Abstract
Langerhans cell sarcoma (LCS) and quintuple cancers are extremely rare. In this report, a case of quintuple cancers including LCS was described. An 80‐year‐old man had squamous cell carcinoma of the nasal skin, colon and rectum adenocarcinomas, and T‐cell/histiocyte‐rich large B‐cell lymphoma. As swelling of multiple submental lymph nodes was observed, fine‐needle aspiration was carried out. Many large cells with high‐grade nuclear atypia and abundant cytoplasm were observed. Lymphocytes and eosinophils were observed in the background. Although a malignant tumor was suspected, a definite diagnosis could not be made. In a biopsy sample, the tumor cells were positive for vimentin, CD68, S‐100, CD1a, and CD163 and negative for epithelial, lymphocyte, and melanoma markers in immunohistochemistry. A diagnosis of LCS was made from the immunohistochemical findings and high mitotic rate with atypical forms. The patient died about 2 months after the first medical examination. Metastasis of LCS was confirmed in many organs by autopsy. LCS has a poor prognosis. In cases with the above‐described cytological findings, LCS should be added to the list of differential diagnosis. The cytological findings presented here may be useful for determining appropriate clinical management such as staging of the disease and follow‐up of the neoplasm. Diagn. Cytopathol. 2017;45:441–445. © 2017 The Authors Diagnostic Cytopathology Published by Wiley Periodicals, Inc.
Keywords: Langerhans cell sarcoma, quintuple cancer, fine‐needle aspiration cytology, touch smear cytology
Langerhans cells (LCs) are antigen‐presenting dendritic cells. According to the recent World Health Organization (WHO) classification,1 LC tumors have been difficult to differentiate from histologically diagnosed non‐Hodgkin lymphoma, melanoma, sarcoma, and undifferentiated carcinomas due to their similarities. Immunostaining for CD1a, S‐100 protein, CD163, and langerin (CD207) is useful for establishing a diagnosis of LC tumor. LC tumors are classified into Langerhans cell histiocytosis (LCH) and Langerhans cell sarcoma (LCS). Differential diagnosis between LCH and LCS is based on malignant potential. Although diagnosis is difficult due to the similarity of cytological and histological features, mitotic activity would become a differential point. In LCH, mitotic activity is variable and without atypical forms. The mitotic rate of LCS is high, usually >50 per 10 high‐power field (HPF).
LCS is extremely rare.1, 2, 3 It can appear in any age group (range, 2–88 years), and it is predominant in females.1 LCS occurs commonly in the skin, soft tissue, and lymph nodes with multiorgan involvement including the liver, spleen, lungs, and bones.1, 2, 3 The organs most frequently involved are the skin (about 60% of cases) and lymph nodes (about 50% of cases).2 Patients presenting with multiorgan involvement have a very poor prognosis.2 Early detection and accurate diagnosis are important for improving the prognosis. However, there has been only one report on cytological findings of LCS.3 Although BRAF mutations in histiocytic proliferative diseases are restricted to lesions of LC type, the positive rate of BRAF mutations in LCS is lower than that in LCH.4
Quintuple primary cancers are rare.5, 6, 7, 8, 9, 10, 11 We experienced a case of quintuple tumors including LCS. It was difficult to make a diagnosis of LCS by fine‐needle aspiration (FNA) of a neck lymph node. We report the cytological findings of LCS by FNA and touch smear cytology.
Case Report
Clinical Summary
An 80‐year‐old Japanese man was admitted to our hospital with a nasal skin tumor. He had past medical histories of adenocarcinomas and T‐cell/histiocyte‐rich large B‐cell lymphoma. His family history was unremarkable. Swelling of multiple submental lymph nodes was revealed by computed tomography (Fig. 1A). The diameter of a lymph node was 16 mm (Fig. 1B). Diagnosis by FNA of a lymph node was difficult. A diagnosis of LCS was made by biopsy. The condition of the patient rapidly deteriorated and he died about 2 months after the first medical examination. Metastasis of LCS was confirmed in many organs by autopsy. Written informed consent was obtained from his daughter. The study was approved by the Institutional Review Boards of Kushiro City General Hospital.
Figure 1.
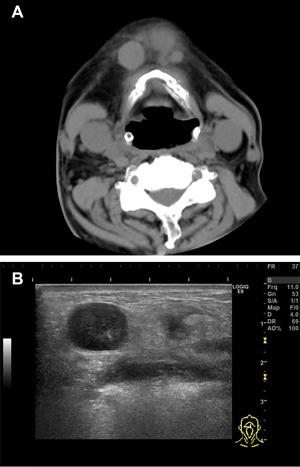
Head and neck computed tomography showing swelling of multiple lymph nodes in the submandibular zone (A). Ultrasonographic image showing a lymph node measuring 16 mm in diameter (B). [Color figure can be viewed at wileyonlinelibrary.com]
Cytological Findings
Many large cells were found with mature lymphocytes by FNA from the swelling lymph node (Figs. 2A and B). The large cells had abundant cytoplasm. Nuclei were pleomorphic. These atypical cells had nuclear grooves (Fig. 2B; red arrow). Multinucleated cells were observed (Fig. 2B; green arrow). These neoplastic cells had fine chromatin granules in touch smear cytology of the lymph node biopsy (Figs. 2C and D). These atypical cells had a small nucleolus, and mitosis was observed (Figs. 2C and D; yellow arrow). There was no atypical mitosis. Several nuclear inclusion bodies were also confirmed (Figs. 2C and D; light blue arrow). There were many eosinophils and mature lymphocytes in the background. The possibility of malignant lymphoma or histiocytic neoplasm was considered. However, the possibility of poorly differentiated carcinoma or malignant melanoma could not be completely ruled out. Because we failed to prepare cellblock material from the cytology specimen for immunocytochemistry, a diagnosis was not obtained.
Figure 2.
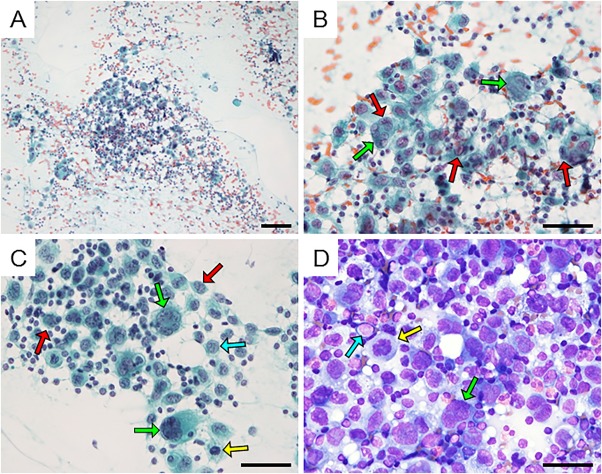
Cytological findings of the lymph node from FNA (A and B, Papanicolaou staining). A large cell with abundant cytoplasm is seen backed by lymphocytes. The nucleus of a tumor cell has a groove (red arrow). A multinucleated tumor cell is seen (green arrow). Cytological findings of imprint cytology (C, Papanicolaou staining; D, Giemsa staining). These atypical cells have a small nucleolus, and mitosis is observed (yellow arrow). Several nuclear inclusion bodies were also confirmed (light blue arrow). Bar: 100 µm (A), 50 µm (B), 50 µm (C and D). [Color figure can be viewed at wileyonlinelibrary.com]
Histological Findings
The cut surface of the lymph node obtained by biopsy was solid and light grayish in color without necrosis or hemorrhage (Fig. 3A). Microscopically, large atypical cells were observed in the lymph node, similar to the results of cytodiagnosis (Fig. 3B). These atypical cells had invaded the area outside the lymph node. The mitotic rate was high (58/10HPF) and atypical mitotic forms were observed (Fig. 3B). Immunohistochemically, the tumor cells were positive for vimentin, CD68, and CD163 and focally positive for S‐100 and CD1a (Figs. 3C–G). Epithelial markers (AE1/AE3, CK7, CK20, and p40), lymphocyte markers (CD3, CD20, CD45, and CD79a), and melanoma markers (HMB45 and Melan‐A) were negative (data not shown). The possibility of a proliferative disease of histiocytes was considered from the positive results for CD68, and the possibility of a malignant tumor was considered from the finding of extranodal invasion. LCS was diagnosed by a high mitotic rate with an atypical form and positivity for CD1a and CD163. Metastasis of LCS was confirmed by the autopsy in many organs including the heart, bilateral lungs, liver, pancreas, bilateral kidneys, left adrenal gland, spleen, bone marrow, and systemic lymph nodes.
Figure 3.
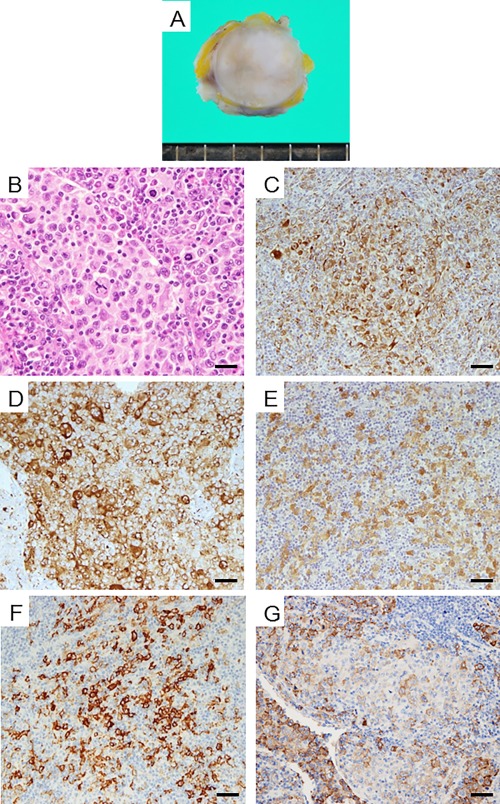
Macroscopic findings of the lymph node (A). The cut section appeared light gray and solid. There is no necrosis or bleeding. Histological findings (B; H&E staining). Cells had diffusely multiplied. There are tumor cells with abundant cytoplasm. Multinucleated tumor cells and mitotic figures are seen. Immunohistochemical findings (C∼F). Tumor cells showing positive staining for vimentin (C), CD68 (D), S‐100 protein (E), CD1a (F), and CD163 (G). Bar: 20 µm (B) and 50 µm (C–F). [Color figure can be viewed at wileyonlinelibrary.com]
Past Material and Genetic Test Findings
Our case was a case of quintuple cancers. The patient had two moderately differentiated adenocarcinomas (sigmoid colon, 12 years ago, pT2 pN0 M0 Stage I, Supporting Information, Fig. 1A; rectum, 12 years ago, pT3 pN0 M0 Stage IIA, Supporting Information, Fig. 1B). He also had T‐cell/histiocyte‐rich large B‐cell lymphoma according to the WHO classification updated in 2008 (12 years ago, Supporting Information, Figs. 1C–F). The tumor cells were positive for CD79a. CD3‐positive cells and CD68‐positive cells were mixed. He achieved complete remission (CR) after chemotherapy. He had two recurrences (8 and 6 years ago). He underwent chemotherapy and achieved CR again. The nasal skin tumor (at the same time as the LCS, Supporting Information, Figs. 1G and H) was squamous cell carcinoma (SCC). The tumor had keratosis and immunoreactivity of P40.
No genetic mutations of KRAS and BRAF V600E were detected in these five tumor materials, including the LCS.
Discussion
LCS is an extremely rare tumor, and it has a very poor prognosis. Our patient died about 2 months after the first medical examination. Early diagnosis is important to improve the prognosis. As described above, atypical tumor cells were observed in cytology, suggesting that the tumor was a histiocytic neoplasm. Although a diagnosis of LCS only by these cytological findings is difficult,4 immunocytochemistry using cell block materials may be useful for an accurate preoperative diagnosis. In cases with the above‐described cytological findings, LCS should be added to the list of differential diagnosis. If cellblock materials had been prepared in this case, they might have been useful for diagnosis. In addition, cytology can be very useful for staging of the disease and follow‐up of the neoplasm. The cytological findings described in this report may be useful for determining appropriate clinical management in the future.
Quintuple cancers are rare.5, 6, 7, 8, 9, 10, 11 Our case had 3 epithelial cancers and 2 tumors of the hematopoietic and lymphoid tissues. It has been reported that KRAS gene mutation was found in colon adenocarcinoma12 and that BRAF gene mutation was found in both LCS and colon adenocarcinoma.4, 12 However, KRAS and BRAF gene mutations were not detected in our case. There have been several reports on LCS arising from other tumors of hematopoietic and lymphoid tissues.13, 14, 15, 16 However, there has been no report on LCS related to T‐cell/histiocyte‐rich large B‐cell lymphoma. Some novel genetic mutations that can give rise to multiple neoplasms might have been hidden in his background.
Supporting information
Supporting Information
Conflicts of interest: There are no conflicts of interest.
Disclosure of grants or other funding: None.
References
- 1. Jaffe R, Weiss LM, Faccetti F. Tumors derived from Langerhans cells In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumors of the hematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. p 358–362. [Google Scholar]
- 2. Zwerdling T, Won E, Shane L, Javahara R, Jaffe R. Langerhans cell sarcoma: Case report and review of world literature. J Pediatr Hematol Oncol 2014;36:419–425. [DOI] [PubMed] [Google Scholar]
- 3. López‐Ferrer P, Jiménez‐Heffernan JA, Alves‐Ferreira J, Vicandi B, Viguer JM. Fine needle aspiration cytology of Langerhans cell sarcoma. Cytopathology 2008;19:59–61. [DOI] [PubMed] [Google Scholar]
- 4. Bubolz AM, Weissinger SE, Stenzinger A, et al. Potential clinical implications of BRAF mutations in histiocytic proliferations. Oncotarget 2014;30:4060–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warren S, Gates O. Multiple primary cancer. Am J Cancer 1932; 16:1358. [Google Scholar]
- 6. Baigrie RJ. Seven different primary cancers in a single patient. A case report and review of multiple primary malignant neoplasia. Eur J Surg Oncol 1991;17:81–83. [PubMed] [Google Scholar]
- 7. Cercato MC, Colella E, Ferraresi V, et al. Report of two cases of quintuple primary malignancies and review of the literature. Anticancer Res 2008;28:2953–2958. [PubMed] [Google Scholar]
- 8. Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 2003;21:1174–1179. [DOI] [PubMed] [Google Scholar]
- 9. Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 1998;95:6870–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–932. [DOI] [PubMed] [Google Scholar]
- 11. Komiyama S, Nishio E, Ichikawa R, et al. Asymptomatic synchronous quintuple primary cancers. Gynecol Obstet Invest 2012;74:324–328. [DOI] [PubMed] [Google Scholar]
- 12. Kawazoe A, Shitara K, Fukuoka S, et al. A retrospective observational study of clinicopathological features of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with metastatic colorectal cancer. BMC Cancer 2015; doi:10.1186/s12885-015-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambrosio MR, De Falco G, Rocca BJ, et al. Langerhans cell sarcoma following marginal zone lymphoma: Expanding the knowledge on mature B cell plasticity. Virchows Arch 2015;467:471–480. [DOI] [PubMed] [Google Scholar]
- 14. Chen W, Jaffe R, Zhang L, et al. Langerhans cell sarcoma arising from chronic lymphocytic lymphoma/small lymphocytic leukemia: Lineage analysis and BRAF V600E mutation study. N Am J Med Sci 2013;5:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furmanczyk PS, Lisle AE, Caldwell RB, et al. Langerhans cell sarcoma in a patient with hairy cell leukemia: Common clonal origin indicated by identical immunoglobulin gene rearrangements. J Cutan Pathol 2012;39:644–650. [DOI] [PubMed] [Google Scholar]
- 16. Ratei R, Hummel M, Anagnostopoulos I, et al. Common clonal origin of an acute B‐lymphoblastic leukemia and a Langerhans' cell sarcoma: Evidence for hematopoietic plasticity. Haematologica 2010;95:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


