Abstract
The role of myeloid‐derived suppressor cells (MDSCs) in cancer development has become clear over recent years, and MDSC targeting is an emerging opportunity for enhancing the effectiveness of current anticancer therapies. As MDSCs are not only able to limit anti‐tumour T‐cell responses, but also to promote tumour angiogenesis and invasion, their monitoring has prognostic and predictive value. Herein, we review the key features of MDSCs in cancer promotion. © 2017 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: myeloid‐derived suppressor cells, tumour promotion, immune suppression
Myeloid‐derived suppressor cells in the tumour microenvironment
The phenotype of a tumour microenvironment is shaped not only by the epithelial component, but also by the interaction with mesenchymal cells and the inflammatory infiltrate. In recent years, the contribution of these components to cancer progression has been evaluated, including ligands, cell–cell adhesion molecules, metabolites, oxygen, and multiple soluble factors, together with the expansion of immune cells (such as B cells, natural killer cells, macrophages, Th2 cells, Th17 cells, and regulatory T cells). Given the prominence of the immune infiltrate in the tumour microenvironment, evaluating the so‐called ‘immunoscore’ has been proposed as a computer‐based analysis to quantify the immune component for improved patient management 1.
Myeloid‐derived suppressor cells (MDSCs) represent a major population of regulatory cells that are expanded in different types of cancer and impair antitumour innate and adaptive immune responses. Many studies have underscored complex MDSC phenotypes that are not restricted to a single defined myeloid cell population, but rather show a plastic phenotype that is responsive to tumour‐derived soluble factors. These phenotypes can be reduced to three main subsets: granulocytic MDSCs [polymorphonuclear (PMN)‐MDSCs], monocytic MDSCs (Mo‐MDSCs), and early‐stage MDSCs (e‐MDSCs) 2, 3 (Figure 1). MDSCs originate from myeloid progenitors in the bone marrow, and are expanded and activated by stromal and tumour‐derived factors; the different composition of soluble factors mobilizing MDSCs dictates the phenotypic features of MDSC subsets observed in each patient. The pathological activation of myeloid precursors causes their acquisition of suppressive activity towards immune cells through a number of mechanisms, depending on cell surface interactions, the release of short‐lived soluble mediators, and the activation of enzymes (such as programmed death‐ligand 1/2, release of reactive oxygen species and reactive nitrogen species, and activation of arginase 1, indoleamine‐2,3‐dioxygenase, and inducible nitric oxide synthase; reviewed in 2). Besides these strategies of immune suppression, MDSCs are also able to promote tumour growth by acting on cancer cells or by triggering mechanisms responsible for tumour dissemination (Figure 1). Here, we will discuss some new advances in the field, documenting an enlargement of the sphere of influence of MDSCs.
Figure 1.
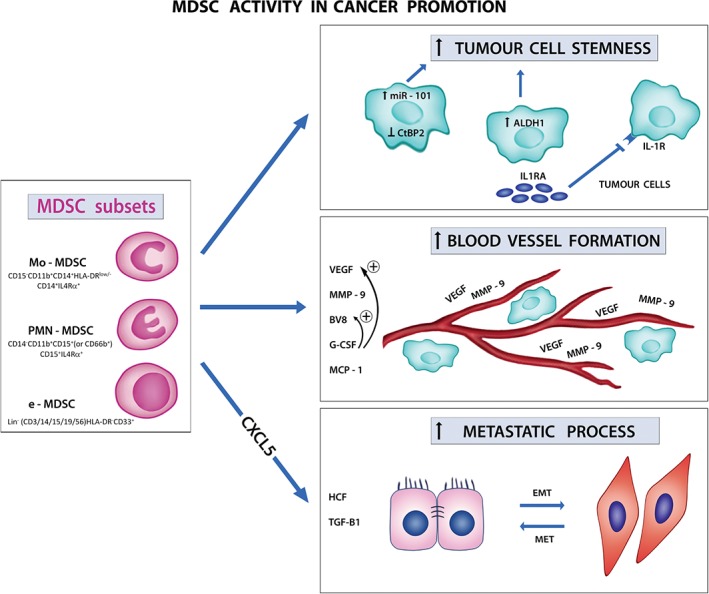
MDSC activity in cancer promotion. Different subsets of MDSCs are recruited to the tumour microenvironment and promote tumour progression in several ways: (1) they sustain tumour cell stemness by acting directly on tumour cells (upper panel on the right); (2) they promote blood vessel formation through the secretion of pro‐angiogenetic factors (central panel on the right); (3) they support EMT to enhance metastatic potential (lower panel on the right). HCF, hepatocyte growth factor; MET, epithelial‐mesenchymal transition.
Direct effects of MDSCs on cancer cells
MDSCs may enhance the stemness of cancer cells 4, 5. In particular, MDSCs triggered miRNA101 expression in ovarian cancer cells, and promoted cancer stemness by targeting the corepressor C‐terminal‐binding protein 2 (CtBP2). High levels of miRNA101 were associated with reduced overall survival in ovarian cancer patients; moreover, high CD33+ MDSC infiltration correlated with low CtBP2 levels, suggesting that the combination of CtBP2 and MDSCs allow for improved prognostic stratification of ovarian cancer 4. In line with these data, STAT3+ Mo‐MDSCs expanded after exposure to human pancreatic cancer (PC) cells and increased the frequency of aldehyde dehydrogenase‐1 (ALDH1)‐positive cancer stem cells; PC patients with a predominant CD14+ tissue infiltrate had reduced survival, and the CD14+ cell subset correlated with tumour ALDH1 expression 5. MDSCs may also promote tumour progression in PTEN‐null prostate tumour models by opposing senescence of cancer cells through the release of interleukin‐1 receptor antagonist (IL‐1RA) in the tumour microenvironment and the consequent effects on IL‐1Rα signalling 6. It is of note that IL‐1RA also inhibited docetaxel‐induced senescence in human prostate cancer cells, and patients with high levels of intratumoural IL‐1RA did not respond to docetaxel and had short disease‐free survival as compared with patients with normal IL‐1RA levels, and tumour‐infiltrating CD33+ myeloid cells were inversely correlated with the presence of p16INK4A+ senescent cells 6.
Promotion of angiogenesis
MDSCs may also have a profound influence on tumour angiogenesis; in fact, murine MDSCs are not only a rich source of matrix metalloproteinase 9 (MMP9), which can increase the bioavailability of vascular endothelial growth factor (VEGF), but also have the potential to differentiate into endothelial‐like cells 7. In this context, the cytokine Bv8 appears to be an important mediator of myeloid cell mobilization and myeloid cell‐dependent tumour angiogenesis, as Bv8 expression is upregulated in MDSCs following implantation of tumour cells, and anti‐Bv8 treatment of mice implanted with human tumours reduced angiogenesis and tumour growth. Moreover, MDSCs mediated tumour refractoriness to bevacizumab, an anti‐VEGF neutralizing monoclonal antibody, and the molecular mechanisms responsible for resistance to anti‐VEGF therapy induced by MDSCs reside in the release of cytokines, such as granulocyte colony‐stimulating factor (G‐CSF) and monocyte chemoattractant protein 1 (MCP‐1), and the proinflammatory factors macrophage inflammatory protein 2 and interleukin‐1 receptor (IL‐1R) 7.
Effects on the pre‐metastatic niche
There is increasing evidence from preclinical studies that MDSCs play an important role in the pre‐metastatic niche. An example is provided by a spontaneous model of uveal melanoma, in which PMN‐MDSCs attracted by CXCL5 and infiltrating primary tumours were able to actively promote cancer cell dissemination by inducing epithelial–mesenchymal transition (EMT); transcriptome analysis revealed that the main inducers of EMT were hepatocyte growth factor and transforming growth factor (TGF)‐β1 secreted by PMN‐MDSCs, and epidermal growth factor released by tumour cells 8. In addition, in another spontaneous model of breast cancer, tumour‐induced interleukin‐17‐producing γδ T cells drove systemic expansion and polarization of neutrophils to PMN‐MDSCs, which, in turn, contributed to suppress a CD8+ response and metastasis formation in distant organs 9.
These data indicate that MDSCs, which have been historically recognized as the most potent inhibitors of the T‐cell response, are actually able to orchestrate more potent strategies to redefine the outcome of tumour development, by contributing to cancer progression and metastasis. Future studies are required to unveil the role of MDSC activity in the pre‐metastatic niche and provide new immune target therapies to improve metastatic disease management.
Author contributions statement
The authors contributed in the following way: SS, LP, SM: wrote the manuscript.
Acknowledgements
This work was supported by grants from Italian Association for Cancer Research (AIRC, grant IG2015‐17400) and by grant CPDA‐144873 (University of Padova; 2014). Artwork by Carla Brighenti.
No conflicts of interest were declared.
References
- 1. Galon J, Mlecnik B, Bindea G, et al Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronte V, Brandau S, Chen SH, et al Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7 : 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solito S, Marigo I, Pinton L, et al Myeloid‐derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319 : 47–65. [DOI] [PubMed] [Google Scholar]
- 4. Cui TX, Kryczek I, Zhao L, et al Myeloid‐derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 2013; 39 : 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panni RZ, Sanford DE, Belt BA, et al Tumor‐induced STAT3 activation in monocytic myeloid‐derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother 2014; 63 : 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Mitri D, Toso A, Chen JJ, et al Tumour‐infiltrating Gr‐1+ myeloid cells antagonize senescence in cancer. Nature 2014; 515 : 134–137. [DOI] [PubMed] [Google Scholar]
- 7. Shojaei F, Zhong C, Wu X, et al Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol 2008; 18 : 372–378. [DOI] [PubMed] [Google Scholar]
- 8. Toh B, Wang X, Keeble J, et al Mesenchymal transition and dissemination of cancer cells is driven by myeloid‐derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9 : e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coffelt SB, Kersten K, Doornebal CW, et al IL‐17‐producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522 : 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]


