Abstract
Lyme disease is the most common tick-borne disease in temperate regions of North America, Europe and Asia, and the number of reported cases has increased in many regions as landscapes have been altered. Although there has been extensive work on the ecology and epidemiology of this disease in both Europe and North America, substantial uncertainty exists about fundamental aspects that determine spatial and temporal variation in both disease risk and human incidence, which hamper effective and efficient prevention and control. Here we describe areas of consensus that can be built on, identify areas of uncertainty and outline research needed to fill these gaps to facilitate predictive models of disease risk and the development of novel disease control strategies. Key areas of uncertainty include: (i) the precise influence of deer abundance on tick abundance, (ii) how tick populations are regulated, (iii) assembly of host communities and tick-feeding patterns across different habitats, (iv) reservoir competence of host species, and (v) pathogenicity for humans of different genotypes of Borrelia burgdorferi. Filling these knowledge gaps will improve Lyme disease prevention and control and provide general insights into the drivers and dynamics of this emblematic multi-host–vector-borne zoonotic disease.
This article is part of the themed issue ‘Conservation, biodiversity and infectious disease: scientific evidence and policy implications'.
Keywords: epidemiology, Borrelia burgdorferi, Ixodes, emerging infectious disease, dilution effect
1. Introduction
Lyme disease is the most common tick-borne pathogen in temperate forested regions of North America, Europe and Asia. There are tens of thousands of clinical cases reported annually, and estimates that account for underreporting suggest there are hundreds of thousands of infections each year [1–4]. The number of reported cases have tripled in the USA in the last 20 years [1] and have increased in some regions in Europe [5,6]. The increase in Lyme disease incidence has been attributed to a range of factors that also contribute to the enormous spatial variation across North America and Eurasia. These include land use or land cover change, habitat fragmentation, changes in vegetation structure, changes in host communities due to these factors or from hunting, increased diagnosis and reporting, and climate change [4,7–14]. Substantial improvements in control are needed to limit and reverse the increase in incidence of Lyme disease.
Globally, Lyme disease is caused by some members of the Borrelia burgdorferi sensu lato (s.l.) species complex including Borrelia burgdorferi sensu stricto (termed Borrelia burgdorferi in the following for simplicity) in North America (and possibly B. mayonii, although this causes a disease somewhat distinct from typical Lyme disease [15]) and five species in Europe, B. afzelii, B. garinii, B. burgdorferi, B. spielmanii and B. bavariensis [2]. The bacterial species causing Lyme disease have been present in North America and Eurasia for millenia [16,17], but Borrelia burgdorferi was only identified as the cause of Lyme disease three decades ago [18]. Patterns of tick abundance prior to the past few decades are unknown, but in the northeastern USA, widespread forest clearing for agriculture and the near extirpation of deer by hunters in the eighteenth and nineteenth centuries are thought to have greatly reduced tick density [10,19–21]. Subsequently, both the tick and the pathogen are believed to have reinvaded as deer populations recovered, and the area became reforested [10]. The expansion of both ticks and Lyme disease over the past three decades continues today in North America with similar patterns in parts of Europe [22–24], which results in highly variable geographical levels of disease risk and disease reporting, creating challenges for clinicians in newly invaded regions who use history of tick exposure as a guide in diagnosis [2].
Developing novel control methods and predicting disease risk to better target control interventions require understanding pathogen and disease dynamics. The multitude of factors influencing Lyme disease incidence is emblematic of many zoonotic pathogens. For zoonotic diseases with multiple wildlife hosts, such as Ebola and Monkeypox viruses, a key challenge is to determine the reservoirs for the pathogen, the contribution of each species in increasing or decreasing transmission, and the processes governing the populations and behaviour of these key species. For vector-borne zoonotic diseases, such as Chagas, West Nile encephalitis and Leishmaniasis, one must also understand the factors that influence vector populations, and their contact with hosts, including humans and other species. Finally, to determine the impact of multi-host zoonotic vector-borne diseases on human health, one needs to understand the factors influencing the probability and severity of disease once a person becomes infected.
For Lyme disease, there are no vaccines currently available for humans, so control is limited to reducing the risk of infection in the environment and encouraging the public to take preventive actions to avoid exposure to infected ticks [25–28]. To effectively target prevention and control, public health organizations need spatio-temporal estimates of Lyme disease risk. Although the local density of infected ticks determines Lyme disease risk, there is only very limited systematic surveillance of tick populations. This gap could be partly filled by predictive models, but models require an understanding of Lyme disease ecology, and specifically the determinants of variation in tick abundance and infection prevalence.
There have been a number of reviews of the nearly four decades of research on Lyme disease ecology and epidemiology. The dominant processes affecting Lyme disease ecology include forest fragmentation and reforestation, hunting, and climate change which influence transmission through their effects on vertebrate host populations, tick survival and human behaviour. An early review [29] described hypothesized links between deer and tick abundance, proposed potential reservoir host species, and described potential climate and microclimate influences on ticks. Substantial research in both Europe and North America over the next three decades identified important habitats for ticks and for exposure of humans to infected ticks, microclimate influences on tick behaviour and survival, specificity of different host species for different Borrelia species, and outlined control methods and their efficacy by examining effects on different components of the transmission cycle [25–28,30,31].
Despite this large body of knowledge, substantial gaps in our understanding still exist regarding the key drivers of spatio-temporal variation in risk (the density of infected ticks) and Lyme disease incidence [25,31,32]. This uncertainty has resulted in strong disagreements in the literature about many aspects of Lyme disease ecology (e.g. [21,33,34] and subsequent rebuttals). We sought to determine what areas of knowledge might be agreed upon by a large fraction of Lyme disease researchers, and what research would be required to reduce uncertainties that led to disagreements. The first author (A.M.K.) submitted a survey (electronic supplementary material) to 19 scientists studying Lyme disease ecology in Europe, and eastern and western North America. Responses from the 12 scientists who agreed to participate were compiled and a synthesis document was sent back to respondents for comment and revision until consensus emerged, with respondents as authors. Responses highlighted several key areas of uncertainty including: the effects of host abundance, climate and climate change on tick abundance and phenology; the influence of host community composition on infection prevalence in ticks and specifically the role of different species in infecting ticks in both Europe and North America, which is known only for a few locations; the drivers of bacterial strain diversity resulting in differential pathogenicity; factors influencing human exposure to ticks; and the potential for ecological restoration of communities to reduce Lyme disease incidence (table 1). Our aim is to synthesize knowledge of Lyme disease ecology to precisely identify these knowledge gaps. We outline the research needed to enable more accurate predictions of disease risk in space and time, and which will facilitate the development of new methods for preventing and controlling this important disease.
Table 1.
Seven key research gaps in Lyme disease ecology.
| (1) The relationship between deer abundance and the abundance of larval Ixodes ticks across the full range of deer density, and in the presence of alternative large mammal hosts. |
| (2) Predictive models for determining nymphal tick abundance from larval tick abundance, including density-dependent survival and feeding success of larval ticks on hosts, the time required for larval ticks to encounter a host, and quantitative relationships between tick activity and survival and temperature and vapour pressure deficit that incorporate effects of both mean and variation around the mean. |
| (3) The factors determining the abundance of important hosts for larval ticks across time and space, including along gradients of land cover/land use. |
| (4) The factors influencing tick burdens, both relative and absolute, on different species across a range of host communities and abundances. |
| (5) The eco-evolutionary origin and maintenance of strain or genotype diversity within Borrelia species and differences in pathogenicity in humans, reservoir competence of host species for these genotypes (i.e. duration and probability of transmitting the bacteria to a feeding tick), and the sensitivity and specificity of diagnostics for these different strains. |
| (6) The factors influencing human and tick behaviour that determine contact between humans and questing nymphal ticks. |
| (7) The degree to which the restoration of predator communities of the dominant hosts for infecting ticks—ungulates, rodents and shrews—would reduce tick abundance or infection prevalence. |
2. Few vectors, many hosts
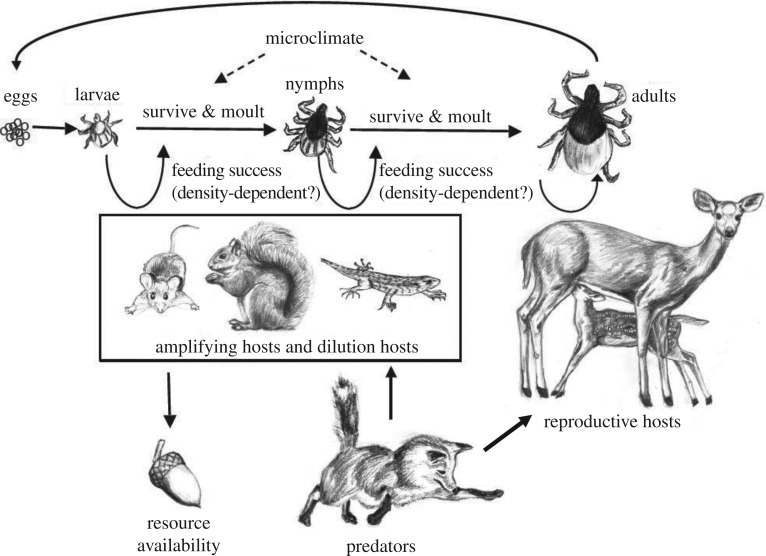
Globally, only four species of Ixodid ticks (I. ricinus in Europe, I. scapularis in eastern North America, I. pacificus in western North America and I. persulcatus in Asia) serve as important vectors for transmitting Lyme disease spirochetes to humans, and in most places, only a single species of tick is responsible for most human cases of infection [2,23]. Although variation within each tick species exists in questing behaviour and thermal tolerances [35,36], having a single tick vector simplifies the ecology of transmission and enables research and control to focus on the single locally important species. All four tick species have three feeding stages (larvae, nymph, adult) which take a single blood meal from a wide range of hosts before moulting to the next stage (larvae and nymphs), or reproducing and dying (adult females; adult males attach to a host but rarely feed; figure 1). The majority of human cases of Lyme disease are thought to be caused by bites from nymphal stage ticks, because infection prevalence in larvae is essentially zero (vertical transmission is low or zero for Borrelia species causing Lyme disease [37,38]) and adult ticks are relatively large, so they are more often noticed and removed by humans before they can attach and feed for the 18–48 h required to transmit the bacterium [2,26,39].
Figure 1.
Schematic of the ecology of Lyme disease. The tick vector has three stages, larvae, nymphs and adults, which each take one blood meal (except adult males) before moulting into the next stage or reproducing and dying (adult females). Adult female ticks feed primarily on deer, whereas the other two stages feed from a wide range of vertebrates including mammals, birds and reptiles with widely varying probabilities of infecting ticks as described in the main text, figure 4 and the electronic supplementary material. Host species are eaten by a suite of interacting predators, and their populations are influenced by fluctuations in food availability. Original illustrations by Yiwei Wang and Taal Levi.
The risk of human infection thus is thought to increase with the density of questing infected nymphs in the environment, which is the product of the density of nymphs and their infection prevalence, and varies at a very local scale. Throughout the rest of the paper, Lyme disease risk refers to this quantity—the density of questing infected nymphs. The most frequently used methods to measure tick abundance and to collect ticks to estimate prevalence are drag sampling or flagging, which are simple low-cost techniques [39,40]. These methods provide a useful index of Lyme disease risk that is often correlated with human incidence at local scales, within some states, and at the county scale across the eastern USA [41–43]. However, the relationship between incidence and the density of infected nymphs is sometimes weak [39,41,44,45], which may be due to: variation in human behaviours that expose them to ticks (table 1) [26,46]; variation in the amount or type of vegetation that interferes with the contact between the dragging cloth and the substrate [40,47]; variation in disease severity caused by different Borrelia genotypes in ticks (genotypes of a subset of infected ticks should be identified, when possible) [41,48–51]; temporal variation in questing tick density on short time scales, challenges in collecting sufficient nymphs to estimate prevalence in areas of low to moderate nymph density [52,53]; variation in the locations or habitat types where ticks are sampled and where people spend time outdoors; and challenges in determining where a patient became infected [39]. Despite these complications, most efforts to understand the causes of spatial and temporal variation in Lyme disease incidence still focus on the drivers of tick abundance and nymphal infection prevalence.
3. Predicting tick distributions and abundance
The distribution and abundance of tick populations depend on the interaction of large-scale climate influences, local-scale microclimates, habitat characteristics (including tick predators) and host densities. These factors interact to determine tick-feeding success and the survival of ticks between feedings (figure 1).
(a). The importance of deer
Cervids (deer; Odocoileus spp. in North America and Cervus elaphus and Capreolus capreolus in Europe) are incompetent hosts for Borrelia [54], but are widely regarded as the most important host for adult ticks in most areas. Habitats that contain very few deer often have much lower tick abundance and Lyme disease risk than those that have moderate or high densities of deer (summarized in table S1 in [11]), although exceptions do exist [55,56]. Low tick abundance occurs both on islands where deer are absent as well as highly urbanized areas of cities (e.g. New York City, Paris, Washington DC, London, San Francisco). Overall, although some medium-bodied mammals host adult ticks, there is only limited evidence to suggest that non-deer hosts can support significant Ixodes populations in woodland habitats [55,56].
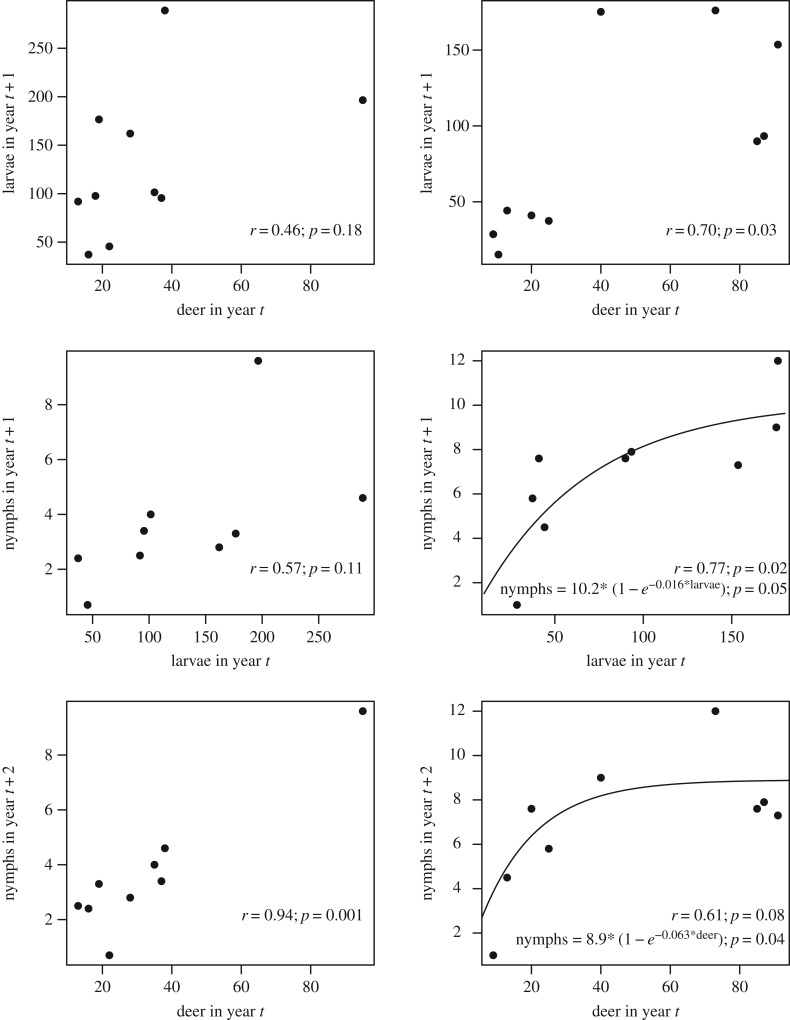
Although deer are important in feeding ticks, it is far less clear how larval and nymphal tick abundance varies with deer density at moderate or higher deer densities. Lyme disease cases at large scales were uncorrelated with deer abundance over space and time [11], and in one study, removal of 70% of deer had no effect on tick density [57]. This is initially surprising because increasing deer density should increase tick survival by reducing the time questing adults are waiting to attach to deer. In addition, on some hosts, an increase in individual tick burdens leads to a reduction in feeding success or moulting success either due to an increase in ‘resistance’ in hosts or due to increased grooming behaviour [58,59]. Reduced individual tick burdens could lower host resistance as ticks are distributed among more deer and would lead to increases in tick survival and density with increased deer abundance. However, density-dependent feeding success has never been experimentally investigated in wild deer species, and does not occur on all species [58,60–62]. In summary, if there is little variation in feeding success in adult ticks on deer with increasing tick burden, if deer density is high enough that adult ticks encounter deer quickly, or if weather and other factors have substantial effects on larval survival then there may be only weak correlations between nymphal tick abundance and deer density above low deer densities (figure 2) [11,34,64,65]. In areas with abundant deer, nymph densities may be more sensitive to changes in the density of hosts for larval ticks and other factors influencing larval tick survival than deer density [66]. If so, deer reduction programmes may have limited success unless they greatly reduce deer densities [26,57].
Figure 2.
Data illustrating the correlations between deer density and nymphal tick density at two sites in Connecticut (Redrawn from [63]). The top row of panels shows the relationship between deer density (deer km−2) and larval tick density (ticks 100 m−2) 1 year later that result from adult ticks (density not reported) feeding on deer in the previous year. The second row shows the relationship between larval tick density and nymphal tick density that fed larvae would moult into 1 year later. Finally, the bottom row shows the relationship between deer density and nymphal tick density 2 years later. Simple Pearson's correlation statistics are shown for each relationship, and where relationships appeared to be nonlinear and potentially asymptotic, a simple nonlinear function was fit to the data (with the p-value corresponding to the slope coefficient).
The relationship between tick density and deer abundance is sometimes nonlinear, with tick abundance saturating at moderate deer abundance and showing little variation as deer abundance increases further (figure 2) [26]. However, where and when saturation occurs, and the deer density above which tick abundance does not increase, are unknown. Uncertainty in the precise relationship between deer density and tick density is one of the most important gaps in our knowledge of Lyme disease ecology (table 1).
Determining how deer density influences the abundance of larval ticks through both encounter probability with adult ticks and density-dependent feeding success on deer would ideally be investigated by first analysing previous studies and using the insight gained to design observational and experimental studies of variation in deer density, measured during adult questing periods, with larval tick density the following year (as in figure 2), after accounting for variation in the density of hosts for larval ticks (electronic supplementary material). Observational studies that take advantage of local variation in deer density due to variation in the abundance of deer predators (e.g. wolves in the Midwestern USA or Europe), or temporal variation in deer density caused by severe winter die-offs, might be especially valuable, as long as they can also account for correlated impacts of cold winters on mortality of ticks. It should also be noted that short-term decreases in deer densities can result in short term increased host-seeking adult tick densities because ticks that would have fed on deer are still questing [67]. This underscores the importance of examining the effect of variation in deer on larval tick density (not adult tick density) the following year (figure 2).
If one could predict questing larval tick abundance based on local-scale deer density, then nymphal tick density could be predicted using a dynamic life-cycle model [66,68,69] that incorporates the product of three probabilities for larvae: (i) surviving the period to find and attach to a host [70], (ii) successfully feeding [71], and (iii) surviving for long enough to moult into the nymphal stage.
(b). Larval and nymphal tick survival and feeding success
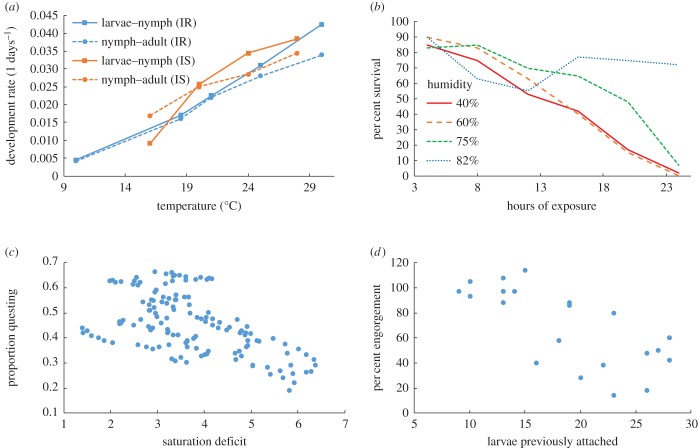
There have been numerous detailed studies of microclimate drivers on tick survival and abundance in both the laboratory and the field (reviewed in [72–74]). Microclimate conditions can impact tick survival directly by increasing tick mortality rates, and indirectly by influencing activity and host finding rates, which in turn affect survival (figure 3) [68,69]. Experiments indicate that both cold and hot temperatures significantly decrease both tick survival and host-seeking activity [72–74]. Similarly, while low humidity (or increased saturation/vapour pressure deficit) reduces activity and can kill ticks quickly through dessication, high levels of rainfall also inhibit activity. These laboratory studies provide mechanisms to potentially explain correlations between climate variables and tick survival or abundance [52,73,80]. However, when adverse climate conditions occur, ticks often seek microclimate refuges in leaf litter or detritus that have milder conditions and this reduces the impacts of extreme temperature and humidity on survival [81,82]. This microclimate selection by ticks can sometimes make it difficult to identify links between tick abundance and larger scale weather, as can fine-scale variation in climate that is not captured by using weather station data.
Figure 3.
Microclimate and density-dependent factors influencing tick development, survival, activity and feeding success. (a) Inter-stage development rates of l. ricinus (blue) and l. scapularis (orange) at different temperatures (redrawn from [75] and [76]). (b) Survival of nymphal l. scapularis ticks after 4–24 h exposure to four different humidity treatments (redrawn from [77]). (c) Proportion of ticks questing plotted against vapour pressure deficit, a measure of dessication stress (redrawn from [78] after correcting for mortality [68]). (d) Per cent of larval Ixodes trianguliceps ticks successfully engorging when feeding on Clethrionomys glareolus (now Myodes glareolus) that had been fed on by variable number of ticks 14 days previously (redrawn from [79]).
In summary, microclimates, modulated by leaf litter in woodland habitats, determine tick development, survival rates and questing behaviour. Quantitative relationships form the basis for predictive models of tick survival rates when they are questing for hosts (the time spent questing is determined by host density; electronic supplemental material) and for development rates [69,72,83]. Scaling up these microclimate models to larger spatial scales by combining remotely sensed climate and vegetation data could help provide more mechanistic explanations for the correlations between large-scale climate indices and Lyme incidence or local measures of risk [73,80,84–87]. Slightly simpler models have already formed the basis for models predicting the distribution, spread and phenology of ticks with climate change [69,88–91].
Even if a tick survives to attach to a host, it still may not survive to obtain a blood meal. The probability of ticks successfully feeding or moulting varies significantly among host individuals and species [71] and laboratory studies indicate some mammals and birds may even kill substantial numbers of ticks through grooming [60,71,92–95]. As noted above, on some host species, feeding success decreases with increasing larval tick burdens through increased host grooming and acquired immunological resistance (figure 3) [58,59,79,96,97]. This density-dependent feeding success could partially regulate tick populations [98], but in some species feeding or moulting success shows no relationship or it may even increase with tick burdens (density-dependent facilitation), including on white-footed mice and sheep [58,60–62].
Models of tick population dynamics to predict tick abundance already exist [68,69,99], but these need further calibration and validation. Data on key missing variables, including the rates at which ticks attach to hosts, could be obtained by experimentally manipulating the abundance of important hosts (i.e. those that feed a substantial fraction of ticks) and tick abundance, and measuring changes in questing tick populations and the fraction of ticks that survive to the next stage, while accounting for inter-annual and inter-site variations in microclimate. Experiments done across a range of regions and habitats would provide opportunities to calibrate and validate the predictive models, and determine the processes that regulate tick populations.
4. Predicting nymphal infection prevalence from vector–host–pathogen interactions
To predict the infection prevalence of nymphal ticks requires data on the fraction of larval ticks that feed on each host species, the fraction of hosts of each species that are infected, and the reservoir competence of these hosts for transmitting Borrelia spirochetes. Host reservoir competence is most fully characterized by experimental infection studies (including single and repeated infections with infected nymphs) and xenodiagnosis with uninfected larvae over the subsequent weeks or months [54,100–103]. Instantaneous host infectiousness (the probability of infecting a feeding tick at a single point in time) can be estimated by capturing animals in the field and holding them for a sufficient period for attached larval ticks to become engorged and fall off the host [92,104]. Infection can then be measured in these ticks after they digest the blood meal and moult to nymphs, and the fraction that are infected is an estimate of instantaneous host infectiousness, after correcting for hosts that were uninfected [105]. Although this approach provides a rigorous estimate of the fraction of larval ticks feeding on a host species that will become infected at that site at the time of sampling, this method provides only a single point estimate and thus does not allow one to estimate the duration and pattern of infectiousness over time, which varies among host species and strains or genotypes of Borrelia bacteria. Instantaneous estimates also do not allow one to account for variation in the time since infection and the number of previous infections among individuals [106–109]. Thus, it is difficult to extrapolate results from this method to other sites or time periods where the frequency of host infection is higher or lower. For both methods, one must also estimate the fraction of each species infected at a location over time which could potentially be predicted using data on the density of infected nymphs and the feeding preferences of nymphal ticks (discussed below).
One challenge for all methods of measuring reservoir competence is genetic variation among and within Borrelia species. Some genospecies in Europe are specialized for different host species [110,111], while there is increasing evidence that strains of B. burgdorferi in North America are associated with certain host species [104,112]. In addition, different genospecies and strains of B. burgdorferi s.l. differ in the likelihood they cause disseminated disease and specific clinical manifestations in humans [2,41]. The mechanisms creating and maintaining this strain diversity are unclear, and variation in Borrelia should be taken into account when quantifying host reservoir competence and the contribution of species to transmission of strains likely to cause disease in humans. In addition, there may be substantial variation in reservoir competence among both individuals and populations within a host species, but the magnitude and cause of this variation is very poorly known.
Feeding patterns of larval ticks can be estimated by a comprehensive study of the density and tick burdens of potential vertebrate hosts [65,92–94,113,114]. Identifying the importance of hosts for feeding larval ticks through blood meal analysis of host DNA remaining in nymphal ticks from their larval blood meal is another method that holds promise, but has so far met with technical challenges (e.g. low sensitivity and specificity and challenges with contamination) [115]. Understanding larval tick feeding patterns involves multiple measurements, because the fraction of ticks feeding on each host species likely changes as host and tick abundance changes [72,94,98,116,117]. For example, larval tick burdens on mice and chipmunks fluctuated as a function of each others' density, suggesting that a fraction of ticks that do not feed on one host may redistribute themselves on other hosts [66,116]. However, in California, near removal of a key host for larval ticks (Sceloporus lizards) did not lead to substantial redistribution of ticks to other hosts that were examined [118], demonstrating the need to understand the drivers of tick distributions among hosts, including the possibility of host preferences in ticks.
Estimates of host abundance and either tick burdens or feeding patterns can be used to quantify relative feeding preferences (or more accurately, host utilization, since host defences, such as grooming, are likely very important in determining feeding success) of ticks [62]. Studies from sites with variation in host abundance could then determine whether host utilizations are relatively consistent across different sites and at larger spatial scales, as has been found for a mosquito-borne pathogen, West Nile virus [119,120]. Experiments manipulating host abundance and measuring tick-feeding patterns, including the fraction that fail to feed [118], could offer substantial insight into host utilization patterns across ranges of host abundance. Consistent host utilization patterns could lead to predictive relationships of host contributions for feeding larval ticks, as a function of host abundance (but see below for challenges in estimating host abundance).
(a). Determining reservoir and dilution hosts
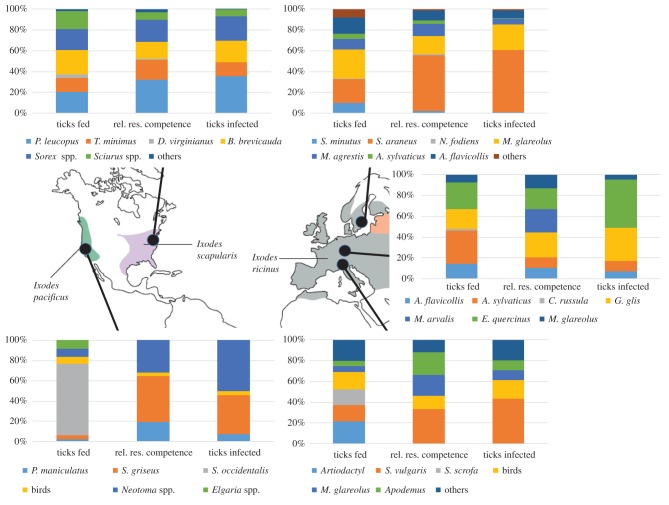
Previous studies have quantified the importance of different hosts in transmission in some areas in both Europe and North America (figure 4). In the northeastern USA, early studies suggested that white-footed mice (Peromyscus leucopus) played an important role in infecting larval ticks, especially in years when they are abundant (e.g. following oak-masting years [121,122]). Their importance in infecting ticks was attributed to their very high and long-lasting infectiousness to feeding ticks, their abundance and their moderate tick burdens [123]. More comprehensive studies in southeastern New York that collected data on each of the traits required to quantify the role of each species in infecting larval ticks (reservoir competence, infection prevalence, abundance, tick burden, and feeding and moulting success) have illustrated a more complex pattern [92]. When white-footed mice are at high abundance they are the most important host infecting larval ticks, but when mice are at lower abundance, other species with substantial tick burdens and moderate to high reservoir competence, including shrews and chipmunks, are often responsible for infecting a larger fraction of larvae than mice [92,124,125]. These studies demonstrate the importance of collecting and integrating the full suite of variables needed to quantify the contribution of hosts in infecting vectors, and highlight the importance of host–vector contact [126]. In other systems, such as West Nile virus, the most important host species for infecting mosquitoes, the American robin, was often a very small fraction (less than 10%) of the host community, but was greatly overutilized by mosquitoes [119,120].
Figure 4.
Role of hosts in contributing to the population of infected nymphal ticks at sites in North America and Europe. The first column in each plot shows the fraction of ticks that fed on each host. The second column shows the relative reservoir competence (duration and probability of infecting feeding ticks) of each host (for the actual reservoir competence, see the electronic supplemental material), and the third column shows the estimated fraction of infected nymphal ticks arising from feeding on each species. See the electronic supplemental material for detailed data, calculations and sources. Map of tick distributions reprinted from [2].
In western North America, tree squirrels (Sciurus spp.) and woodrats (Neotoma spp.) are important for infecting ticks whereas some lizards (Sceloporus occidentalis and Elgaria spp.) are important in reducing infection prevalence because they feed a large fraction of larval ticks but do not infect them (figure 4) [93,94]. Interestingly, these lizard species actually lead to lower prevalence in adult ticks than nymphs through borreliacidal factors in their blood that clear infected nymphs of Borrelia that feed on them [127]. In other regions lizards (Eumeces spp.) are moderately competent reservoirs; they infected 24% of larval ticks feeding on them over the 30–40 days following infection [128], which is a higher level of reservoir competence than several squirrel species and many other mammals [92].
In Europe, the most important reservoir hosts differed among regions. Garden dormice (Eliomys quercinus) and edible dormice (Glis glis) were the most important reservoir hosts in sites in France and Germany, respectively [114]. By contrast, red squirrels (Sciurus vulgaris) appear to be important hosts in Switzerland [129–132] and shrews infected the largest fraction of larval ticks at a site over 2 years in Sweden [65,133] (figure 4).
These studies of host competence and tick burdens suggest that in areas or regions where squirrels (Sciurus spp.) are the most reservoir competent hosts feeding a significant portion of larval ticks (western North America and parts of Europe; figure 4) nymphal infection prevalence will be lower than in areas where host species that feed most larval ticks are more infectious (e.g. white-footed mice, chipmunks and shrews in northeast North America [92]). Further studies of the role of each species in infecting ticks would allow one to predict the importance of different taxa in different regions, and the consequences of changes in host community composition that often accompany land use change [92].
Although past studies have provided insight into which host species are important for infecting larval ticks at single sites, predicting local spatio-temporal patterns of nymphal infection prevalence requires consistent host utilization (and estimates of host utilizations), predictions or estimates of host community composition and abundance over space and time, and estimates of host competence and feeding/moulting success, as described above. Two studies have assembled this type of data. One showed that model-predicted prevalence explained approximately 25% of variation in observed prevalence across 26 forest fragments in the northeast USA and 45% of the temporal variation across one set of sites over 10 years [53]. In the other study, predicted prevalence explained 99% of the spatial variation in observed prevalence in one set of seven plots in 1 year, but a non-significant amount of variation in three other sets of seven plots [93]. These studies indicate that local-scale spatial variation in prevalence is only partly predictable with data on host–vector–pathogen interactions, but that temporal patterns appear to be more predictable. This suggests that collecting host–vector–pathogen interaction data from targeted hotspots could provide useful predictions of temporal variation in Lyme disease risk. In addition, spatial variation in prevalence at larger (regional) spatial scales is often much more consistent (e.g. nymphal infection prevalence is substantially lower in western than eastern North America [52,93,94,118]).
(b). The dilution effect hypothesis
Given how widespread and infectious white-footed mice are in the northeast USA, the presence of additional species that either feed larval ticks (and infect a smaller fraction of them), reduce the abundance of mice through competition or predation, or reduce the density of ticks through grooming, might lead to lower Borrelia infection prevalence in, or abundance of, nymphal ticks. This idea was called the ‘dilution effect’ [134,135] because these other species dilute the effect of white-footed mice in infecting ticks. This hypothesis was subsequently tested in a study using 37 forest fragments in the northeastern USA that quantified host community composition, and nymphal infection prevalence at each site [53]. Prevalence in nymphs increased, rather than decreased, with the richness of small mammal species, and was not significantly correlated with two other host species groups (large mammals or birds). In addition, as previously noted, observed prevalence was significantly correlated with an estimate of predicted prevalence that used data on species abundances, reservoir competence and tick burdens [53]. This study shows that variation in host competence influences nymphal infection prevalence, but prevalence was not reduced by higher host richness. This suggests that to predict nymphal infection prevalence, and to target control efforts (e.g. by manipulating or vaccinating hosts), it is much more useful to determine the identity and abundance of species and the role they play in feeding and infecting ticks, rather than how many host species are present at a site. In addition, it remains to be determined how nymph density varies with host composition and diversity.
Several studies have examined drivers of temporal or spatial variation in the density of individual key hosts for larval ticks (e.g. white-footed mice that infect many ticks and some lizards that feed many ticks but do not infect them) [34,121,136]. These data, when combined with data on host feeding utilization, infection prevalence and duration and magnitude of infectiousness, could be used to make predictions of nymphal infection prevalence across space or time [34,92,109]. However, there is enormous variation in abundance of most host species across different habitat types and over time, and host abundance is rarely measured or monitored in a systematic way. Thus, prediction of host abundance, even to an order of magnitude is difficult, in part due to the complex web of species interactions and variable environments that these species exist in, and also because of, our poor understanding of factors influencing abundance such as predator–prey relationships [11,66,121,137,138]. Despite this variation in host abundance, variation in tick infection prevalence is often relatively small [123,139], suggesting that exact predictions of host abundance may not be necessary to obtain reasonable estimates of nymphal infection prevalence. In fact, the relatively low variance in infection prevalence over time within some regions (e.g. twofold to fourfold) compared with variation in tick abundance (e.g. 10-fold or higher) has led some to suggest that both science and management should focus on tick abundance [33,72]. However, comparisons of the variation in nymphal prevalence and abundance with large datasets across landscape gradients have yet to be done, and could shed substantial insight on this question.
5. Lyme disease ecology in human-altered ecosystems
The current ecological dynamics of Lyme disease are likely very different than they were in past centuries. Clearing of forests and hunting is thought to have nearly eliminated deer from large regions in eastern North America and Europe in the eighteenth and nineteenth centuries [29,140]. During the same period many predators of deer in these regions were extirpated, including wolves (Canis lupus), bears (Ursus arctos in Europe, Ursus americanus in Eastern North America) and cougars (Puma concolor) in Eastern North America. Some predators of small mammals were also greatly reduced in abundance including both fishers (Pekania pennanti) and marten (Martes americana). In the twentieth century, habitat alteration, including agriculturalization and reforestation, probably led to increased ungulate abundance and altered small mammal communities [20,141,142]. Altered species interactions among predators such as wolves, coyotes and foxes [143], may have allowed deer and rodent populations to increase substantially, and reduction or elimination of species that might compete with rodents (and deer) for food (e.g. passenger pigeons eating acorns) may also have increased the abundance of important hosts for ticks and B. burgdorferi [11].
More recently, urbanization and habitat fragmentation have greatly altered Lyme disease ecology, but with mixed and uncertain effects on different aspects of transmission. Many studies have examined fragmentation and Lyme disease incidence at broad (e.g. USA county) scales and have found positive correlations [44,84,144–147]. However, the causes of these county-level correlations are difficult to determine because more local studies at the scale on which infection occurs have found mixed results or contradictory patterns between human incidence and the density of infected nymphs. For example, increased fragmentation, measured as patch size or isolation, increased both tick abundance and prevalence in some studies [44,148] but was uncorrelated with infection prevalence in others [53,149]. In another study, per cent forest cover was uncorrelated with human incidence, perhaps because forest cover was also uncorrelated with prevalence or abundance in nymphs [43].
Variation in human behaviour in different landscapes may partly explain these contradictory patterns. Although the density of infected nymphs is generally higher in forested areas than nearby edges [150], in the northeastern USA the majority of exposure to ticks occurs at forest edges near people's residences [39]. The importance of human behaviour was also evident in opposite correlations between measures of fragmentation on tick abundance and prevalence, and human incidence of Lyme disease at the township scale [44]. These results suggest that patterns of human disease incidence are best explained by simultaneously considering factors that influence the density of infected nymphal ticks and variation in human behaviour that brings people in contact with habitats where ticks reside (table 1) [25,39]. Otherwise, control efforts to reduce tick abundance may not actually reduce incidence [45]. An important detail in future studies is to determine the most probable location where patients became infected, rather than geographically assigning cases to their home address [151].
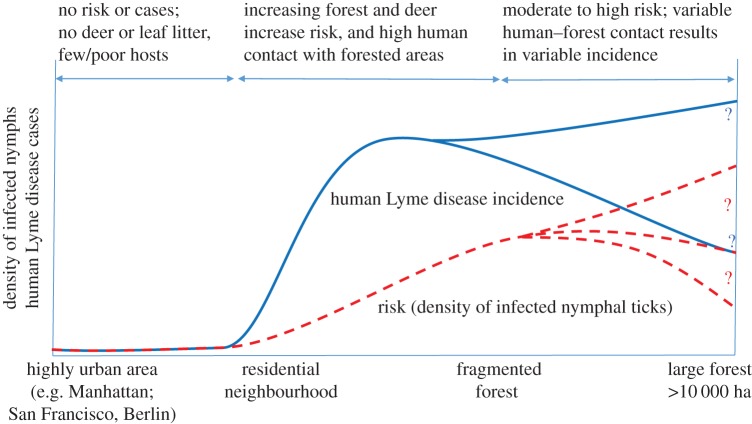
Integrating previous research suggests a suite of possible relationships between urbanization, Lyme disease risk and human incidence, and identifies areas of substantial uncertainty in the relationships between tick density, infection prevalence and human exposure to infection (figure 5). The absence of appreciable disease risk from highly urbanized areas is likely consistent across continents. By contrast, there is substantial variation and uncertainty in how the density of infected ticks changes between semi-urban fragmented forests and intact forests because multiple factors are operating, sometimes in opposite directions. Fragmentation alters the vertebrate host community as well as vegetation structure, leaf litter and microclimates for ticks. An increase in edge habitat with fragmentation will initially increase the diversity of vertebrate hosts for ticks and Borrelia (birds and mammals), but completely urbanized or agricultural landscapes have few (but sometimes highly abundant) hosts [152]. Although the impacts of fragmentation on broad-scale changes in host communities might be predicted based on trophic relationships [90,92], detailed predictions of how individual host species densities will change with fragmentation are not yet possible, despite their importance in Lyme disease ecology. For example, in some locations deer abundance is highest in partly fragmented landscapes [153,154] which might increase larval tick abundance, whereas leaf litter and favourable microclimates are highest in intact forests, which leads to higher tick survival. Similarly, mouse density (relative or absolute), which is sometimes correlated with nymphal infection prevalence [92], is highly variable and hard to predict. Although it is much lower in intact forests than in some small forest fragments, it is highly variable in small fragments, and it is uncorrelated with patch size in patches larger than 1 ha [155–157]. Further, in some forested areas, mouse density is higher in forests with higher rodent species diversity, in contrast with the predictions from fragmentation studies [158]. Thus, while the elimination of Lyme disease risk from highly urbanized areas relatively clear, much more work is needed to understand the effect of fragmentation on tick abundance and survival, host abundance and tick–host interactions in order to be able to predict Lyme disease risk in forested landscapes [21].
Figure 5.
Summary of patterns of Lyme disease risk (density of infected nymphal ticks) and human incidence across a fragmentation gradient from highly urban areas (far left) to large forested areas (right) based on the available literature (see text). The mechanisms underlying the trends in both risk and incidence are given above the graph. The two blue and three red curves ending with question marks illustrate the variability and uncertainty in the pattern of incidence and disease risk in large forested areas. (Online version in colour.)
6. Controlling Lyme disease
We have outlined research needed to identify and predict Lyme disease risk in the environment and to understand the mechanisms leading to high risk. This knowledge could be used to develop new control methods and to more effectively target control efforts using existing methods. Many techniques have been explored or proposed for controlling Lyme disease [25–28], including vaccination of people or mice [159–161], reduced deer abundance (through either hunting or predator reintroduction) to reduce tick abundance [63,64,162], reduced tick abundance on deer, mice or in the environment by treatment with acaricides [160,162–166], increased public education [160,167,168] and landscape alteration through reducing fragmentation [145], controlled burning [169,170], or vegetation and leaf litter removal [171,172]. It is worth noting that any strategy that reduces tick abundance will also decrease disease risk for the many other pathogens transmitted by these Ixodes ticks [28].
The comparative cost-effectiveness of the proposed strategies is not clear [25], and many strategies have adverse effects (e.g. mortality of non-target organisms) or are only effective in certain landscapes, or at certain spatial scales (e.g. individual properties) [162,173,174]. Given the dual importance of the density of infected ticks and human behaviour resulting in exposure to ticks [45,46], a combined approach is likely to be most effective [25]. A recent review assembled the efficacy of dozens of methods and experiments for reducing the density of nymphal ticks or infected nymphal ticks [26]. Although there is some variation from study to study, and regionally, promising strategies for reducing infected nymph density include treating small mammal hosts (via bait boxes or treated cotton) or deer (via ‘4-poster’ feeding stations) with chemical or biological agents, public education campaigns to increase personal protective behaviours, clearing vegetation or leaf litter near houses, and where landowners are willing, application of acaricides, including both moderately effective natural product-based options (e.g. entomopathogenic fungi) and more effective and longer lasting synthetic acaricides [26]. It is worth noting that it is primarily up to individuals to reduce Lyme disease risk through these approaches, which contrasts with mosquito control, which is usually guided by county or state health departments [27].
7. Key information gaps
The research summarized above points to a strong foundation of knowledge upon which to build. However, substantial uncertainty in seven critical aspects of Lyme disease ecology (table 1) prevent accurate quantitative predictions of disease risk in space and time and make targeting of species for either vaccination or manipulation through habitat alteration difficult. Filling these gaps would substantially improve our ability to predict the location and timing of hot spots of Lyme disease and to target control efforts at the most important parts of the transmission cycle. Given the differences in host communities, ticks, vegetation, climate and human behaviour across the vast range of this disease, there will be different drivers at both regional and local scales. Efficient prevention and control will thus require combining insights from general patterns gained from the richness of studies conducted across multiple continents with an understanding of the local ecology and human behaviour. This is a daunting challenge, but the public health impact of Lyme disease warrants the substantial effort needed by disease ecologists and epidemiologists.
Supplementary Material
Supplementary Material
Acknowledgements
We thank K. Lafferty and C. Woods who helped draft the questions (electronic supplementary material) that formed the basis for this paper.
Authors' contributions
A.M.K. drafted a questionnaire (electronic supplementary material) and invited 19 contributors from Europe and North America to respond. A.M.K. collated the responses and drafted a manuscript. All authors provided feedback on and revised multiple drafts of the manuscript.
Competing interests
We have no competing interests.
Funding
We acknowledge financial support from the NSF (grants nos EF-0914866, 0914376), NIH (grants 1R01AI090159, 5R01GM105246), the Bay Area Lyme Foundation and the US Geological Survey.
Disclaimer
Use of trade or product names does not imply support by the US Government.
References
- 1.CDC. 2016. Lyme disease. See http://www.cdc.gov/lyme/index.html (27 May 2016).
- 2.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379, 461–473. ( 10.1016/s0140-6736(11)60103-7) [DOI] [PubMed] [Google Scholar]
- 3.Mead PS. 2015. Epidemiology of Lyme disease. Infect. Dis. Clin. N. Am. 29, 187–210. ( 10.1016/j.idc.2015.02.010) [DOI] [PubMed] [Google Scholar]
- 4.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg. Infect. Dis. 21, 1625–1631. ( 10.3201/eid2109.150417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith R, O'Connell S, Palmer S. 2000. Lyme disease surveillance in England and Wales, 1986–1998. Emerg. Infect. Dis. 6, 404–407. ( 10.3201/eid0604.000416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennet L, Halling A, Berglund J. 2006. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur. J. Clin. Microbiol. Infect. Dis. 25, 426–432. ( 10.1007/s10096-006-0167-2) [DOI] [PubMed] [Google Scholar]
- 7.Ertel S-H, Nelson RS, Cartter ML. 2012. Effect of surveillance method on reported characteristics of Lyme disease, Connecticut, 1996–2007. Emerg. Infect. Dis. 18, 242–247. ( 10.3201/eid1802.101219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naleway AL, Belongia EA, Kazmierczak JJ, Greenlee RT, Davis JP. 2002. Lyme disease incidence in Wisconsin: a comparison of state-reported rates and rates from a population-based cohort. Am. J. Epidemiol. 155, 1120–1127. ( 10.1093/aje/155.12.1120) [DOI] [PubMed] [Google Scholar]
- 9.Orloski KA, Campbell GL, Genese CA, Beckley JW, Schriefer ME, Spitalny KC, Dennis DT. 1998. Emergence of Lyme disease in Hunterdon County, New Jersey, 1993: a case-control study of risk factors and evaluation of reporting patterns. Am. J. Epidemiol. 147, 391–397. ( 10.1093/oxfordjournals.aje.a009462) [DOI] [PubMed] [Google Scholar]
- 10.Barbour AG, Fish D. 1993. The biological and social phenomenon of Lyme disease. Science 260, 1610–1616. ( 10.1126/science.8503006) [DOI] [PubMed] [Google Scholar]
- 11.Levi T, Kilpatrick AM, Mangel M, Wilmers CC. 2012. Deer, predators, and the emergence of Lyme disease. Proc. Natl Acad. Sci. USA 109, 10 942–10 947. ( 10.1073/pnas.1204536109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownstein JS, Holford TR, Fish D. 2005. Effect of climate change on Lyme disease risk in North America. Ecohealth 2, 38–46. ( 10.1007/s10393-004-0139-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medlock JM, Leach SA. 2015. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 15, 721–730. ( 10.1016/s1473-3099(15)70091-5) [DOI] [PubMed] [Google Scholar]
- 14.Ogden NH. 2014. Lyme disease and climate change. In Climate change and global health (ed. Beard CB.), p. 328 Wallingford, UK: CABI. [Google Scholar]
- 15.Pritt BS, et al. 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect. Dis. 16, 556–564. ( 10.1016/s1473-3099(15)00464-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, Barbour A, Kurtenbach K, Fish D. 2009. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc. Natl Acad. Sci. USA 106, 15 013–15 018. ( 10.1073/pnas.0903810106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margos G, et al. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl Acad. Sci. USA 105, 8730–8735. ( 10.1073/pnas.0800323105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. ( 10.1126/science.7043737) [DOI] [PubMed] [Google Scholar]
- 19.Matuschka FR, Spielman A. 1986. The emergence of Lyme disease in a changing environment in North America and central Europe. Exp. Appl. Acarol. 2, 337–353. ( 10.1007/bf01193900) [DOI] [PubMed] [Google Scholar]
- 20.Alverson WS, Waller DM, Solheim SL. 1988. Forests too deer: edge effects in northern Wisconsin. Conserv. Biol. 2, 348–358. ( 10.1111/j.1523-1739.1988.tb00199.x) [DOI] [Google Scholar]
- 21.Wood CL, Lafferty KD. 2013. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol. 28, 239–247. ( 10.1016/j.tree.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 22.Walter KS, Pepin KM, Webb CT, Gaff HD, Krause PJ, Pitzer VE, Diuk-Wasser MA. 2016. Invasion of two tick-borne diseases across New England: harnessing human surveillance data to capture underlying ecological invasion processes. Proc. R. Soc. B 283, 20160834 ( 10.1098/rspb.2016.0834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisen RJ, Eisen L, Beard CB. 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 53, 349–386. ( 10.1093/jme/tjv237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kugeler KJ, Farley GM, Forrester JD, Mead PS. 2015. Geographic distribution and expansion of human Lyme disease, United States. Emerg. Infect. Dis. 21, 1455–1457. ( 10.3201/eid2108.141878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisen RJ, Piesman J, Zielinski-Gutierrez E, Eisen L. 2012. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J. Med. Entomol. 49, 11–22. ( 10.1603/me11138) [DOI] [PubMed] [Google Scholar]
- 26.Eisen L, Dolan MC. 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 53, 1063–1092. ( 10.1093/jme/tjw103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piesman J. 2006. Strategies for reducing the risk of Lyme borreliosis in North America. Int. J. Med. Microbiol. 296, 17–22. ( 10.1016/j.ijmm.200.11.007) [DOI] [PubMed] [Google Scholar]
- 28.Piesman J, Eisen L. 2008. Prevention of tick-borne diseases. Annu. Rev. Entomol. 53, 323–343. ( 10.1146/annurev.ento.53.103106.093429) [DOI] [PubMed] [Google Scholar]
- 29.Spielman A, Wilson ML, Levine JF, Piesman J. 1985. Ecology of Ixodes dammini-borne human babesiosis and lyme disease. Annu. Rev. Entomol. 30, 439–460. ( 10.1146/annurev.ento.30.1.439) [DOI] [PubMed] [Google Scholar]
- 30.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129, S191–S220. ( 10.1017/s0031182003004694) [DOI] [PubMed] [Google Scholar]
- 31.Ostfeld RS. 2010. Lyme disease: the ecology of a complex system. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Millins C, Gilbert L, Medlock J, Hansford K, Thompson DBA, Biek R. 2017. Effects of conservation management of landscapes and vertebrate communities on Lyme borreliosis risk in the United Kingdom. Phil. Trans. R. Soc. B 372, 20160123 ( 10.1098/rstb.2016.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randolph SE, Dobson ADM. 2012. Pangloss revisitied: a critique of the dilution effect and the biodiversity-buffers-infection paradigm. Parasitology 139, 847–863. ( 10.1017/S0031182012000200) [DOI] [PubMed] [Google Scholar]
- 34.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 4, 1058–1068. ( 10.1371/journal.pbio.0040145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, Tsao JI. 2015. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS ONE 10, e0127450 ( 10.1371/journal.pone.0127450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginsberg HS, Rulison EL, Azevedo A, Pang GC, Kuczaj IM, Tsao JI, LeBrun RA. 2014. Comparison of survival patterns of northern and southern genotypes of the North American tick Ixodes scapularis (Acari: Ixodidae) under northern and southern conditions. Paras. Vec. 7, 394 ( 10.1186/1756-3305-7-394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter D, Debski A, Hubalek Z, Matuschka F-R. 2012. Absence of Lyme disease spirochetes in larval ixodes ricinus ticks. Vector-Borne Zoonotic Dis. 12, 21–27. ( 10.1089/vbz.2011.0668) [DOI] [PubMed] [Google Scholar]
- 38.Rollend L, Fish D, Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks and Tick-Borne Diseases 4, 46–51. ( 10.1016/j.ttbdis.2012.06.008) [DOI] [PubMed] [Google Scholar]
- 39.Eisen L, Eisen RJ. 2016. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J. Med. Entomol. 7, 1230–1235. ( 10.1093/jme/tjw092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rulison EL, Kuczaj I, Pang G, Hickling GJ, Tsao JI, Ginsberg HS. 2013. Flagging versus dragging as sampling methods for nymphal Ixodes scapularis (Acari: Ixodidae). J. Vector Ecol. 38, 163–167. ( 10.1111/j.1948-7134.2013.12022.x) [DOI] [PubMed] [Google Scholar]
- 41.Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. 2012. Geographic variation in the relationship between human lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg. 86, 1062–1071. ( 10.4269/ajtmh.2012.11-0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J. Clin. Microbiol. 36, 1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mather TN, Nicholson MC, Donnelly EF, Matyas BT. 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 144, 1066–1069. ( 10.1093/oxfordjournals.aje.a008879) [DOI] [PubMed] [Google Scholar]
- 44.Brownstein JS, Skelly DK, Holford TR, Fish D. 2005. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 146, 469–475. ( 10.1007/s00442-005-0251-9) [DOI] [PubMed] [Google Scholar]
- 45.Hinckley A, et al. 2016. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J. Infect. Dis. 214, 182–188. ( 10.1093/infdis/jiv775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, O'Keefe CF, Diuk-Wasser MA. 2014. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS ONE 9, e0084758 ( 10.1371/journal.pone.0084758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobson ADM. 2013. Ticks in the wrong boxes: assessing error in blanket-drag studies due to occasional sampling. Paras. Vec. 6, 347 ( 10.1186/1756-3305-6-347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strle K, Jones KL, Drouin EE, Li X, Steere AC. 2011. Borrelia burgdorferi RST1 (OspC Type A) genotype is associated with greater inflammation and more severe Lyme disease. Am. J. Pathol. 178, 2726–2739. ( 10.1016/j.ajpath.2011.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogden NH, Feil EJ, Leighton PA, Lindsay LR, Margos G, Mechai S, Michel P, Moriarty TJ. 2015. Evolutionary aspects of emerging Lyme disease in Canada. Appl. Environ. Microbiol. 81, 7350–7359. ( 10.1128/aem.01671-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH Jr. 2011. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2, 123–128. ( 10.1016/j.ttbdis.2011.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girard YA, Travinsky B, Schotthoefer A, Fedorova N, Eisen RJ, Eisen L, Barbour AG, Lane RS. 2009. Population structure of the Lyme borreliosis spirochete Borrelia burgdorferi in the Western black-legged tick (Ixodes pacificus) in Northern California. Appl. Environ. Microbiol. 75, 7243–7252. ( 10.1128/aem.01704-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diuk-Wasser MA, et al. 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in Eastern United States. Am. J. Trop. Med. Hyg. 86, 320–327. ( 10.4269/ajtmh.2012.11-0395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logiudice K, Duerr STK, Newhouse MJ, Schmidt KA, Killilea ME, Ostfeld RS. 2008. Impact of host community composition on Lyme disease risk. Ecology 89, 2841–2849. ( 10.1890/07-1047.1) [DOI] [PubMed] [Google Scholar]
- 54.Telford SR, Mather TN, Moore SI, Wilson ML, Spielman A. 1988. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 39, 105–109. [DOI] [PubMed] [Google Scholar]
- 55.Jaenson TGT, Talleklint L. 1996. Lyme borreliosis spirochetes in Ixodes ricinus (Acari: Ixodidae) and the varying hare on isolated islands in the Baltic Sea. J. Med. Entomol. 33, 339–343. ( 10.1093/jmedent/33.3.339) [DOI] [PubMed] [Google Scholar]
- 56.Junttila J, Peltomaa M, Soini H, Marjamaki M, Viljanen MK. 1999. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J. Clin. Microbiol. 37, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson ML, Levine JF, Spielman A. 1984. Effect of deer reduction on abundance of the deer tick (Ixodes dammini). Yale J. Biol. Med. 57, 697–705. [PMC free article] [PubMed] [Google Scholar]
- 58.Dizij A, Kurtenbach K. 1995. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Ixodes ricinus L, the main European vector of Borrelia burgdorferi. Paras. Immunol. 17, 177–183. ( 10.1111/j.1365-3024.1995.tb00887.x) [DOI] [PubMed] [Google Scholar]
- 59.Levin ML, Fish D. 1998. Density-dependent factors regulating feeding success of Ixodes scapularis larvae (Acari: Ixodidae). J. Parasitol. 84, 36–43. ( 10.2307/3284526) [DOI] [PubMed] [Google Scholar]
- 60.Hazler KR, Ostfeld RS. 1995. Larval density and feeding success of Ixodes scapularis on two species of Peromyscus. J. Parasitol. 81, 870–875. ( 10.2307/3284032) [DOI] [PubMed] [Google Scholar]
- 61.Ogden NH, Casey ANJ, French NP, Adams JDW, Woldehiwet Z. 2002. Field evidence for density-dependent facilitation amongst Ixodes ricinus ticks feeding on sheep. Parasitology 124, 117–125. ( 10.1017/s0031182001001081) [DOI] [PubMed] [Google Scholar]
- 62.Slowik TJ, Lane RS. 2009. Feeding preferences of the immature stages of three western North American Ixodid ticks (Acari) for avian, reptilian, or rodent hosts. J. Med. Entomol. 46, 115–122. ( 10.1603/033.046.0115) [DOI] [PubMed] [Google Scholar]
- 63.Stafford KC, Denicola AJ, Kilpatrick HJ. 2003. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) with reduction of white-tailed deer. J. Med. Entomol. 40, 642–652. ( 10.1603/0022-2585-40.5.642) [DOI] [PubMed] [Google Scholar]
- 64.Stafford KC, Williams SC. 2014. Deer, ticks, and Lyme disease. New Haven, CT: The Connecticut Agricultural Experiment Station. [Google Scholar]
- 65.Talleklint L, Jaenson TGT. 1994. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari: Ixodidae), in Sweden. J. Med. Entomol. 31, 880–886. ( 10.1093/jmedent/31.6.880) [DOI] [PubMed] [Google Scholar]
- 66.Levi T, Keesing F, Holt RD, Barfield M, Ostfeld RS. 2016. Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecol. Appl. 26, 484–498. ( 10.1890/15-0122.1) [DOI] [PubMed] [Google Scholar]
- 67.Ginsberg HS, Zhioua E. 1999. Influence of deer abundance on the abundance of questing adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 36, 376–381. ( 10.1093/jmedent/36.3.376) [DOI] [PubMed] [Google Scholar]
- 68.Dobson ADM, Finnie TJR, Randolph SE. 2011. A modified matrix model to describe the seasonal population ecology of the European tick Ixodes ricinus. J. Appl. Ecol. 48, 1017–1028. ( 10.1111/j.1365-2664.2011.02003.x) [DOI] [Google Scholar]
- 69.Ogden NH, et al. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 35, 375–389. ( 10.1016/j.ijpara.2004.12.013) [DOI] [PubMed] [Google Scholar]
- 70.Berger KA, Ginsberg HS, Dugas KD, Hamel LH, Mather TN. 2014. Adverse moisture events predict seasonal abundance of Lyme disease vector ticks (Ixodes scapularis). Paras. Vec. 7, 181 ( 10.1186/1756-3305-7-181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunner JL, Cheney L, Keesing F, Killilea M, Logiudice K, Previtali A, Ostfeld RS. 2011. Molting success of Ixodes scapularis varies among individual blood meal hosts and species. J. Med. Entomol. 48, 860–866. ( 10.1603/me10256) [DOI] [PubMed] [Google Scholar]
- 72.Randolph SE. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129, S37–S65. ( 10.1017/S0031182004004925) [DOI] [PubMed] [Google Scholar]
- 73.Eisen RJ, Eisen L, Ogden NH, Beard CB. 2016. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol. 53, 250–261. ( 10.1093/jme/tjv199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ostfeld RS, Brunner JL. 2015. Climate change and Ixodes tick-borne diseases of humans. Phil. Trans. R. Soc. B 370, 20140051 ( 10.1098/rstb.2014.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O'Callaghan CJ, Waltner-Toews D, Barker IK. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 41, 622–633. ( 10.1603/0022-2585-41.4.622) [DOI] [PubMed] [Google Scholar]
- 76.Campbell JA. 1948. The life history and development of the sheep tick Ixodes ricinus Linaeus in Scotland, under natural and controlled conditions. PhD thesis, University of Edinburgh, UK. [Google Scholar]
- 77.Rodgers SE, Zolnik CP, Mather TN. 2007. Duration of exposure to suboptimal atmospheric moisture affects nymphal blacklegged tick survival. J. Med. Entomol. 44, 372–375. ( 10.1603/0022-2585%282007%2944%5B372%3Adoetsa%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 78.Perret JL, Rais O, Gern L. 2004. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J. Med. Entomol. 41, 361–365. ( 10.1603/0022-2585-41.3.361) [DOI] [PubMed] [Google Scholar]
- 79.Randolph SE. 1994. Density-dependent acquired resistance to ticks in natural hosts, independent of concurrent infection with Babesia microti. Parasitology 108, 413–419. ( 10.1017/S003118200007596X) [DOI] [PubMed] [Google Scholar]
- 80.Diuk-Wasser MA, et al. 2010. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob. Ecol. Biogeogr. 19, 504–514. ( 10.1111/j.1466-8238.2010.00526.x) [DOI] [Google Scholar]
- 81.Padgett KA, Lane RS. 2001. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J. Med. Entomol. 38, 684–693. ( 10.1603/0022-2585-38.5.684) [DOI] [PubMed] [Google Scholar]
- 82.Lindsay LR, Mathison SW, Barker IK, McEwen SA, Gillespie TJ, Surgeoner GA. 1999. Microclimate and habitat in relation to Ixodes scapularis (Acari: Ixodidae) populations on Long Point, Ontario, Canada. J. Med. Entomol. 36, 255–262. ( 10.1093/jmedent/36.3.255) [DOI] [PubMed] [Google Scholar]
- 83.Dobson ADM, Auld S. 2016. Epidemiological implications of host biodiversity and vector biology: key insights from simple models. Am. Nat. 187, 405–422. ( 10.1086/685445) [DOI] [PubMed] [Google Scholar]
- 84.Tran PM, Waller L. 2013. Effects of landscape fragmentation and climate on Lyme disease incidence in the Northeastern United States. Ecohealth 10, 394–404. ( 10.1007/s10393-013-0890-y) [DOI] [PubMed] [Google Scholar]
- 85.Brownstein JS, Holford TR, Fish D. 2003. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 111, 1152–1157. ( 10.1289/ehp.6052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schauber EM, Ostfeld RS, Evans AS. 2005. What is the best predictor of annual Lyme disease incidence: weather, mice, or acorns? Ecol. Appl. 15, 575–586. ( 10.1890/03-5370) [DOI] [Google Scholar]
- 87.Subak S. 2003. Effects of climate on variability in Lyme disease incidence in the northeastern United States. Am. J. Epidemiol. 157, 531–538. ( 10.1093/aje/kwg014) [DOI] [PubMed] [Google Scholar]
- 88.Ogden NH, et al. 2006. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 36, 63–70. ( 10.1016/j.ijpara.2005.08.016) [DOI] [PubMed] [Google Scholar]
- 89.Ogden NH, et al. 2008. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr. 7, 24 ( 10.1186/1476-072x-7-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simon JA, et al. 2014. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 7, 750–764. ( 10.1111/eva.12165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levi T, Keesing F, Oggenfuss K, Ostfeld RS. 2015. Accelerated phenology of blacklegged ticks under climate warming. Phil. Trans. R. Soc. B 370, 20130556 ( 10.1098/rstb.2013.0556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567–571. ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swei A, Briggs CJ, Lane RS, Ostfeld RS. 2012. Impacts of an introduced forest pathogen on the risk of Lyme disease in California. Vector Borne Zoonotic Dis. 12, 623–632. ( 10.1089/vbz.2011.0783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salkeld D, Lane R. 2010. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology 91, 293–298. ( 10.1890/08-2106.1) [DOI] [PubMed] [Google Scholar]
- 95.Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H, Ostfeld RS. 2009. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B 276, 3911–3919. ( 10.1098/rspb.2009.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hughes VL, Randolph SE. 2001. Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: a force for aggregated distributions of parasites. J. Parasitol. 87, 49–54. ( 10.1645/0022-3395%282001%29087%5B0049%3Atdiaar%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 97.Randolph SE. 1979. Population regulation in ticks: the role of acquired resistance in natural and unnatural hosts. Parasitology 79, 141–156. ( 10.1017/S0031182000052033) [DOI] [PubMed] [Google Scholar]
- 98.Goodwin BJ, Ostfeld RS, Schauber EM. 2001. Spatiotemporal variation in a Lyme disease host and vector: black-legged ticks on white-footed mice. Vector-Borne Zoonotic Dis. 1, 129–138. ( 10.1089/153036601316977732) [DOI] [PubMed] [Google Scholar]
- 99.Dobson ADM, Randolph SE. 2011. Modelling the effects of recent changes in climate, host density and acaricide treatments on population dynamics of Ixodes ricinus in the UK. J. Appl. Ecol. 48, 1029–1037. ( 10.1111/j.1365-2664.2011.02004.x) [DOI] [Google Scholar]
- 100.Richter D, Spielman A, Komar N, Matuschka FR. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6, 133–138. ( 10.3201/eid0602.000205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donahue JG, Piesman J, Spielman A. 1987. Reservoir competence of white-footed mice for Lyme disease. Am. J. Trop. Med. Hyg. 36, 92–96. [DOI] [PubMed] [Google Scholar]
- 102.Ginsberg HS, Buckley PA, Balmforth MG, Zhioua E, Mitra S, Buckley FG. 2005. Reservoir competence of native North American birds for the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 42, 445–449. ( 10.1603/0022-2585%282005%29042%5B0445%3Arconna%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 103.Mather TN, Wilson ML, Moore SI, Ribeiro JMC, Spielman A. 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130, 143–150. ( 10.1093/oxfordjournals.aje.a115306) [DOI] [PubMed] [Google Scholar]
- 104.Brisson D, Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168, 713–722. ( 10.1534/genetics.104.028738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brunner JL, LoGiudice K, Ostfeld RS. 2008. Estimating reservoir competence of Borrelia burgdorferi hosts: prevalence and infectivity, sensitivity, and specificity. J. Med. Entomol. 45, 139–147. ( 10.1093/jmedent/45.1.139) [DOI] [PubMed] [Google Scholar]
- 106.Schauber EM, Ostfeld RS. 2002. Modeling the effects of reservoir competence decay and demographic turnover in Lyme disease ecology. Ecol. Appl. 12, 1142–1162. ( 10.1890/1051-0761%282002%29012%5B1142%3AMTEORC%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 107.Burgdorfer W, Schwan TG. 1991. Lyme borreliosis: a relapsing fever-like disease? Scand. J. Infect. Dis. 77, 17–22. [PubMed] [Google Scholar]
- 108.Hanincova K, Ogden NH, Diuk-Wasser M, Pappas CJ, Iyer R, Fish D, Schwartz I, Kurtenbach K. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74, 153–157. ( 10.1128/aem.01567-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ogden NH, Bigras-Poulin M, O'Callaghan CJ, Barker IK, Kurtenbach K, Lindsay LR, Charron DF. 2007. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology 134, 209–227. ( 10.1017/s0031182006001417) [DOI] [PubMed] [Google Scholar]
- 110.Hanincova K, Schafer SM, Etti S, Sewell HS, Taragelova V, Ziak D, Labuda M, Kurtenbach K. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126, 11–20. ( 10.1017/s0031182002002548) [DOI] [PubMed] [Google Scholar]
- 111.Hanincova K, Taragelova V, Koci J, Schafer SM, Hails R, Ullmann AJ, Piesman J, Labuda M, Kurtenbach K. 2003. Association of Borrelia garinii and B-valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69, 2825–2830. ( 10.1128/aem.69.5.2825-2830.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 12, 604–611. ( 10.3201/eid1204.051016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casher L, Lane R, Barrett R, Eisen L. 2002. Relative importance of lizards and mammals as hosts for ixodid ticks in northern California. Exp. Appl. Acarol. 26, 127–143. ( 10.1023/a:1020911306291) [DOI] [PubMed] [Google Scholar]
- 114.Richter D, Matuschka F-R. 2012. Differential contribution of various dormice to the natural transmission cycle of Lyme disease spirochetes in Central Europe. Peckiana 8, 235–244. [Google Scholar]
- 115.Estrada-Pena A, Gray JS, Kahl O, Lane RS, Nijhof AM. 2013. Research on the ecology of ticks and tick-borne pathogens—methodological principles and caveats. Front. Cell. Infect. Microbiol. 3, 29 ( 10.3389/fcimb.2013.00029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brunner JL, Ostfeld RS. 2008. Multiple causes of variable tick burdens on small-mammal hosts. Ecology 89, 2259–2272. ( 10.1890/07-0665.1) [DOI] [PubMed] [Google Scholar]
- 117.Eisen RJ, Eisen L, Lane RS. 2001. Prevalence and abundance of Ixodes pacificus immatures (Acari: Ixodidae) infesting western fence lizards (Sceloporus occidentalis) in northern California: temporal trends and environmental correlates. J. Parasitol. 87, 1301–1307. ( 10.1645/0022-3395%282001%29087%5B1301%3Apaaoip%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 118.Swei A, Ostfeld RS, Lane RS, Briggs CJ. 2011. Impact of the experimental removal of lizards on Lyme disease risk. Proc. R. Soc. B 278, 2970–2978. ( 10.1098/rspb.2010.2402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 273, 2327–2333. ( 10.1098/rspb.2006.3575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kilpatrick AM. 2011. Globalization, land use, and the invasion of West Nile virus. Science 334, 323–327. ( 10.1126/science.1201010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones CG, Ostfeld RS, Richard MP, Schauber EM, Wolff JO. 1998. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 279, 1023–1026. ( 10.1126/science.279.5353.1023) [DOI] [PubMed] [Google Scholar]
- 122.Ostfeld RS, Jones CG, Wolff JO. 1996. Of mice and mast. Bioscience 46, 323–330. ( 10.2307/1312946) [DOI] [Google Scholar]
- 123.Fish D. 1995. Environmental risk and prevention of Lyme disease. Am. J. Med. 98, S2–S9. ( 10.1016/s0002-9343(99)80038-2) [DOI] [PubMed] [Google Scholar]
- 124.Brisson D, Dykhuizen DE, Ostfeld RS. 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. R. Soc. B 275, 227–235. ( 10.1098/rspb.2007.1208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schulze TL, Jordan RA, Schulze CJ. 2005. Host associations of Ixodes scapularis (Acari: ixodidae) in residential and natural settings in a Lyme disease-endemic area in New Jersey. J. Med. Entomol. 42, 966–973. ( 10.1603/0022-2585%282005%29042%5B0966%3Ahaoisa%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 126.Kilpatrick AM, Salkeld DJ, Titcomb G, Hahn MB. 2017. Conservation of biodiversity as a strategy for improving human health and well-being. Phil. Trans. R. Soc. B 372, 20160131 ( 10.1098/rstb.2016.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lane RS, Quistad GB. 1998. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol. 84, 29–34. ( 10.2307/3284524) [DOI] [PubMed] [Google Scholar]
- 128.Levin M, Levine JF, Yang S, Howard P, Apperson CS. 1996. Reservoir competence of the southeastern five-lined skink (Eumeces inexpectatus) and the green anole (Anolis carolinensis) for Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 54, 92–97. [DOI] [PubMed] [Google Scholar]
- 129.Cadenas FM, Rais O, Humair P-F, Douet V, Moret J. 2007. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J. Med. Entomol. 44, 1109–1117. ( 10.1093/jmedent/44.6.1109) [DOI] [PubMed] [Google Scholar]
- 130.Richter D, Schlee DB, Matuschka FR. 2011. Reservoir competence of various rodents for the Lyme disease spirochete Borrelia spielmanii. Appl. Environ. Microbiol. 77, 3565–3570. ( 10.1128/aem.00022-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matuschka FR, Fischer P, Heiler M, Richter D, Spielman A. 1992. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J. Infect. Dis. 165, 479–483. ( 10.1093/infdis/165.3.479) [DOI] [PubMed] [Google Scholar]
- 132.Gern L et al. 1998. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentbl. Bakteriol. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 287, 196–204. [DOI] [PubMed] [Google Scholar]
- 133.Talleklint L, Jaenson TGT. 1997. Infestation of mammals by Ixodes ricinus ticks (Acari: Ixodidae) in south-central Sweden. Exp. Appl. Acarol. 21, 755–771. ( 10.1023/a:1018473122070) [DOI] [PubMed] [Google Scholar]
- 134.Ostfeld R, Keesing F. 2000. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. ( 10.1139/z00-172) [DOI] [Google Scholar]
- 135.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 136.Swei A, Ostfeld RS, Lane RS, Briggs CJ. 2011. Effects of an invasive forest pathogen on abundance of ticks and their vertebrate hosts in a California Lyme disease focus. Oecologia 166, 91–100. ( 10.1007/s00442-010-1796-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brunner JL, Duerr S, Keesing F, Killilea M, Vuong H, Ostfeld RS. 2013. An experimental test of competition among mice, chipmunks, and squirrels in deciduous forest fragments. PLoS ONE 8, e0066798 ( 10.1371/journal.pone.0066798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levi T, Barfield M, Holt RD, Kilpatrick AM, Mangel M, Wilmers CC. 2015. Threshold levels of generalist predation determine consumer response to resource pulses. Oikos 124, 1436–1443. ( 10.1111/oik.01487) [DOI] [Google Scholar]
- 139.Ostfeld RS, Glass GE, Keesing F. 2005. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol. Evol. 20, 328–336. ( 10.1016/j.tree.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 140.Andersen R, Duncan P, Linnell JDC. 1998. The European roe deer: the biology of success. Oslo, Norway: Scandinavian University Press. [Google Scholar]
- 141.Hargis CD, Bissonette JA, Turner DL. 1999. The influence of forest fragmentation and landscape pattern on American martens. J. Appl. Ecol. 36, 157–172. ( 10.1046/j.1365-2664.1999.00377.x) [DOI] [Google Scholar]
- 142.Payer DC, Harrison DJ. 2003. Influence of forest structure on habitat use by American marten in an industrial forest. For. Ecol. Manage. 179, 145–156. ( 10.1016/s0378-1127(02)00517-0) [DOI] [Google Scholar]
- 143.Levi T, Wilmers CC. 2012. Wolves-coyotes-foxes: a cascade among carnivores. Ecology 93, 921–929. ( 10.1890/11-0165.1) [DOI] [PubMed] [Google Scholar]
- 144.Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. 1991. Landscape ecology of lyme-disease in a residential area of Westchester-County, New York. Am. J. Epidemiol. 133, 1105–1113. ( 10.1093/oxfordjournals.aje.a115823) [DOI] [PubMed] [Google Scholar]
- 145.Jackson LE, Hilborn ED, Thomas JC. 2006. Towards landscape design guidelines for reducing Lyme disease risk. Int. J. Epidemiol. 35, 315–322. ( 10.1093/ije/dyi284) [DOI] [PubMed] [Google Scholar]
- 146.Seukep SE, Kolivras KN, Hong Y, Li J, Prisley SP, Campbell JB, Gaines DN, Dymond RL. 2015. An examination of the demographic and environmental variables correlated with Lyme disease emergence in Virginia. Ecohealth 12, 634–644. ( 10.1007/s10393-015-1034-3) [DOI] [PubMed] [Google Scholar]
- 147.Eisen RJ, Lane RS, Fritz CL, Eisen L. 2006. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. Am. J. Trop. Med. Hyg. 75, 669–676. [PubMed] [Google Scholar]
- 148.Allan BF, Keesing F, Ostfeld RS. 2003. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 17, 267–272. ( 10.1046/j.1523-1739.2003.01260.x) [DOI] [Google Scholar]
- 149.Zolnik CP, Falco RC, Kolokotronis S-O, Daniels TJ. 2015. No observed effect of landscape fragmentation on pathogen infection prevalence in blacklegged ticks (Ixodes scapularis) in the Northeastern United States. PLoS ONE 10, e0139473 ( 10.1371/journal.pone.0139473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Horobik V, Keesing F, Ostfeld RS. 2006. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest-field edges. Ecohealth 3, 262–268. ( 10.1007/s10393-006-0065-1) [DOI] [Google Scholar]
- 151.Connally NP, Ginsberg HS, Mather TN. 2006. Assessing peridomestic entomological factors as predictors for Lyme disease. J. Vector Ecol. 31, 364–370. ( 10.3376/1081-1710%282006%2931%5B364%3Aapefap%5D2.0.co%3B2) [DOI] [PubMed] [Google Scholar]
- 152.Tews J, Brose U, Grimm V, Tielborger K, Wichmann MC, Schwager M, Jeltsch F. 2004. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31, 79–92. ( 10.1046/j.0305-0270.2003.00994.x) [DOI] [Google Scholar]
- 153.Masse A, Cote SD. 2009. Habitat selection of a large herbivore at high density and without predation: trade-off between forage and cover? J. Mammal. 90, 961–970. ( 10.1644/08-MAMM-A-148.1) [DOI] [Google Scholar]
- 154.Yahner RH. 1988. Changes in wildlife communities near edges. Conserv. Biol. 2, 333–339. ( 10.1111/j.1523-1739.1988.tb00197.x) [DOI] [Google Scholar]
- 155.Nupp TE, Swihart RK. 1996. Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can. J. Zool. 74, 467–472. ( 10.1139/z96-054) [DOI] [Google Scholar]
- 156.Nupp TE, Swihart RK. 1998. Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. J. Mammal. 79, 1234–1243. ( 10.2307/1383014) [DOI] [Google Scholar]
- 157.Krohne DT, Hoch GA. 1999. Demography of Peromyscus leucopus populations on habitat patches: the role of dispersal. Can. J. Zool. Rev. Can. Zool. 77, 1247–1253. ( 10.1139/cjz-77-8-1247) [DOI] [Google Scholar]
- 158.Bouchard C, Beauchamp G, Leighton PA, Lindsay R, Belanger D, Ogden NH. 2013. Does high biodiversity reduce the risk of Lyme disease invasion? Paras. Vec. 6, 195 ( 10.1186/1756-3305-6-195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl Acad. Sci. USA 101, 18 159–18 164. ( 10.1073/pnas.0405763102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen AK, Mead PS, Beard CB. 2011. The Lyme disease vaccine—a public health perspective. Clin. Infect. Dis. 52, S247–S252. ( 10.1093/cid/ciq115) [DOI] [PubMed] [Google Scholar]
- 161.Richer LM, Brisson D, Melo R, Ostfeld RS, Zeidner N, Gomes-Solecki M. 2014. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J. Infect. Dis. 209, 1972–1980. ( 10.1093/infdis/jiu005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ginsberg HS. 2014. Tick control: trapping, biocontrol, host management and other alternative strategies. In Biology of ticks, 2nd edn (eds Sonenshine DE, Roe RM), pp. 409–444. New York, NY: Oxford University Press. [Google Scholar]
- 163.Brei B, et al. 2009. Evaluation of the United States Department of Agriculture Northeast area-wide tick control project by meta-analysis. Vector-Borne Zoonotic Dis. 9, 423–430. ( 10.1089/vbz.2008.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hoen AG, et al. 2009. Effects of tick control by acaricide self-treatment of white-tailed deer on host-seeking tick infection prevalence and entomologic risk for Ixodes scapularis-borne pathogens. Vector-Borne Zoonotic Dis. 9, 431–438. ( 10.1089/vbz.2008.0155) [DOI] [PubMed] [Google Scholar]
- 165.Dolan MC, Maupin GO, Schneider BS, Denatale C, Hamon N, Cole C, Zeidner NS, Stafford KC. 2004. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J. Med. Entomol. 41, 1043–1054. ( 10.1603/0022-2585-41.6.1043) [DOI] [PubMed] [Google Scholar]
- 166.Ostfeld RS, Price A, Hornbostel VL, Benjamin MA, Keesing F. 2006. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience 56, 383–394. ( 10.1641/0006-3568%282006%29056%5B0383%3ACTATZW%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 167.Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, Heimer R. 2009. Peridomestic Lyme disease prevention results of a population-based case-control study. Am. J. Prev. Med. 37, 201–206. ( 10.1016/j.amepre.2009.04.026) [DOI] [PubMed] [Google Scholar]
- 168.Daltroy LH, Phillips C. 2007. A controlled trial of a novel primary prevention program for Lyme disease and other tick-borne illnesses. Health Educ. Behav. 34, 531–542. ( 10.1177/1090198106294646) [DOI] [PubMed] [Google Scholar]
- 169.Gleim ER, Conner LM, Berghaus RD, Levin ML, Zemtsova GE, Yabsley MJ. 2014. The phenology of ticks and the effects of long-term prescribed burning on tick population dynamics in Southwestern Georgia and Northwestern Florida. PLoS ONE 9, e0112174 ( 10.1371/journal.pone.0112174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Padgett KA, Casher LE, Stephens SL, Lane RS. 2009. Effect of prescribed fire for tick control in California Chaparral. J. Med. Entomol. 46, 1138–1145. ( 10.1603/033.046.0522) [DOI] [PubMed] [Google Scholar]
- 171.Williams SC, Ward JS, Worthley TE, Stafford KC. 2009. Managing Japanese Barberry (Ranunculales: Berberidaceae) infestations reduces blacklegged tick (Acari: Ixodidae) abundance and infection prevalence with Borrelia burgdorferi (Spirochaetales: Spirochaetaceae). Environ. Entomol. 38, 977–984. ( 10.1603/022.038.0404) [DOI] [PubMed] [Google Scholar]
- 172.Schulze TL, Jordan RA, Hung RW. 1995. Suppression of subadult Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. J. Med. Entomol. 32, 730–733. ( 10.1093/jmedent/32.5.730) [DOI] [PubMed] [Google Scholar]
- 173.Hayes EB, Piesman J. 2003. Current concepts—how can we prevent Lyme disease? N. Engl. J. Med. 348, 2424–2430. ( 10.1056/NEJMra021397) [DOI] [PubMed] [Google Scholar]
- 174.Talleklint L, Jaenson TGT. 1995. Control of Lyme borreliosis in Sweden by reduction of tick vectors: an impossible task? Int. J. Angiol. 4, 34–37. ( 10.1007/BF02043504) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.