ultrasound signals in mechanically vibrated tissues. Ultrasound Med Biol. 1990;16:231–239. doi: 10.1016/0301-5629(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 35.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 36.Kim K, Weitzel WF, Rubin JM, Xie H, Chen X, O’Donnell M. Vascular intramural strain imaging using arterial pressure equalization. Ultrasound Med Biol. 2004;30:761–771. doi: 10.1016/j.ultrasmedbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand palpation imaging. Ultrasound Med Biol. 2003;29:427–435. doi: 10.1016/s0301-5629(02)00733-0. [DOI] [PubMed] [Google Scholar]
- 38.Morikawa H, Fukuda K, Kobayashi S, Fujii H, Iwai S, Enomoto M, Tamori A, Sakaguchi H, Kawada N. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. J Gastroenterol. 2011;46:350–358. doi: 10.1007/s00535-010-0301-x. [DOI] [PubMed] [Google Scholar]
- 39.Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, Pennanen MF. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 40.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE. In vivo visualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol. 2008;53:279–293. doi: 10.1088/0031-9155/53/1/020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 43.Goya C, Kilinc F, Hamidi C, Yavuz A, Yildirim Y, Cetincakmak MG, Hattapoglu S. Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am J Roentgenol. 2015;204:324–329. doi: 10.2214/AJR.14.12493. [DOI] [PubMed] [Google Scholar]
- 44.Liu B, Zheng Y, Huang G, Lin M, Shan Q, Lu Y, Tian W, Xie X. Breast Lesions: Quantitative Diagnosis Using Ultrasound Shear Wave Elastography-A Systematic Review and Meta--Analysis. Ultrasound Med Biol. 2016;42:835–847. doi: 10.1016/j.ultrasmedbio.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Rubin JM. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology. 2013;267:757–766. doi: 10.1148/radiol.13121775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Keshavarzi NR, Rubin JM. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33:2115–2123. doi: 10.7863/ultra.33.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumgart DC, Müller HP, Grittner U, Metzke D, Fischer A, Guckelberger O, Pascher A, Sack I, Vieth M, Rudolph B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology. 2015;275:889–899. doi: 10.1148/radiol.14141929. [DOI] [PubMed] [Google Scholar]
- 48.Fufezan O, Asavoaie C, Tamas A, Farcau D, Serban D. Bowel elastography - a pilot study for developing an elastographic scoring system to evaluate disease activity in pediatric Crohn’s disease. Med Ultrason. 2015;17:422–430. doi: 10.11152/mu.2013.2066.174.bwe. [DOI] [PubMed] [Google Scholar]
- 49.Fraquelli M, Branchi F, Cribiù FM, Orlando S, Casazza G, Magarotto A, Massironi S, Botti F, Contessini-Avesani E, Conte D, et al. The Role of Ultrasound Elasticity Imaging in Predicting Ileal Fibrosis in Crohn’s Disease Patients. Inflamm Bowel Dis. 2015;21:2605–2612. doi: 10.1097/MIB.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 50.Havre RF, Leh S, Gilja OH, Ødegaard S, Waage JE, Baatrup G, Nesje LB. Strain assessment in surgically resected inflammatory and neoplastic bowel lesions. Ultraschall Med. 2014;35:149–158. doi: 10.1055/s-0032-1325535. [DOI] [PubMed] [Google Scholar]
- 51.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 52.Rustemovic N, Cukovic-Cavka S, Brinar M, Radić D, Opacic M, Ostojic R, Vucelic B. A pilot study of transrectal endoscopic ultrasound elastography in inflammatory bowel disease. BMC Gastroenterol. 2011;11:113. doi: 10.1186/1471-230X-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giannetti A, Biscontri M, Matergi M. Feasibility of real-time strain elastography in colonic diseases. J Ultrasound. 2014;17:321–330. doi: 10.1007/s40477-014-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cârțână ET, Gheonea DI, Săftoiu A. Advances in endoscopic ultrasound imaging of colorectal diseases. World J Gastroenterol. 2016;22:1756–1766. doi: 10.3748/wjg.v22.i5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ignee A, Jenssen C, Hocke M, Dong Y, Wang WP, Cui XW, Woenckhaus M, Iordache S, Saftoiu A, Schuessler G, et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017;6:55–60. doi: 10.4103/2303-9027.200216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Havre R, Gilja OH. Elastography and strain rate imaging of the gastrointestinal tract. Eur J Radiol. 2014;83:438–441. doi: 10.1016/j.ejrad.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, Serra C, Dietrich CF, Sporea I, Saftoiu A, et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound - Part 1: Examination Techniques and Normal Findings (Long version) Ultraschall Med. 2016 doi: 10.1055/s-0042-115853. Epub ahead of print. [DOI] [Google Scholar]


