Abstract
Objective
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). We compared patient‐reported outcomes (PROs) in patients with RA treated with tofacitinib or placebo in combination with conventional disease‐modifying antirheumatic drugs (DMARDs).
Methods
In a 12‐month, phase III randomized controlled trial (ORAL Sync), patients (n = 795) with active RA and previous inadequate response to therapy with ≥1 conventional or biologic DMARD were randomized 4:4:1:1 to tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg BID, placebo advanced to 5 mg BID, or placebo to 10 mg BID, in combination with stable background DMARD therapy. PROs included patient global assessment of arthritis (PtGA), patient assessment of arthritis pain (Pain), physical function (Health Assessment Questionnaire disability index [HAQ DI]), health‐related quality of life (Short Form 36 health survey [SF‐36]), fatigue (Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT‐F]), and sleep (Medical Outcomes Study Sleep [MOS Sleep]).
Results
At month 3, statistically significant improvements from baseline versus placebo were reported in PtGA, Pain, HAQ DI, all 8 SF‐36 domains, FACIT‐F, and MOS Sleep with tofacitinib 10 mg BID, and in PtGA, Pain, HAQ DI, 7 SF‐36 domains, FACIT‐F, and MOS Sleep with tofacitinib 5 mg BID. Improvements were sustained to month 12. Significantly more tofacitinib‐treated patients reported improvements of greater than or equal to the minimum clinically important differences at month 3 versus placebo in all PROs, except the SF‐36 role‐emotional domain (significant for tofacitinib 10 mg BID).
Conclusion
Patients with active RA treated with tofacitinib combined with background conventional DMARD therapy reported sustained, significant, and clinically meaningful improvements in PROs versus placebo.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation, joint destruction, and impairment in physical function and health‐related quality of life (HRQOL) 1. Improvements in HRQOL, pain, physical function, and fatigue are often more important and meaningful to patients than improvements in composite disease activity measures when evaluating new therapies 2. This patient preference is reflected by Outcome Measures in Rheumatology, American College of Rheumatology, and European League Against Rheumatism recommendations, and in US Food and Drug Administration guidance, all of which highlight the importance of including clinically relevant patient‐reported outcomes (PROs) when designing clinical trials of potential RA treatments 2.
Box 1. Significance & Innovations.
At month 3, treatment with tofacitinib 5 mg twice daily and 10 mg twice daily resulted in significantly greater improvements from baseline in patient global assessment of arthritis (PtGA), patient assessment of arthritis pain (Pain), and Health Assessment Questionnaire disability index (HAQ DI) score compared with placebo (P < 0.0001). The proportion of patients reporting improvements greater than the minimum clinically important differences in PtGA, Pain, and HAQ DI at month 3 was significantly greater with both doses of tofacitinib versus placebo (P < 0.0001).
Treatment with tofacitinib 5 mg twice daily and 10 mg twice daily resulted in significant improvements (P < 0.05) compared with placebo at month 3 in Short Form 36 health survey (SF‐36) physical component and mental component scores, and 7 of 8 SF‐36 domains with 5 mg twice daily and all 8 SF‐36 domains with 10 mg twice daily.
At month 3, significantly greater mean improvements from baseline (P < 0.001) in Functional Assessment of Chronic Illness Therapy–Fatigue and Medical Outcomes Study Sleep scores were reported by patients treated with both doses of tofacitinib versus placebo.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. Tofacitinib 5 mg twice daily (BID) and tofacitinib 10 mg BID have demonstrated consistent efficacy in reducing the signs and symptoms of RA and produced improvements in PROs, with manageable safety profiles across 6 phase III studies 3, 4, 5, 6, 7, 8, either as monotherapy or in combination with conventional disease‐modifying antirheumatic drugs (DMARDs).
The phase III ORAL Sync 12‐month, randomized controlled trial (RCT) assessed tofacitinib treatment in combination with stably dosed DMARDs in adult patients with active RA and previous inadequate responses to DMARDs and/or biologic DMARDs. Tofacitinib 5 mg BID and 10 mg BID demonstrated rapid, significant, and clinically meaningful improvements in signs and symptoms of RA and physical function, with a safety profile consistent with that observed in other phase III studies 4. Patients treated with tofacitinib reported mean changes from baseline in the Health Assessment Questionnaire disability index (HAQ DI) and the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F), comprising 13 items assessing fatigue to give a total score ranging 0–52, with higher scores indicating lower fatigue, which were statistically significant compared with placebo 4. Here we present further PRO data, including HRQOL, from the ORAL Sync study.
Patients and methods
Study design and participants
ORAL Sync was a 12‐month, double‐blind, RCT conducted across 114 centers worldwide between May 2009 and January 2011 (full details of the study design have been reported previously [4]). The trial was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines and was approved by the institutional review board or independent ethics committee for each study center. All patients provided written, informed consent.
Patients were ages ≥18 years with active RA, defined by ≥4 tender joints, ≥4 swollen joints (68‐ or 66‐joint count), and an erythrocyte sedimentation rate ≥28 mm/hour (Westergren method) or a C‐reactive protein level >7 mg/liter. Patients must have had an inadequate response to ≥1 conventional or biologic DMARD before baseline, and continued stable background DMARD therapy throughout the trial. The background DMARD therapy included methotrexate, sulfasalazine, antimalarials, and other nonimmunosuppressive conventional DMARDs, administered alone or in combination. Key exclusion criteria included hemoglobin level <90 grams/liter, hematocrit <30%, leukocyte count <3.0 × 109 cells/liter, neutrophil count <1.2 × 109 cells/liter, platelet count <100 × 109 cells/liter, estimated glomerular filtration rate ≤40 ml/minute per 1.73 m2 (Cockcroft‐Gault equation), aspartate aminotransferase or alanine aminotransferase levels >1.5 times the upper limit of normal, and evidence of active infection, including inadequately treated latent or active Mycobacterium tuberculosis.
Patients were randomized 4:4:1:1 to receive tofacitinib 5 mg or 10 mg BID, or placebo advanced to 5 mg or 10 mg BID. At month 3, placebo nonresponders (without ≥20% reductions from baseline in swollen and tender joint counts) were advanced blindly to tofacitinib 5 mg or 10 mg BID; tofacitinib nonresponders at month 3 continued the same treatment. At month 6, all patients still receiving placebo were advanced blindly to tofacitinib 5 mg or 10 mg BID.
Assessment of PROs
All PROs were predefined as secondary trial outcomes and reported at month 3, i.e., before advancement of placebo nonresponders from placebo. Patient global assessment of arthritis (PtGA) and patient assessment of arthritis pain (Pain) used a 100‐mm visual analog scale (VAS); physical function was assessed by HAQ DI. HRQOL was evaluated using the Medical Outcomes Study (MOS) Short Form 36 health survey (SF‐36) questionnaire (acute version), comprising 8 domains (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, and mental health) scored 0–100, with higher scores indicating better HRQOL. In addition, the individual SF‐36 domain scores were combined into physical component summary (PCS) and mental component summary (MCS) scores. Sleep was assessed by the MOS Sleep scale, which queries 6 aspects of sleep to give a total score ranging 12–71, with lower scores indicating better sleep.
Minimum clinically important differences (MCIDs) were defined as ≥10‐mm decreases from baseline in PtGA and Pain VAS scores, ≥0.22‐point decrease from baseline in HAQ DI, ≥2.5‐point increases from baseline in SF‐36 PCS and MCS scores, ≥5‐point increases from baseline in SF‐36 domain scores, and ≥4‐point increase from baseline in FACIT‐F 9. No MCID has been defined for the MOS Sleep scale.
Statistical analysis
Data are presented for the full analysis set (all patients randomized who received ≥1 dose of study drug with ≥1 post‐baseline assessment) without imputation for missing values. Imputation for missing values is normally applied to model the placebo response, assuming placebo patients had not advanced to tofacitinib treatment after month 3; however, all PROs as secondary outcomes were reported at month 3 before advancement of placebo nonresponders. Estimates of mean changes from baseline for each treatment and mean differences from placebo at month 3 were derived from the model as least squares means (LSMs), with corresponding SEs. The percentages of patients reporting improvements ≥MCID in PROs at month 3 were compared between the tofacitinib and placebo treatment groups (except for MOS Sleep) in a post hoc analysis using the normal approximation to the binomial. Statistical significance was declared at a P value of less than or equal to 0.05, with no correction for multiple comparisons. The number needed to treat (NNT) was calculated as 1/(proportion of patients receiving placebo not reporting changes ≥MCID minus the proportion of patients using tofacitinib not reporting improvements ≥MCID).
Correlation analyses at month 3
Correlation analyses (Pearson's correlation) were performed to investigate potential interactions between PROs at month 3. Changes from baseline in each PRO were correlated pairwise, both among themselves and with the change from baseline in clinical efficacy (assessed using the Simplified Disease Activity Index [SDAI]) at month 3. Results of analyses were presented for each treatment group using descriptive statistics.
Results
Patients
Patient disposition and demographics have been reported previously 4. Of 792 patients treated, 315 received tofacitinib 5 mg BID, 318 received 10 mg BID, and 159 received placebo. At month 3, there were 297 patients (94.3%), 295 patients (92.8%), and 149 patients (93.7%) in the respective treatment groups.
Baseline values
Baseline patient demographics were similar across treatment groups: mean age ranged 51.9–52.7 years, the proportion of women was 77.4–83.8%, and the mean disease duration was 8.1–9.9 years 4. Overall, 6.0–7.3% of patients had received previous treatment with tumor necrosis factor inhibitor (TNFi) therapy, and 0–7.6% had received non‐TNFi biologic agents. The mean number of failed DMARDs was 1.3–1.4 (4). At baseline, mean PtGA and Pain scores ranged 57.1–60.2, HAQ DI 1.35–1.44, SF‐36 PCS and MCS scores 32.0–32.7 and 40.9–41.7, respectively, FACIT‐F 28.7–29.7, and MOS Sleep scores 39.8–41.1, indicating a substantial burden of disease (Table 1).
Table 1.
Baseline values and LSM changes from baseline, percentage of patients with improvement ≥MCID, and NNT at month 3 for PRO measures (full analysis set, no imputation), for patients receiving tofacitinib 5 mg or 10 mg twice daily, or placeboa
| Baseline | Change from baseline at month 3 | Patients reporting improvements ≥MCID at month 3 | NNT to achieve MCID | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRO measure | 5 mg (n = 312)b | 10 mg (n = 315)b | placebo (n = 158) | 5 mg (n = 294)b | 10 mg (n = 292)b | placebo (n = 147) | 5 mg (n = 294)b | 10 mg (n = 292)b | placebo (n = 148) | 5 mg (n = 294)b | 10 mg (n = 292)b |
| PtGA | 59.0 ± 22.9 (n = 311) | 60.2 ± 22.5 | 57.9 ± 23.3 | −24.8 ± 1.2 (n = 293)c | −28.2 ± 1.3c | −12.5 ± 1.7 (n = 148) | 68.6 (n = 293)c | 72.6c | 49.3 | 5.2 (n = 293) | 4.3 |
| Pain | 57.1 ± 23.8 (n = 311) | 58.6 ± 22.2 | 57.1 ± 22.8 | −24.2 ± 1.2 (n = 293)c | −26.8 ± 1.3c | −11.4 ± 1.7 (n = 148) | 67.9 (n = 293)c | 69.5c | 46.0 | 4.6 (n = 293) | 4.2 |
| HAQ DI | 1.44 ± 0.69 (n = 311) | 1.43 ± 0.68 | 1.35 ± 0.66 (n = 157) | −0.46 ± 0.03 (n = 292)c | −0.56 ± 0.03c | −0.21 ± 0.04 | 66.1 (n = 292)c | 70.2c | 44.9 (n = 147) | 4.7 (n = 292) | 4.0 |
| HRQOL (SF‐36) | |||||||||||
| PCS | 32.4 ± 7.8 | 32.0 ± 7.5 | 32.7 ± 7.6 | 5.9 ± 0.4 (n = 293)c | 7.5 ± 0.4 (n = 290)c | 2.4 ± 0.6 (n = 146) | 64.5 (n = 293)d | 74.1 (n = 290)c | 47.3 (n = 146) | 5.8 (n = 293) | 3.7 (n = 290) |
| MCS | 40.9 ± 12.6 | 41.6 ± 11.1 | 41.7 ± 11.6 | 4.4 ± 0.5 (n = 293)e | 4.4 ± 0.5 (n = 290)e | 1.6 ± 0.7 (n = 146) | 58.4 (n = 293)d | 53.8 (n = 290)e | 41.8 (n = 146) | 6.0 (n = 293) | 8.3 (n = 290) |
| PF | 32.5 ± 9.6 | 31.7 ± 9.6 | 32.8 ± 9.6 | 4.5 ± 0.5d | 6.4 ± 0.5 (n = 291)c | 1.7 ± 0.7 | 42.5e | 53.3 (n = 291)c | 32.0 | 9.5 | 4.7 (n = 291) |
| RP | 33.7 ± 9.7 | 33.2 ± 9.4 | 33.9 ± 9.6 | 5.7 ± 0.5d | 7.4 ± 0.5c | 2.6 ± 0.7 | 45.9e | 54.1c | 30.6 (n = 147) | 6.5 | 4.3 |
| BP | 33.4 ± 7.3 | 33.9 ± 7.3 | 34.2 ± 7.5 | 7.3 ± 0.4c | 8.7 ± 0.5c | 3.9 ± 0.6 | 47.6c | 52.7c | 28.6 (n = 147) | 5.3 | 4.1 |
| GH | 34.0 ± 9.1 | 34.3 ± 8.6 | 34.7 ± 8.3 | 5.3 ± 0.4c | 5.6 ± 0.4 (n = 291)c | 1.3 ± 0.6 | 46.6c | 46.1 (n = 291)c | 22.5 (n = 147) | 4.1 | 4.2 (n = 291) |
| VT | 40.8 ± 10.3 | 40.9 ± 8.9 | 41.3 ± 9.4 | 6.3 ± 0.5c | 6.5 ± 0.5c | 2.6 ± 0.7 | 55.4c | 57.2c | 34.7 (n = 147) | 4.8 | 4.4 |
| SF | 36.2 ± 11.2 | 37.0 ± 10.3 | 36.9 ± 11.6 | 5.2 ± 0.5c | 5.9 ± 0.5c | 1.7 ± 0.7 | 55.8e | 60.3d | 42.9 (n = 147) | 7.7 | 5.7 |
| RE | 35.5 ± 13.7 | 34.9 ± 13.0 | 35.4 ± 13.0 | 3.9 ± 0.6 (n = 293) | 5.3 ± 0.6 (n = 291)e | 2.3 ± 0.9 (n = 146) | 39.3 (n = 293) | 47.1 (n = 291)e | 34.3 (n = 146) | 20.0 (n = 293) | 7.8 (n = 291) |
| MH | 39.9 ± 12.5 | 41.1 ± 10.5 | 41.5 ± 11.4 | 4.8 ± 0.5c | 4.7 ± 0.5d | 1.5 ± 0.7 | 50.3c | 52.1c | 29.3 (n = 147) | 4.7 | 4.4 |
| FACIT‐F | 29.0 ± 11.1 | 28.7 ± 9.5 (n = 314) | 29.7 ± 9.0 | 5.8 ± 0.5c | 6.9 ± 0.5 (n = 291)c | 2.1 ± 0.6 | 53.7e | 63.6 (n = 291)c | 40.8 (n = 147) | 7.7 | 4.4 (n = 291) |
| MOS Sleep | 41.1 ± 20.7 | 40.9 ± 18.5 (n = 313) | 39.8 ± 18.3 | −6.2 ± 0.8 (n = 292)d | −7.4 ± 0.8 (n = 290)c | −1.6 ± 1.1 (n = 146) | NA | NA | NA | NA | NA |
Values are: mean ± SD (baseline), LSM ± SE (month 3), % (patients reporting improvements). LSM = least squares mean; MCID = minimum clinically important difference; NNT = number needed to treat; PRO = patient‐reported outcome; PtGA = patient global assessment of arthritis; Pain = patient assessment of arthritis pain; HAQ DI = Health Assessment Questionnaire disability index; HRQOL = health‐related quality of life; SF‐36 = Short Form 36 health survey; PCS = physical component score; MCS = mental component score; PF = physical functioning; RP = role‐physical; BP = bodily pain; GH = general health; VT = vitality; SF = social functioning; RE = role‐emotional; MH = mental health; FACIT‐F = Functional Assessment of Chronic Illness Therapy–Fatigue; MOS = Medical Outcomes Study; NA = not available.
Tofacitinib twice daily.
P < 0.0001 versus placebo.
P < 0.001.
P < 0.05.
Month 3 values
PtGA
At month 3, treatment with tofacitinib 5 and 10 mg BID resulted in significantly greater improvements from baseline in PtGA versus placebo (Figure 1A; P < 0.0001 versus placebo for both), with significantly more (P <0.0001) patients reporting improvements ≥MCID; NNTs were 5.2 and 4.3, respectively (Table 1).
Figure 1.
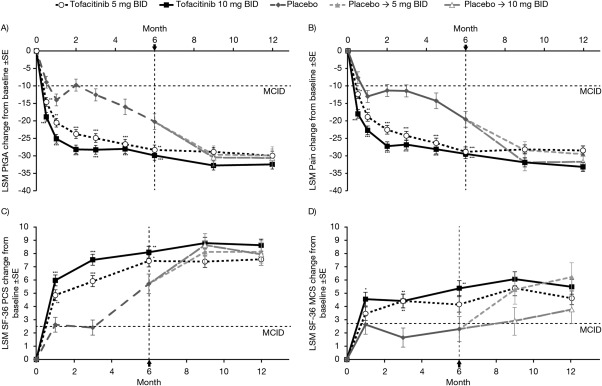
Least squares mean (LSM) change from baseline over time for: A, patient global assessment of arthritis (PtGA), B, patient assessment of arthritis pain (Pain), C, Short Form 36 health survey (SF‐36) physical component summary (PCS) score, and D, SF‐36 mental component summary (MCS) score (full analysis set, no imputation). Horizontal dotted lines represent the minimum clinically important differences (MCIDs). Arrow and vertical dotted line denote completion of advancement to tofacitinib for patients randomized to placebo. Advancement to tofacitinib was at month 3 for placebo nonresponders and at month 6 for all remaining placebo‐treated patients. BID = twice daily; * = P < 0.05; ** = P < 0.01; *** = P < 0.0001 versus placebo.
Pain
Patients in both tofacitinib groups reported significantly greater improvements from baseline in Pain at month 3 compared with placebo (Figure 1B; P < 0.0001 versus placebo for both), with significantly more (P < 0.0001) patients reporting improvements ≥MCID; NNTs were 4.6 for tofacitinib 5 mg BID and 4.2 for 10 mg BID (Table 1).
HAQ DI
Treatment with tofacitinib 5 and 10 mg BID resulted in significantly greater LSM changes from baseline in HAQ DI versus placebo (P < 0.0001 versus placebo for both) at month 3, with significantly more (P < 0.0001) patients reporting changes ≥MCID; NNTs were 4.7 and 4.0, respectively (Table 1).
SF‐36 PCS and MCS
LSM changes from baseline in SF‐36 PCS and MCS scores at month 3 were significantly greater for tofacitinib‐treated patients versus placebo (Figures 1C and 1D; P < 0.0001 for PCS, and P < 0.05 for MCS), with significantly more patients reporting improvements in PCS and MCS scores ≥MCID (5 mg BID: P < 0.001 for both; 10 mg BID: P < 0.0001 for PCS, and P < 0.05 for MCS); NNTs were 5.8 and 3.7 for PCS, and 6.0 and 8.3 for MCS, respectively (Table 1).
SF‐36 domain scores
At baseline, mean scores across all SF‐36 domains indicated substantial impairment in HRQOL compared with an age‐ and sex‐matched US normative population (Figure 2). At month 3, improvements reported by patients treated with tofacitinib 5 mg BID in 7 of 8 domains were statistically significant versus placebo (P < 0.001 for all domains except role‐emotional), and across all 8 domains (P < 0.001; P < 0.05 for role‐emotional) with tofacitinib 10 mg BID (Table 1).
Figure 2.
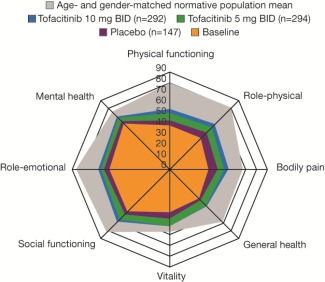
Spydergram representing mean overall Short Form 36 health survey domain scores at month 3 (full analysis set, no imputation). Baseline values (orange) were pooled (with weighting) across all 3 treatment groups. BID = twice daily.
A significantly greater proportion of patients reported improvements ≥MCID at month 3 across all domains except role‐emotional with tofacitinib 5 mg BID (P < 0.05) and all 8 domains with 10 mg BID (P < 0.001; P < 0.05 for role‐emotional); NNTs ranged 4.1–20.0 and 4.1–7.8, respectively (Table 1).
FACIT‐F
Patients receiving both doses of tofacitinib reported significantly greater improvements (P < 0.0001) from baseline in FACIT‐F at month 3 versus placebo.
Significantly more patients reported improvements ≥MCID (P < 0.05 for 5 mg BID, and P < 0.0001 for 10 mg BID); NNTs were 7.7 and 4.4, respectively (Table 1).
MOS Sleep
At month 3, significantly greater LSM changes from baseline (P < 0.001 for 5 mg BID, and P < 0.0001 for 10 mg BID) in MOS Sleep score were reported by tofacitinib‐treated patients compared with placebo (Table 1).
Month 12 values
Reported improvements in all PROs were sustained to month 12 (Figure 1).
Correlation analyses
Correlations were generally positive or negative as expected. For a PRO where an improvement was denoted by positive change from baseline, there was a negative correlation with change in SDAI, for which negative values indicate an improvement, etc. The vast majority of all the correlations were between 0.3 and 0.6 in absolute value. Among domains and components of the SF‐36, the domains of physical functioning, bodily pain, and vitality had the largest correlations in absolute value with SDAI (0.32–0.42) for tofacitinib 5 mg BID and 10 mg BID. The largest correlations in absolute value for PROs with SDAI were observed for PtGA and Pain (0.44–0.59), followed by HAQ DI (0.45–0.47) and FACIT‐F (0.35–0.41) for tofacitinib 5 mg BID and 10 mg BID.
Discussion
PROs assessing HRQOL and fatigue are considered important in RCTs of RA treatments because, in addition to being incorporated into composite disease activity measures, they reflect aspects of disease impact (such as physical function, physical and emotional wellbeing, pain, and fatigue) that are likely more important to patients than joint counts or laboratory tests 2.
In this study, baseline SF‐36 scores reflected a substantial burden of disease compared with a benchmark age‐ and sex‐matched normative population. Treatment with tofacitinib 5 and 10 mg BID in combination with DMARDs resulted in statistically significant improvements versus placebo at month 3 in PtGA, Pain, HAQ DI, SF‐36 PCS and MCS scores, 7 and 8 SF‐36 domains, respectively, FACIT‐F, and MOS Sleep. These improvements resulted in generally small, clinically meaningful NNTs and were sustained to month 12. Sleep disturbance is associated with disease activity and pain in RA and affects mood 10; it is encouraging, therefore, that in this trial, reported improvements in HRQOL and fatigue were accompanied by similar changes in MOS Sleep. Together, these results demonstrate a broad benefit of tofacitinib treatment on the physical and mental burden of RA.
The clinical importance of these results is supported by significantly greater proportions of patients in both tofacitinib treatment groups reporting improvements ≥MCID, with correspondingly small NNTs. With the exception of the role‐emotional domain with the 5 mg BID dose, reported improvements in PROs were statistically significant and clinically meaningful with both tofacitinib doses, generally higher with 10 mg BID, and consistent with the primary results of the trial 4.
Results of this trial are also consistent with those reported in the other phase III RCTs of tofacitinib in RA, where statistically significant and clinically meaningful improvements across a range of PROs were reported in methotrexate‐inadequate responders (ORAL Standard trial [NCT00853385]) 8 and biologic DMARD‐inadequate responders (bDMARD‐IR; ORAL Step trial [NCT00960440]) 7. Data from the present trial are also consistent with those from RCTs of bDMARDs in DMARD‐IR populations, including adalimumab 11, etanercept 12, golimumab 13, infliximab 14, and tocilizumab 15.
A limitation of this study was the short duration of placebo exposure, with advancement from placebo occurring in 2 stages at months 3 and 6. Therefore, although improvements in PROs were reported to month 12, direct comparison with all placebo‐treated patients beyond month 3 was not possible. A second limitation was that enrollment in the study was not stratified by background DMARD therapy, which varied among patients, with most receiving methotrexate, alone or in combination with other conventional DMARDs. In this analysis, missing data were not imputed; however, LSM change from baseline was calculated and is considered to be less sensitive to missing data than use of arithmetic means.
In conclusion, this RCT demonstrated that tofacitinib 5 and 10 mg BID in combination with DMARDs resulted in significant and clinically meaningful improvements versus placebo across a broad range of PROs in conventional‐and bDMARD‐IR patients with active RA. These changes from baseline were sustained through month 12 and provide further evidence that treatment with tofacitinib improves pain, physical function, HRQOL, fatigue, and sleep, in addition to underlying disease activity.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Wallenstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Strand, Kremer, Gruben, Krishnaswami, Zwillich, Wallenstein.
Acquisition of data
Kremer.
Analysis and interpretation of data
Strand, Kremer, Gruben, Krishnaswami, Zwillich, Wallenstein.
ROLE OF THE STUDY SPONSOR
This study was funded by Pfizer Inc. The study was designed by the Pfizer Inc. authors in collaboration with the academic authors. Dr. Gruben performed data and statistical analyses. Medical writing support under the direction of the authors was provided by Daniel Binks, PhD, of Complete Medical Communications and funded by Pfizer Inc. All authors were involved in the interpretation of data and manuscript drafting, reviewing, and development. Publication of this article was not contingent upon approval by Pfizer Inc.
ACKNOWLEDGMENTS
The authors thank the patients, investigators, and study team who were involved in the ORAL Sync study.
ClinicalTrials.gov identifier: NCT00856544.
Supported by Pfizer.
Dr. Strand has received consulting fees and is an advisory board member for AbbVie, Amgen, Biogen Idec, BMS, Crescendo, Genentech/Roche, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer Inc, Regeneron, Sanofi, Takeda, UCB, and Vertex (less than $10,000 each), and has received consulting fees from Alder, AstraZeneca, Biotest, Celgene, Celltrion, Corrona LLC, Horizon, Hospira, Incyte, Merck Serono, and Protalex (less than $10,000 each). Dr. Kremer has received consulting fees and research support from AbbVie, Amgen, BMS, Genentech, Pfizer Inc, and UCB (less than $10,000 each), and has received honoraria or speaking fees from AbbVie (less than $10,000). Drs. Gruben, Krishnaswami, Zwillich, and Wallenstein hold shares in Pfizer Inc.
REFERENCES
- 1. Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health‐related quality of life and productivity. Drugs 2010;70:121–45. [DOI] [PubMed] [Google Scholar]
- 2. Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M, et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis: progress at OMERACT 7. J Rheumatol 2005;32:2250–6. [PubMed] [Google Scholar]
- 3. Fleischmann R, Kremer J, Cush J, Schulze‐Koops H, Connell CA, Bradley JD, et al. Placebo‐controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 4. Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin‐Mola E, et al. Tofacitinib in combination with nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 5. Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 6. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP‐690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four‐month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 7. Strand V, Burmester GR, Zerbini CA, Mebus CA, Zwillich SH, Gruben D, et al. Tofacitinib with methotrexate in third‐line treatment of patients with active rheumatoid arthritis: patient‐reported outcomes from a phase III trial. Arthritis Care Res (Hoboken) 2015;67:475–83. [DOI] [PubMed] [Google Scholar]
- 8. Strand V, van Vollenhoven RF, Lee EB, Fleischmann R, Zwillich SH, Gruben D, et al. Tofacitinib or adalimumab versus placebo: patient‐reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strand V, Boers M, Idzerda L, Kirwan JR, Kvien TK, Tugwell PS, et al. It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible: response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38:1720–7. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol 2006;33:1942–51. [PubMed] [Google Scholar]
- 11. Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health‐related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol 2012;39:63–72. [DOI] [PubMed] [Google Scholar]
- 12. Van der Heijde D, Klareskog L, Singh A, Tornero J, Melo‐Gomes J, Codreanu C, et al. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis 2006;65:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genovese MC, Han C, Keystone EC, Hsia EC, Buchanan J, Gathany T, et al. Effect of golimumab on patient‐reported outcomes in rheumatoid arthritis: results from the GO‐FORWARD study. J Rheumatol 2012;39:1185–91. [DOI] [PubMed] [Google Scholar]
- 14. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004;50:1051–65. [DOI] [PubMed] [Google Scholar]
- 15. Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health‐related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24‐week randomized controlled RADIATE study. Rheumatology (Oxford) 2012;51:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


