ABSTRACT
Antibody disulfide bond reduction during monoclonal antibody (mAb) production is a phenomenon that has been attributed to the reducing enzymes from CHO cells acting on the mAb during the harvest process. However, the impact of antibody reduction on the downstream purification process has not been studied. During the production of an IgG2 mAb, antibody reduction was observed in the harvested cell culture fluid (HCCF), resulting in high fragment levels. In addition, aggregate levels increased during the low pH treatment step in the purification process. A correlation between the level of free thiol in the HCCF (as a result of antibody reduction) and aggregation during the low pH step was established, wherein higher levels of free thiol in the starting sample resulted in increased levels of aggregates during low pH treatment. The elevated levels of free thiol were not reduced over the course of purification, resulting in carry‐over of high free thiol content into the formulated drug substance. When the drug substance with high free thiols was monitored for product degradation at room temperature and 2–8°C, faster rates of aggregation were observed compared to the drug substance generated from HCCF that was purified immediately after harvest. Further, when antibody reduction mitigations (e.g., chilling, aeration, and addition of cystine) were applied, HCCF could be held for an extended period of time while providing the same product quality/stability as material that had been purified immediately after harvest. Biotechnol. Bioeng. 2017;114: 1264–1274. © 2017 The Authors. Biotechnology and Bioengineering Published by Wiley Periodicals Inc.
Keywords: purification, stability, antibody disulfide bond reduction, aggregate
Introduction
Monoclonal antibodies (mAbs) are an important class of biomolecules that are used in the treatment of various diseases such as cancer, multiple sclerosis, rheumatoid arthritis, lupus, and respiratory diseases (Choy et al., 1998; Cobleigh et al., 1999; Haynes et al., 2009; Helliwell and Coles, 2009; Robak and Robak, 2009). During mAb process development, aggregates and fragments have to be removed to adequate levels due to their associated risks with increased immunogenicity and potential effects on drug efficacy (Fan et al., 2012; Rosenberg, 2006). Further, the presence of these product variants can also affect the stability of the product during storage leading to reduced shelf life.
Many commercial manufacturing processes for mAbs involve the use of Chinese Hamster Ovary (CHO) cells for product expression and depth filtration or centrifugation for harvest, followed by purification, and formulation to produce the drug substance. There were several recently reported instances in literature whereby reduction of mAb disulfide (S‐S) bonds is observed during different parts of the process. Hutchinson and co‐workers observed significant mAb fragmentation of an IgG4 molecule with increasing centrifugation shear conditions. They hypothesized that the mechanical forces in the centrifuge were responsible for reducing the molecule into half‐antibodies (Hutchinson et al., 2006). Trexler‐Schmidt and co‐workers further demonstrated that antibody disulfide reduction can be attributed to the cell lysis and the release of intra‐cellular reducing enzymes (primarily thio‐redoxin reductase/thioredoxin) as a result of harsh centrifugation conditions (Trexler‐Schmidt et al., 2010). Hutterer and co‐workers attributed the extent of antibody reduction to be dependent on the cell line and cell culture process (Hutterer et al., 2013). Various methods of minimizing antibody reduction were reported. Maintaining a highly oxidative environment through air sparging was proposed as a solution to shift the equilibrium of the reversible redox reaction toward oxidation (Mun et al., 2015). Addition of chemical inhibitors (e.g., cystine, copper sulfate, EDTA) to act as a competitive inhibitor, directly inhibit responsible enzymes, or remove the metal ions required in the enzymatic pathway was suggested as means of minimizing enzymatic activity (Trexler‐Schmidt et al., 2010). However, while the manufacturing process conditions that cause the antibody reduction (Hutterer et al., 2013), the impact on the molecular structure, as well the susceptibility of various mAb isoforms to reduction (Magnusson et al., 1997; Wang et al., 2015) were well‐characterized, the impact of antibody reduction on purification process performance and long term drug substance stability has not been reported.
During process development of an IgG2 antibody, antibody reduction was observed in the harvested cell culture fluid (HCCF) and was accompanied by an increase in the aggregate levels after the low‐pH viral inactivation step. The long term drug substance stability was also affected as a higher rate of aggregation was observed. This study investigated the link between antibody reduction in the starting HCCF material and aggregation in the downstream process as well as in the drug substance storage stage. Various reduction strategies to prevent the antibody reduction were tested and their impact on process performance and drug substance stability were examined.
Materials and Methods
Cell Culture, Purification, and Formulation Procedures
Cell culture fluid was generated using CHO cells in a 50 L scale fed‐batch process in a stainless steel bioreactor (Applkon; Delft, The Netherlands) using proprietary media and nutrient feeds with an initial working volume of 42 L. Cell separation was performed by an LAPX 404 continuous centrifuge (Alfa Laval; Lund, Sweden). The centrate was then filtered using X0HC POD depth filters followed by SHC sterile membrane filters (Millipore; Billerica, MA). Storage vessels for HCCF include 1 and 2 L PETG bottles (VWR, Bridgeport, NJ, 89096–292 and 89095–290) for small volume aliquots and disposable sterile bags (Sartorius Stedim, Bohemia, NY, FXB110922) for large volume aliquots. For storage conditions where headspace is required in the sterile bags, air was introduced through a 0.2 μm Acro 50 sterile filter (Pall, 4250) until the bag was fully inflated. HCCF was allowed to warm up to room temperature before initiation of purification if it had been stored chilled. Purification was performed at bench scale using an ÄKTA Explorer 100 (GE Healthcare, Piscataway, NJ, 18111241) systems and Vantage L (EMD Millipore, Billerica, MA, 96220250) chromatography columns. Protein A capture was performed using MabSelectSuRe resin (GE Healthcare Piscataway, NJ, 17‐5438‐05) followed by low pH inactivation with 300 mM Glycine, pH 2.35. Intermediate polishing was carried out using Super Q 650‐M resin (Tosoh Bioscience, King of Prussia, PA, 17229) and final polishing utilized POROS 50HS resin (Thermo Fisher Scientific, Waltham, MA, 1335908). Hold studies were performed using 50 mL Flexboy storage bags from Sartorius Stedim. After purification, the samples were concentrated through centrifugation at 4000g to approximately 120 mg/mL using an Amicon Ultra‐15 centrifugal filter unit with an Ultracel‐30 membrane, (Millipore, UFC903096). Samples were then dialyzed overnight into 10 mM histidine buffer at pH 6. The final formulation to 60 mg/mL protein in 10% (w/v) trehalose dihydrate, 0.02% (w/v) polysorbate 80, 10 mM histidine at pH 6 was achieved by mixing in concentrated buffer.
Antibody Aggregation Analysis
The percentage of antibody aggregates was determined using a standard size exclusion chromatography (HP‐SEC) method. An Agilent HPLC system (Agilent 1200 series) was used with a 7.8 mm × 300 mm TSKgel G3000SW XL column (Tosoh Bioscience, 08541) at 1 mL/min flow rate using a mobile phase buffer of 0.1 M sodium phosphate, 0.1 M sodium sulfate, pH 6.8. The absorbance at 280 nm was used to quantify the results.
Reduced Antibody Species Analysis
Samples were diluted to 2.0 mg/mL in 1X PBS and mixed in non‐reducing sample buffer containing N‐Ethylmaleimide (NEM). All samples were heated on a heating block at 100°C for 2 min and the protein ladder was heated on a heating block at 100°C for 5 min. Following denaturation, samples, and the ladder were diluted with ultra‐pure water and loaded on a 96‐well plate. The plate and a chip that contained the gel dye, the destain solution, and the protein express lower maker were placed into a LabChip GX system (Perkin Elmer, Waltham, MA, 124582) for analysis. The GX LabChip was placed in a LabChip GXII analyzer (Perkin Elmer, 124582/b) and read using LabChip GXII software. Protein and fragments were detected by laser‐induced fluorescence and translated into gel‐like images (bands) and electropherograms (peaks).
Free Thiol Quantitation in Harvested Cell Culture Fluid (HCCF)
The amount of free thiol at each site of IgG from HCCF was determined by Lys‐C peptide mapping method under non‐reducing condition. The free cysteine was capped with NEM, and the free thiol per each cysteine‐containing peptide was calculated as the percentage of NEM‐capped peptide. The HCCF was first buffer‐exchanged to phosphate buffer using a 30 kDa MW cut‐off centrifugal device. Prior to digestion with a serine protease, sample was mixed with NEM and guanidine to cap the free cysteine and denature the protein. Following protease digestion, half of each reaction mixture was reduced by the addition of 1,4‐dithiothreitol (DTT). The reduced and non‐reduced digests were both separated by a 1.7 μm, 2.1 × 150 mm Acquity UPLC HSS C18 column (Waters, 176001126) and analyzed by a tunable UV (TUV) detector and an Orbitrap mass spectrometer. The mobile phase A was 0.02% trifluoroacetic acid (TFA) in water and the mobile phase B was 0.02% TFA in acetonitrile. The peptides were eluted at a flow rate of 0.2 mL/min with a gradient of mobile phase B from 0% to 95% over 90 min.
Colorimetric Free Thiol Quantitation in Purification Process Intermediates
The free thiol assay evaluates the integrity of the disulfide connections in a protein by measuring the levels of free thiol groups on unpaired cysteine residues. Samples are incubated under native and denatured conditions with 5, 5′‐dithiobis‐(2‐nitrobenzoic acid (DTNB) that binds to free thiol and releases a colored thiolate ion. The colored thiolate ion is detected with a UV‐visible spectrophotometer. The concentration of free thiol is interpolated from a standard curve and the free thiol‐to‐antibody molar ratio is reported.
Results and Discussion
Observation of Reduced Antibody Species Formation
A platform mAb purification process (Fig. 1) was used to purify the IgG2 monoclonal antibody that had been stored chilled in a storage bag with no headspace for 30 days before purification was initiated. As shown in Table I, Purification Run 1, high fragment levels (89%) were observed in the HCCF and gradually decreased with each step of the purification process. Aggregates levels increased by 1.0% after low pH viral inactivation and were removed during the subsequent polishing steps. As shown in Figure 2a and b, NR‐GX images of the capture product and final chromatography polishing intermediate detected the presence of fragment bands with molecular weights corresponding to combinations of heavy chain (H) and light chain (L) fragments of the intact antibody that can arise as a result of reduction (L, H, L‐L, H‐L, H‐H, H‐H‐L), showing that the inter‐chain disulfide bonds were being reduced. As seen in Figure 2c, similar bands of the reduced species were also detected when the harvested cell culture fluid (HCCF) was analyzed by NR‐GX, indicating that the mAb reduction phenomenon was occurring either in the bioreactor or at the harvest step. The cell culture material was clarified using centrifugation harvest and it has previously been reported by Trexler‐Schmidt and co‐workers that harsh centrifugation conditions can impact product quality through increased cell rupture leading to the release of intra‐cellular host cell proteins such as thioredoxin and thioredoxin reductase. This can subsequently lead to cleavage of inter‐chain disulfide bonds (Trexler‐Schmidt et al., 2010). In addition to thioredoxin and thioredoxin reductase, other reducing enzymes present in the lysed HCCF including gluthathione reductase and protein disulfide isomerase can also cleave disulfide bonds (Guzman, 1997; Ikebuchi et al., 1992).
Figure 1.
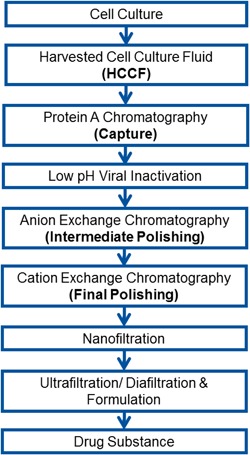
Schematic of platform mAb purification process.
Table I.
Comparison of aggregate and fragment levels in the purification process pre‐ and post‐process optimization
| Purification process intermediate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Purification Run no. | Purification conditions | Purity analysis | HCCF | Capture | Post‐low pH viral inactivation | Intermediate polishing | Final polishing | Drug substance |
| Purification Run 1 | Purification process with HCCF subjected to: (i) 2–8°C storage in 50 L bag without headspace (ii) purification after 30 day hold | HP‐SEC | NA | 96.2 (%mon) | 95.7 (%mon) | 96.0 (%mon) | 98.2 (%mon) | NA |
| 2.2 (%agg) | 3.2 (%agg) | 2.8 (%agg) | 0.5 (%agg) | |||||
| 1.6 (%frag) | 1.1 (%frag) | 1.2 (%frag) | 1.3 (%frag) | |||||
| NR‐GX | 6.4 (%mon) | 13.1 (%mon) | 63.5 (%mon) | NA | 87.6 (%mon) | NA | ||
| 93.6 (%frag) | 86.9 (%frag) | 36.5 (%frag) | 13.4 (%frag) | |||||
| Purification Run 2 | Purification process with HCCF subjected to: (i) 2–8°C storage in 50 L bag with headspace (ii) immediate purification | HP‐SEC | NA | 97.7 (%mon) | 97.2 (%mon) | 97.2 (%mon) | 99.6 (%mon) | 99.5 (%mon) |
| 2.3 (%agg) | 2.8 (%agg) | 2.8 (%agg) | 0.4 (%agg) | 0.5 (%agg) | ||||
| 0.0 (%frag) | 0.0 (%frag) | 0.0 (%frag) | 0.0 (%frag) | 0.0 (%frag) | ||||
| NR‐GX | 87.8 (%mon) | 99.0 (%mon) | 99.3 (%mon) | 99.4 (%mon) | 99.1 (%mon) | NA | ||
| 12.2 (%frag) | 1.0 (%frag) | 0.7 (%frag) | 0.6 (%frag) | 0.9 (%frag) | ||||
| Purification Run 3 | Purification process with HCCF subjected to: (i) 0.4 mM cys/g mAb spike (ii) 2–8°C storage in 50 L bag with headspace (iii) purification after 4 day hold | HP‐SEC | NA | 97.9 (%mon) | 95.9 (%mon) | 96.3 (%mon) | 99.0 (%mon) | 98.4 (%mon) |
| 2.0 (%agg) | 4.0 (%agg) | 3.6 (%agg) | 1.0 (%agg) | 1.5 (%agg) | ||||
| 0.1 (%frag) | 0.1 (%frag) | 0.1 (%frag) | 0.0 (%frag) | 0.1 (%frag) | ||||
| NR‐GX | 83.5 (%mon) | 93.1 (%mon) | 99.8 (%mon) | 100.0 (%mon) | 100.0 (%mon) | 99.8 (%mon) | ||
| 16.5 (%frag) | 6.9 (%frag) | 0.2 (%frag) | 0.0 (%frag) | 0.0 (%frag) | 0.2 (%frag) | |||
| Purification Run 4 | Purification process with HCCF subjected to: (i) 0.8 mM cys/g mAb spike (ii) 2–8°C storage in 2 L bottle with headspace (iii) purification after 2 week hold | HP‐SEC | NA | 98.2 (%mon) | 97.8 (%mon) | NA | 99.2 (%mon) | 99.0 (%mon) |
| 1.8 (%agg) | 2.2 (%agg) | 0.8 (%agg) | 1.0 (%agg) | |||||
| 0.0 (%frag) | 0.0 (%frag) | 0.0 (%frag) | 0.0 (%frag) | |||||
| NR‐GX | 87.6 (%mon) | 99.3 (%mon) | 99.3 (%mon) | 99.3 (%mon) | 99.3 (%mon) | NA | ||
| 13.4 (%frag) | 0.7 (%frag) | 0.7 (%frag) | 0.7 (%frag) | 0.7 (%frag) | ||||
| Free thiol | 2.1% (inter‐chain) 5.4% | 0.1 (mol/mol) | 0.14 (mol/mol) | NA | 0.05 (mol/mol) | NA | ||
| (intra‐chain LC) 30.8% (intra‐chain HC) | ||||||||
Aggregate levels were determined by HP‐SEC. Fragment levels were determined by HP‐SEC and non‐reduced GX (NR‐GX). Free thiol levels in the HCCF and subsequent process intermediates were determined by LC‐MS and a colorimetric assay, respectively (NA, not analyzed).
Figure 2.

Non‐reduced GX (NR‐GX) electropherograms of purification Run 1 (Table I) process intermediates (a) capture product, (b) final polishing product, (c) harvested cell culture fluid (HCCF). Black trace: sample; gray trace: reference standard; L: light chain fragment; H: heavy chain fragment.
Based on the hypothesis that the loss in product quality was driven primarily by the release of intra‐cellular reducing enzymes (either from dead cells in the bioreactor or through harsh centrifuge conditions) disrupting disulfide bonds in the mAb molecule, controls were put in place to slow down the enzymatic reaction by the temperature of the HCCF to 2–8°C. In this study, (Table I, Purification Run 2), storage of the material in a vessel with air‐containing headspace was also employed in an attempt to provide a more oxidative environment. It was reported that providing an oxidative environment can allow for re‐oxidation of reduced disulfide bonds (Mullan et al., 2011; Mun et al., 2015; Wang et al., 2015). As a rule of thumb, the volume of the storage vessel employed was twice the volume of HCCF (e.g., 2 L PETG bottle were used to store 1 L of HCCF, storage bags were filled to half the recommended maximum volume and inflated with filtered air). In addition, the HCCF was purified within 3 h after harvest to minimize the exposure of the mAb to reducing enzymes. As re‐oxidation of reduced species can occur across the duration of the purification process, multiple 1 mL aliquots of purification process intermediates were made as soon as they were available, flash‐frozen (by immersion into a solution of ethanol containing dry ice) and stored at −80°C. When thawed, the aliquots were analyzed immediately in order to get a representative readout of the product quality at each step of the purification process. The approach of sample handling was applied to Purification Run 2 and all subsequent purification Runs.
As seen in Figure 3 and Table I, NR‐GX analysis of the intermediates showed no reduced species (fragments) present across the purification process. In contrast to Purification Run 1 which started with 86.9% fragment in the HCCF and ended with 13.4% at the final polishing step, Purification Run 2 had fragment levels of 12.2% and 1.0% in the HCCF and final polishing step, respectively. Aggregate levels increased by 0.5% (from 2.3% pre‐low pH treatment to 2.8% post‐low pH treatment), which was lower than the 1% increase observed in Purification Run 1. Hence, it is likely that prevention of reduction in the HCCF also helped in minimizing aggregate formation during low pH treatment.
Figure 3.

NR‐GX images of purification process intermediates from purification Run 2 (Table I). HCCF subjected to chilling, headspace aeration, and immediate purification after harvest (L: light chain fragment; H: heavy chain fragment—disulfide link between fragments).
While it is possible to control for reduction through the combined approach in Table I, Purification Run 2 (chilled HCCF, air‐containing headspace, immediate processing), its implementation imposes severe limitations on manufacturing flexibility. Chilling of the HCCF requires increases processing time. Head space aeration potentially becomes less effective as product surface area to volume ratios decrease with increasing processing scale. Larger product hold tanks may be required and lower capacity limits may have to be set due to the need for adequate headspace. While alternatives like air sparging in the hold vessel may be a solution, these may not be available at every facility. Immediate purification of the HCCF becomes challenging when column size limitations in the manufacturing plant may require multiple cycles of the capture step to be performed.
Impact of Cystine Addition on Reduction Control
In order to allow for extended HCCF holds to assure manufacturing flexibility without impacting product quality, L‐cystine spikes into the HCCF were evaluated. L‐cystine was reported to be a potential competitive inhibitor of the reducing enzymes or to act as a surrogate substrate for the enzyme in place of the mAb product (Trexler‐Schmidt et al., 2010).
As part of an initial assessment of cystine levels on reduction control, the level of L‐cystine in the harvested HCCF was adjusted to 0, 0.4, and 0.8 mM L‐cystine per gram of mAb (mM Cys/g mAb) through the addition of L‐cystine into the HCCF immediately after harvest. To provide a worst case scenario for antibody reduction (low oxidative environment), the HCCF aliquots were sealed in flexboy storage bags without headspace and held for 2 weeks at 2–8°C before being analyzed. Figure 4 shows reduced species were not detected at the start of the hold study, but by the end of the hold study, bold bands representing H‐H‐L fragments and faint bands of H‐L fragments were identified in the samples containing 0 (no cystine) and 0.4 mM Cys/g mAb while the sample containing 0.8 mM Cys/g mAb still showed no signs of reduction. Decrease in band intensity with increasing cystine levels also demonstrates that 0.4 mM Cys/g mAb slowed down the rate of reduction during the 2 week hold, but 0.8 mM Cys/g mAb was required for complete prevention of reduction. Disulfide mapping mass spectrometry (MS) provided further evidence that supports the hypothesis of 0.4 mM Cys/g mAb being inadequate for complete reduction mitigation (Fig. 5). Higher levels of inter‐chain free thiol were observed in the 0 and 0.4 mM Cys/g mAb samples by the end of the hold but not in the 0.8 mM Cys/g mAb sample nor in the control starting material (HCCF + 0 mM cys, t = 0). The largest increase in free thiol content occurred at the hinge cysteines, followed by the inter‐chain cysteines (LC Cys 216, HC Cys134). The intra‐chain cysteines on the light chain of the mAb showed smaller increases in free thiol than the inter‐chain cysteines. Little or no increase in free thiol was detected on the intra‐chain cysteines of the mAb heavy chain.
Figure 4.
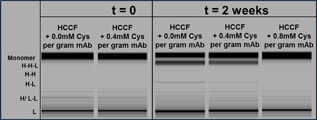
NR‐GX images of harvested cell culture fluid (HCCF) at t = 0 and 2 weeks, containing 0, 0.4, and 0.8 mM cystine per gram mAb. During 2 week hold, HCCF was held at 2–8°C in air‐tight bags. (Note: a separate aliquot of the same HCCF at t = 0 was spiked to 0.4 mM cystine per gram mAb and purified after a 4 day hold at 2–8°C in a vessel with headspace and is listed as purification Run 3 in Table I).
Figure 5.

Mass spectrometry quantification of change in free thiol levels for HCCF held for 2 weeks in the presence of 0, 0.4, and 0.8 mM cystine per gram of mAb.
To assess the impact of cystine addition on purification process performance, HCCF was spiked to 0.4 mM cystine/g mAb (same material as used in the cystine hold study above) and purified after a 4 day hold at 2–8°C in a vessel with headspace (Table I, Purification Run 3). At the start of purification, higher levels of fragment were observed in the HCCF (16.5% frag) as compared to Purification Run 2 (HCCF: 12.2% frag). After low pH treatment, a 2% increase in aggregate was observed. In comparing Purification Runs 1, 2, and 3 in Table I, a trend is observed wherein higher levels of fragment in the HCCF results in higher levels of aggregate after low pH treatment. Given that antibody disulfide bond reduction was observed for Runs 1 and 3 but not Run 2, it can be hypothesized that reduction in the HCCF is related to the higher levels of aggregate formation during low pH treatment.
The purification performance of Run 3 coupled with the observation of reduction for the same HCCF during the cystine hold study indicates that chilling, provision of headspace in the hold vessel and spiking of cys to 0.4 mM/g mAb was insufficient in the prevention of antibody reduction during the HCCF hold. To better understand the contributions of headspace provision to preventing antibody disulfide reduction, a 0.75 mL aliquot of the HCCF from Run 3 was stored at 2–8°C in a 1.5 mL Eppendorf tube for 2 weeks before being analyzed by NR‐GX. Fragment bands were detected in the sample at the end of the 2 week hold but not at the beginning indicated that the provision of headspace was playing a less significant role to the prevention of antibody disulfide bond reduction as compared to spiking adequate levels of cystine. Although it was not evaluated in this study, it is possible that agitating the HCCF (for large volumes) through storage of HCCF in vessels with mixing impellers could make the provision of an oxidative environment a more effective strategy in preventing antibody disulfide bond reduction due to improved oxygen transfer into the HCCF as compared to the small scale systems where constant agitation is difficult to achieve.
Impact of Reduction During HCCF Hold on Aggregation During Low pH Treatment
When the capture product from Purification Run 2 was held in low pH buffers ranging from pH 3.2 to 3.6, an increase in free thiol content was observed with decreasing pH (Fig. 6a). As reducing enzymes such as thioredoxin reductase, glutathione reductase, and protein disulfide isomerase generally exhibit decreased enzymatic activity with decreasing pH (Guzman, 1997; Ikebuchi et al., 1992; Xia et al., 2003), the increase in free thiol is unlikely to be due to enzymatic action but rather due to exposure of the antibody to low pH.
Figure 6.
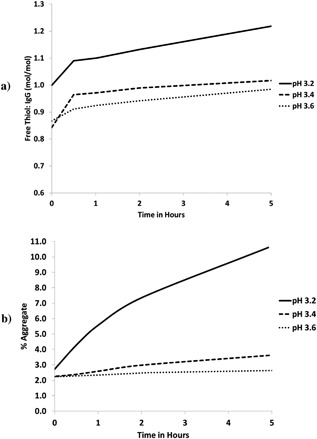
Change in (a) free content and (b) aggregate content with time when protein a product from purification Run 2 is incubated at pH 3.2, 3.4, and 3.6.
An increase in aggregate content was also seen with decreasing pH (Fig. 6b), indicating that there could be a correlation between free thiol content and aggregate content. Franey et al. (2010) have reported similar findings that an increase in free thiol level leads to a corresponding increase in aggregate formation for monoclonal antibodies, with the impact on IgG2 molecules being more severe compared to other IgG formats due to higher number of inter‐chain disulfide bonds. It has been established earlier that reduction during the HCCF hold leads to the generation of reduced species along with increased free thiol. The reduced species with heavy chain subunits (H‐L, H‐H, H‐H‐L) are likely recovered in the protein A product along with intact monomer and undergo low pH treatment. With a higher starting level of free thiol in the protein A product, coupled with further free thiol formation during low pH treatment, increased aggregate formation occurs. Although the exact mechanism was not investigated in this study, Buchanan and co‐workers have demonstrated through site‐directed mutagenesis that having unpaired cysteines on the surface of a mAb can lead to significantly increased rates of aggregation (Buchanan et al., 2013). Hence, a larger increase in aggregate content was observed during low pH treatment for Purification Runs 1 and 3 (which showed reduction in the HCCF), but not Purification Run 2 (no reduction in HCCF due to immediate purification after harvest).
Effect of Increased Cystine Levels and HCCF Hold Conditions on Product Quality
As shown above, spiking of HCCF to 0.4 mM cys/g mAb in combination with low temperature hold and provision of headspace was only able to slow down the rate of reduction and not prevent reduction completely. This resulted in high free thiol levels (as determined by disulfide mapping MS) which caused increased aggregation during low pH treatment. In contrast, 0.8 mM cys/g mAb seemed to provide better reduction mitigation and minimize aggregate formation. This hypothesis was evaluated through the study outlined in Figure 7. To one sample, no L‐cystine (0 mM cystine) was spiked into the mixture; to the other sample, the mixture was spiked to 0.8 mM cystine/g mAb. Half of each sample mixture was purified and formulated to 50 mg/mL immediately, while the other half was held in vessels with headspace for 2 weeks at 2–8°C before purification and formulation. After formulation, all four lots were monitored for aggregation stability for up to 17 months at 2–8°C and 1 month at 25 and 40°C.
Figure 7.
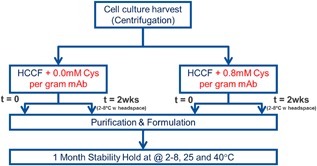
Evaluation schematic to assess the impact of reduction mitigation on purification process performance and formulated product stability.
Table I, Purification Run 4 shows the purification performance for the HCCF lot that was spiked to 0.8 mM cystine/g mAb and stored chilled in a vessel with headspace for 2 weeks before being purified. Fragment levels in the HCCF (13.4% frag) were lower as compared to Run 3 (16.5% frag) even though the material was held for a longer duration under similar conditions. After low pH treatment, aggregate levels only increased by 0.4% (comparable to Run 2), further supporting the theory that prevention of reduction in the HCCF is important in minimizing aggregate formation during low pH treatment.
The free thiol content of the process intermediates generated from the conditions outlined in Figure 7 were measured by mass spectrometry (for HCCF) and a colorimetric assay (for subsequent process intermediates). As seen in Figure 8a, an increase in inter‐chain free thiol content was observed for the HCCF held for 2 weeks at 2–8°C in the absence of cystine. In contrast, HCCF that was spiked to 0.8 mM cys/g mAb and held under similar conditions showed no change in free thiol levels. Similar trends in free thiol levels were also observed in the subsequent process intermediates (Fig. 8b). Capture product generated from HCCF held for 2 weeks in the absence of cystine showed the highest free thiol level. Free thiol content increased for all aliquots after low pH treatment but the capture product with the highest starting free thiol level also showed the largest increase during low pH treatment. Though it was not investigated in this study, existing levels of free thiol in a sample can potentially determine the rate of increase during low pH treatment.
Figure 8.
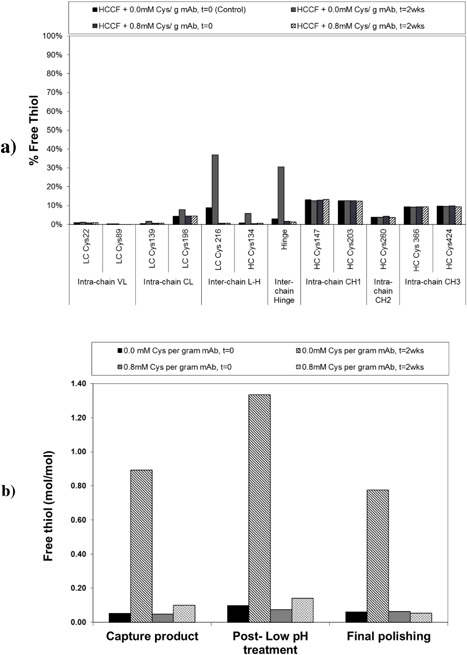
(a) Mass spectrometry quantification of change in free thiol levels for HCCF at t = 0 and after a 2 week hold at 2–8°C in the presence of 0 and 0.8 mM cystine per gram mAb. (b) Free thiol levels determined by colorimetric assay for purification process intermediates generated from HCCF purified immediately and held for 2 weeks in the presence of no cystine (0 mM) and 0.8 mM cystine per gram mAb.
Free thiol levels decreased for all aliquots between the low pH treatment and final polishing chromatography process steps. There are two possible explanations for this observed decrease: (i) fragments and aggregates containing free thiols were removed by the polishing chromatography steps; and (ii) exposure to the atmosphere during purification allowed for disulfide bond formation (i.e., re‐oxidation) between free thiols leading to the reformation of larger fragments, monomer, and aggregates. The intermediate polishing unit operation consisted of an anion exchange (AEX) chromatography step that is operated in flow‐through mode which does not retain any fragment, monomer, or aggregate. The final polishing unit operation consists of a cation exchange (CEX) chromatography step (operated in bind and elute mode) that was designed for aggregate removal. Shown in Supplementary Figure S1a and b are CEX chromatograms from Purification Run 1 (showed the highest degree of antibody disulfide bond reduction and high fragment levels) and Run 2 (showed no signs of antibody disulfide bond reduction and low fragment levels), respectively. In Purification Run 1, a step yield 48% was obtained when the column was loaded to a capacity of 21g/L. In contrast, purification Run 2 gave a step yield of 63% for the same column loaded to a capacity of 16g/L. Protein breakthrough did not occur in the loading and re‐equilibration steps, indicating that all loaded protein was collected back in the elution and strip pool. Compared to Run 2 which showed the typical CEX chromatographic profile for the process, the elution peak (usually composed of monomer) in Run 1 had a lower peak height with significant tailing, while the strip peak (usually composed of monomer and aggregate) was larger than expected. Despite the lack of peaks in the load and re‐equilibration steps, fragment levels by NR‐GX fell from 36.5% at the end of low pH inactivation to 13.4% at the end of CEX. These observations supports the hypothesis that re‐oxidation of the free thiol leading to the formation of monomer or aggregate is likely playing a larger role in causing the observed decrease in free thiol and fragment level for this mAb molecule. During the reoxidation of fragment, different monomer, and aggregate isoforms can arise depending on the site of re‐oxidation on the antibody fragment. These structural differences could result in different retention behavior on the CEX column and potentially explain for the tailing effect observed during the elution step of Run 1.
With higher free thiol levels, there is the increased likelihood that all re‐oxidized species can still possess free thiol residues. The purification process may be optimized for the removal of fragment and aggregate, but may not be able to resolve monomers with different free thiol levels that may subsequently form more aggregate. The formation of the impurities during processing also increases the impurity burden (both fragment and aggregate) on the polishing steps, making it more challenging to achieve robust process control. Further, antibody disulfide bond reduction lead to significantly fragment formation and lower overall process yield as observed in Purification Run 1. Hence, it is important to implement adequate reduction mitigation early on in the process.
The formulated drug substance from each of the four runs purified as described above was held at 25 and 40°C for 1 month and 5°C for 17 months. Aggregate levels were measured using HP‐SEC. As seen in Figure 9a–c, at all temperatures, the material generated from the HCCF that was held for 2 weeks in the absence of cystine had higher starting and final aggregate levels despite going through the same purification and formulation process. In contrast, the material generated from the HCCF that was spiked to 0.8 mM cys/g mAb and held for 2 weeks showed similar starting and final purity levels as the drug substance generated from the HCCF that had been purified immediately. In addition, the rate of aggregate formation was also significantly higher for the sample without cystine and with 2 weeks hold when compared to other samples. Remarkably, the observed trend in the aggregation rates of the drug substances is consistent with the free thiol levels of their corresponding final polishing intermediates. Hence, antibody reduction during the hold of HCCF not only has consequences on the performance of the purification process, it also has a carry‐over effect on the stability of the final drug substance, potentially leading to shorter product shelf life.
Figure 9.

Change in percent aggregate levels over time for drug substance generated from HCCF held in the absence of cystine (0 mM) or in the presence of 0.8 mM cystine per gram mAb at (a) 2–8°C for 17 months (b) 25°C for 1 month (c) 40°C for 1 month.
Conclusions
During purification of an IgG2 antibody, high level of fragment in the process intermediates was detected by HP‐SEC. Further investigations subsequently traced the cause to be antibody reduction in the HCCF. In addition, when the HCCF was reduced, larger increase in aggregate content was observed during low pH treatment. Four different strategies were employed to mitigate antibody reduction by minimizing reducing enzyme activity: (i) shorter hold duration (immediate purification after harvest); (ii) oxidative environment (exposure to air); (iii) low temperature; and (iv) inhibitor addition (cystine addition).
When purification was initiated immediately after harvest, antibody reduction was not observed in the HCCF and aggregate formation during low pH treatment was minimized. This can be attributed to the shorter exposure time of the antibody to reducing enzymes in the HCCF. However, immediate purification may not always be a feasible option, particularly as process scale increases.
Increasing cystine concentrations resulted in lower free thiol levels when the HCCF was held. When HCCF is spiked to 0.8 mM cystine/g mAb, free thiol levels remained constant across a 2 week hold. When a combination of low temperature, exposure to air and 0.4 mM cystine/g mAb spike was applied to a HCCF before purification, antibody reduction progressed at a slower rate, which still resulted in increased aggregate formation during low pH treatment. However, when the cystine concentration was increased to 0.8 mM cystine/g mAb, reduction was not observed. Less aggregate was formed during the low pH step, suggesting a potential link between antibody reduction in the HCCF and aggregate formation during low pH treatment. A pH hold study subsequently demonstrated increasing free thiol levels and aggregate with decreasing pH. This indicates that some reduced species with free thiol which get co‐purified with the intact monomer during protein A chromatography can exacerbate aggregate formation during low pH treatment.
Subsequent decrease in free thiol levels from the low pH treatment step to final polishing chromatography can be attributed to clearance of fragments and aggregates with high free thiol content, or the formation of disulfide bonds between free thiols due to aeration due to exposure to the atmosphere, or potentially both mechanisms in combination. Nonetheless, high free thiol levels increases the likelihood of forming subclasses of monomer that still possess free thiol moieties which may be difficult to remove during polishing, and also adversely affect drug substance stability and lot‐to‐lot consistency.
The carry‐over of product species with high free thiol content from the final polishing product into the drug substance had a subsequent effect on the final product stability with faster rates of aggregation occurring at all temperatures. However, when free thiol levels in the purified material was kept low, similar rates of aggregation were observed regardless of whether the original HCCF was purified immediately or held for an extended period before purification.
Therefore, higher free thiol content after harvest not only increases the burden on the purification process due to higher levels of aggregate, but also limits the stability and shelf‐life of the molecule. As the cause of free thiol increase was ultimately identified to be a result of mAb reduction, proper mitigations should always be considered in the process to prevent antibody reduction during development and manufacturing. By making the product quality less dependent on lot‐to‐lot differences in HCCF hold conditions, greater control of product consistency and quality can be achieved.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Figure S1.
References
- Buchanan A, Clementel V, Woods R, Harn N, Bowen MA, Mo W, Popovic B, Bishop SM, Dall'Acqua W, Minter R, Jermutus L, Bedian V. 2013. Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression. MAbs 5(2):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EH, Kingsley GH, Panayi GS. 1998. Monoclonal antibody therapy in rheumatoid arthritis. Br J Rheumatol 37(5):484–490. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. 1999. Multinational study of the efficacy and safety of humanized anti‐her2 monoclonal antibody in women who have her2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648. [DOI] [PubMed] [Google Scholar]
- Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, Dubinsky WP, Strohl WR, Jordan RE, Zhang N, An Z. 2012. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Res 14(4):R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franey H, Brych SR, Kolvenbach CG, Rajan RS. 2010. Increased aggregation propensity of IgG2 subclass over IgG1: Role of conformational changes and covalent character in isolated aggregates. Protein Sci 19(9):1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman N. 1997. Prolyl Hydroxylase, Protein Disulfide Isomerase and other structurally related proteins. New York: Marcel Dekker; p 159. [Google Scholar]
- Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. 2009. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BAL B/C mice. J Infectious Diseases 200(3):439–447. [DOI] [PubMed] [Google Scholar]
- Helliwell CL, Coles AJ. 2009. Monoclonal antibodies in multiple sclerosis treatment: Current and future steps. Ther Adv Neurol Disord 2(4):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson N, Bingham N, Murrell N, Farid S, Hoare M. 2006. Shear stress analysis of mammalian cell suspensions for prediction of industrial centrifugation and its verification. Biotechnol Bioeng 95(3):483–491. [DOI] [PubMed] [Google Scholar]
- Hutterer KM, Hong RW, Lull J, Zhao X, Wang T, Pei R, Le ME, Borisov O, Piper R, Liu YD, Petty K, Apostol I, Flynn GC. 2013. Monoclonal antibody disulfide reduction during manufacturing. Untangling process effects from product effects. MAbs 5(4):608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi M, Kashiwagi A, Asahina T, Tanaka Y, Takagi Y, Nishio Y, Hidaka H, Kikkawa R, Shigeta Y. 1992. Effect of medium pH on glutathione redox cycle in cultured human umbilical vein endothelial cells. Metabolism 42(9):1121–1126. [DOI] [PubMed] [Google Scholar]
- Magnusson CG, Björnstedt M, Holmgren A. 1997. Human IgG is substrate for the thioredoxin system: Differential cleavage pattern of interchain disulfide bridges in IgG subclasses. Mol Immunol 34(10):709–717. [DOI] [PubMed] [Google Scholar]
- Mullan B, Dravis B, Lim A, Clarke A, Janes S, Lambooy P, Olson D, OɽRiordan T, Ricart B, Tulloch A. 2011. Disulphide bond reduction of a therapeutic monoclonal antibody during cell culture manufacturing operations. BMC Proceedings 5(Suppl 8):P110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun M, Khoo S, Do Minh A, Dvornicky J, Trexler‐Schmidt M, Kao Y‐H, Laird MW. 2015. Air sparging for prevention of antibody disulfide bond reduction in harvested CHO cell culture fluid. Biotechnol Bioeng 112:734–742. [DOI] [PubMed] [Google Scholar]
- Robak E, Robak T. 2009. Monoclonal antibodies for systemic lupus erythematosus (SLE). Curr Drug Targets 10(1):26–37. [DOI] [PubMed] [Google Scholar]
- Rosenberg AS. 2006. Effects of protein aggregates: An immunologic perspective. AAPS J 8(3):E501–E507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler‐Schmidt M, Sargis S, Chiu J, Sze‐Khoo S, Mun M, Kao YH, Laird MW. 2010. Identification and prevention of antibody disulfide bond reduction during cell culture manufacturing. Biotechnol Bioeng 106(3):452–461. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu YD, Cai B, Huang G, Flynn GC. 2015. Investigation of antibody disulfide reduction and re‐oxidation and impact to biological activities. J Pharm Biomed Anal 102:519–528. [DOI] [PubMed] [Google Scholar]
- Xia L, Nordman T, Olsson JM, Damdimopoulos A, Björkhem‐Bergman L, Nalvarte I, Eriksson LC, Arnér ES, Spyrou G, Björnstedt M. 2003. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J Biol Chem 278(4):2141–2146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Figure S1.


