Abstract
Reducing the burden of neglected tropical diseases (NTDs) is one of the key strategic targets advanced by the Sustainable Development Goals. Despite the unprecedented effort deployed for NTD elimination in the past decade, their control, mainly through drug administration, remains particularly challenging: persistent poverty and repeated exposure to pathogens embedded in the environment limit the efficacy of strategies focused exclusively on human treatment or medical care. Here, we present a simple modelling framework to illustrate the relative role of ecological and socio-economic drivers of environmentally transmitted parasites and pathogens. Through the analysis of system dynamics, we show that periodic drug treatments that lead to the elimination of directly transmitted diseases may fail to do so in the case of human pathogens with an environmental reservoir. Control of environmentally transmitted diseases can be more effective when human treatment is complemented with interventions targeting the environmental reservoir of the pathogen. We present mechanisms through which the environment can influence the dynamics of poverty via disease feedbacks. For illustration, we present the case studies of Buruli ulcer and schistosomiasis, two devastating waterborne NTDs for which control is particularly challenging.
This article is part of the themed issue ‘Conservation, biodiversity and infectious disease: scientific evidence and policy implications’.
Keywords: planetary health, environmentally transmitted diseases, coupled ecological–economic systems, sustainable disease control
1. Introduction
More than one billion people, most of them in tropical and subtropical countries, still live in extreme poverty and suffer a disproportionate burden of neglected tropical diseases (NTDs) [1,2]. NTDs consist of parasitic, viral and bacterial infections (table 1) that are responsible for chronic disabling conditions that account for more than 500 000 deaths and nearly 57 million disability-adjusted life-years (DALYs) annually [4,5]. The availability of affordable and safe drug treatments has led to declines in the prevalence of many NTDs, with plans for the elimination of lymphatic filariasis, onchocerciasis and trachoma by 2030 (table 1) [3,6]. Nevertheless, there is growing recognition of the limitations of interventions focused exclusively on treating the human host [3,7]. Most pathogens responsible for NTDs spend part of their life cycle outside the human host and are transmitted through environmental pathways (table 1), such that people may be repeatedly exposed and infected [7]. Moreover, coverage targets for mass drug administration (MDA) are hard to meet in the context of weak healthcare-delivery systems and extreme poverty, and drug resistance can threaten the long-term success of control programmes [8]. Sustainably reducing the burden of many NTDs requires complementary strategies to disrupt disease transmission that are informed by a broader understanding of interactions taking place in coupled natural–human systems [9].
Table 1.
NTDs, causes, global burden and current priorities for disease control (based on WHO 2015 [3]).
| NTD Group | pathogens responsible | transmission route | disease burden | control target and main strategiesa |
|---|---|---|---|---|
| Parasitic infections | ||||
| soil-transmitted helminthiases | Ascaris spp., Trichuris spp., Necator spp. and Ancylostoma spp. | Environmental. Ingestion of eggs and larvae or penetration of the skin | 875 million children at risk | Morbidity control. MDA of PC (albendazole or mebendazole) |
| schistosomiasis | Schistosoma spp. (mostly S. haematobium and S. japonicum) | Waterborne. Direct entry into skin after asexual multiplication in snail hosts | 240 million infected | Elimination (some regions). MDA of PC (praziquantel) |
| lymphatic filariasis | Wuchereria bancrofti, Brugia malayi and B. timori | Vector-borne. Mosquito bites (Anopheles spp., Culex spp. and Aedes spp.) | more than 120 million infected | Elimination. MDA of PC (albendazole + diethylcarbamazine or ivermectin) |
| onchocerciasis | Onchocerca volvulus | Vector-borne. Bites of blackflies (Simulium spp.) | 37 million infected | Elimination. MDA of PC (ivermectin) |
| dracunculiasis (Guinea worm) | Dracunculus medinensis | Waterborne. Drinking water with infected copepods | 100–200 cases/year | Eradication. Active surveillance, water filters, and improved drinking water sources |
| foodborne trematodiases | Clonorchis spp., Opisthorchis spp., Fasciola spp. and Paragonimus spp. | Foodborne. Consumption of contaminated fish, seafood or vegetables | unclear; more than 56 million in 2005 | Morbidity control. MDA of PC (praziquantel or triclabendazole) |
| taeniasis/cisticercosis | Taenia solium | Foodborne or faecal–oral. Consumption of pork or ingestion of parasite eggs | unclear; responsible for 1.5 million epilepsy cases | Reduce transmission. Veterinary controls of pork meat; treatment of infected pigs and humans |
| echinococcosis | Echinococcus granulosus and E. multilocularis | Foodborne. Accidental ingestion of items contaminated with eggs | unclear; 30/100 000 person-year, endemic areas | Reduce transmission. Veterinary controls of livestock; dog vaccination and regular treatment |
| Protozoan infections | ||||
| human African trypanosomiasis | Trypanosoma brucei gambiense and T. brucei rhodesiense | Vector-borne. Bites of tse-tse flies (Glossina spp.) | 20 000 cases/year | Elimination. Early diagnosis and treatment (pentamidine, suramin, melarsoprol or eflornithine) |
| Chagas disease | Trypanosoma cruzi | Vector-borne. Faeces of haematophagous triatomines | seven million infected | Reduce transmission. Sustained vector control; early diagnosis and treatment (benznidazole or nifurtimox) |
| leishmaniasis | Leishmania spp. | Vector-borne. Bites of sandflies (Phlebotomus spp.) | 1.3 million cases/year | Elimination (some regions). Surveillance, early diagnosis and treatment (liposomal amphotericin B) |
| Bacterial infections | ||||
| Buruli ulcer | Mycobacterium ulcerans | Waterborne. Vector-borne and/or direct environmental transmission (unclear) | 5000 cases/year | Prevention of disability. Early diagnosis and treatment (streptomycin + rifampin) |
| leprosy | Mycobacterium leprae | Person-to-person. Skin contact or respiratory route (unclear) | 200 000 cases/year | Elimination. Early diagnosis and treatment (multidrug therapy) |
| trachoma | Chlamydia trachomatis | Person-to-person. Direct personal contact, shared towels and cloths, or flies | more than 21 million with active trachoma | Elimination. MDA of PC (azithromycin) |
| yaws (endemic treponematoses) | Treponema pallidum | Person-to-person. Skin contact | 50 000–80 000 cases/year | Eradication. MDA of PC (azithromycin); early diagnosis and treatment |
| Viral infections | ||||
| dengue and chikungunya | Arbovirus (dengue virus and chikungunya virus) | Vector-borne. Mosquito bites (Aedes spp.) | 390 million dengue infections/year | Reduce mortality, morbidity and transmission. Integrated surveillance and vector control |
| rabies | rabies virus | Zoonosis. Bite from infected dogs | 60 000 deaths/year | Elimination. Mass vaccination of domestic dogs |
aMDA of PC, mass drug administration of preventive chemotherapy.
Here, we present a conceptual framework based on a simple model to account for ecological and socio-economic drivers of NTDs for designing context-specific control strategies. As examples, we present the cases of Buruli ulcer and schistosomiasis, two NTDs associated with land-use change in hydrological ecosystems, which represent different ends of the spectrum of diseases for which transmission is highly dependent on the environment (referred to hereafter as ‘environmentally transmitted’). Buruli ulcer is caused by Mycobacterium ulcerans which, like other ‘sapronotic’ pathogens [10], can be considered ubiquitous and free-living within the environment (i.e. not dependent on the level of infection within humans or any other known host) in certain regions. It afflicts only a few thousand people per year, but each case of infection can cause permanent disability with catastrophic socio-economic effects on households. Schistosomiasis, which is caused by Schistosoma spp., is representative of a larger class of parasitic worms and vector-borne pathogens whose presence in the environment depends upon the local prevalence of infected people. Schistosomiasis infects hundreds of millions of people each year and is responsible for a high burden of chronic morbidity globally. Integration of ecological knowledge and socio-economic feedbacks into a common framework can thus inform the most effective action targets and lead to better interventions for control of NTDs and other infectious diseases of poverty.
2. A framework for coupled ecological and socio-economic determinants of disease
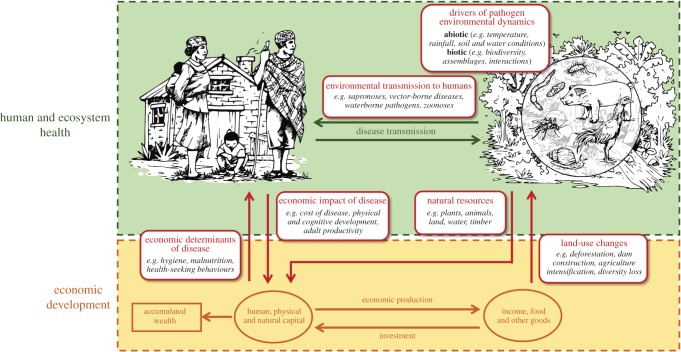
Economic systems of the rural poor are often coupled with natural systems through subsistence-resource exploitation (e.g. agriculture, pastoralism and fishing) and exposure to ambient pathogens (figure 1) [11,12]. This is particularly relevant in the tropics, where climatic conditions are favourable for many pathogens and their vectors (e.g. flies, mosquitoes and ticks), and the diversity of mammal and bird species hosts a greater diversity of pathogens in this region [13–16]. Land-use change for the purpose of economic development (e.g. expansion of road networks, dams and agricultural land conversion) can further affect environmentally transmitted parasites and pathogens through impacts on physicochemical conditions of water and soil [15], ecological community composition (e.g. loss of biodiversity), or inter- and intra-species interactions (e.g. predation and migration) [17,18].
Figure 1.
Coupled natural and human systems: feedbacks between ecosystems, infectious diseases and economic development. Many human pathogens in tropical regions, including those that cause NTDs, spend part of their lifecycle outside the human host, where environmental conditions drive their ecology and transmission (upper panel). Ecosystem and disease dynamics influence key forms of capital necessary for economic development of at-risk human communities (lower panel). Simultaneously, economic activities and resources affect those same dynamics by altering vulnerability to disease and introducing land-use change to ecosystems.
(a). Model of environmental transmission of disease
Building on traditional systems in disease ecology and epidemiology [19–21], we first present a general infectious disease model that accounts for direct and environmental pathways of disease transmission. The model is meant to represent the dynamics of NTDs and other tropical diseases of poverty across a wide spectrum of transmission strategies, spanning from human-to-human contagious diseases (e.g. leprosy and trachoma) to parasitic worms (e.g. schistosomiasis), waterborne bacteria (e.g. Buruli ulcer), foodborne pathogens (e.g. echinococcosis), vector-borne diseases (e.g. dengue and malaria), or zoonotic and multi-host pathogens (e.g. rabies and Nipah virus):
| 2.1a |
and
| 2.1b |
The state variables I and S represent the infected and susceptible proportions of the human population, respectively; W is the environmental reservoir of the pathogen; βD is the direct (human-to-human) transmission rate; βE is the transmission rate through the environmental reservoir; γ is the rate of recovery (either into an immune class in an SIR model or back to the susceptible class in an SIS model without immunity); λ is the rate or production of infectious propagules released in the environment (either as free-living stages, such as for soil-transmitted helminths, or pathogen abundance in vectors or intermediate hosts); σ is the fraction of the propagules that reach the environment; V is a parameter proportional to the abundance of vectors (in the case of dengue, chikungunya, etc.), to the abundance of intermediate hosts (in the case of trematode infections, such as schistosomiasis, fascioliasis, etc.), or to habitat suitability for pathogen environmental persistence (in the case of soil-borne or waterborne diseases, such as intestinal nematode infections and cholera, respectively); Ω is the recruitment rate of infective propagules associated with exogenous processes not directly related to disease prevalence in the population; δ is the mortality of infectious stages in the environment. From this general framework, we define two parameters (or ‘meta-parameters’) that characterize the role of the environment on transmission:
| 2.2a |
and
| 2.2b |
Parameter ɛ is an index of environmental transmission that ranges from zero for direct transmission (i.e. person-to-person) to unity for complete environmental transmission (i.e. pathogens with obligatory non-human hosts, reservoirs or vectors). Parameter φ represents the degree of exogenous versus focal transmission among the environmentally transmitted diseases (ETDs). It ranges from zero when the abundance of infectious propagules in the environmental reservoir is solely a function of the local number/fraction of infected people, with no exogenous recruitment (Ω = 0 and λ > 0), to unity when the pathogen's environmental load depends exclusively on its own free-living replication in the environment or on other environmental factors (Ω > 0 and λ = 0). ‘Sapronotic’ pathogens—those that can replicate within the environment free of any known host (a characteristic of almost all fungal pathogens, about one-third of bacterial pathogens and several parasites that infect man [10])—have by definition φ = 1, while directly transmitted diseases have, by the nature of the close contact required for contagion, φ → 0. For other parasites and pathogens, for example, those that experience intermediate levels of focal to exogenous transmission, the value of φ depends on the scale of analysis.
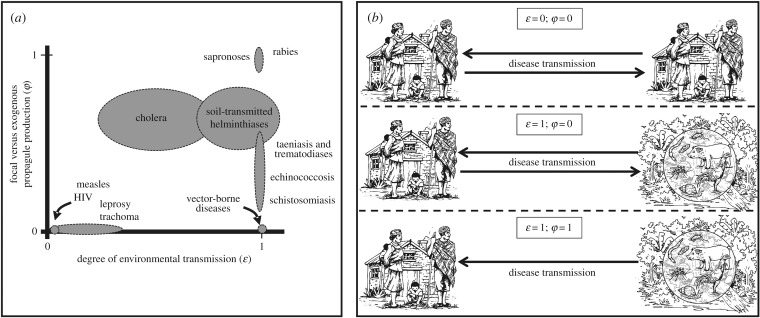
Figure 2 provides a simple qualitative description of common infective agents in tropical regions according to their transmission strategies based on parameters ɛ and φ. Many pathogens responsible for mortality in developing countries have low ɛ and φ, such as childhood diseases (measles and whooping cough), respiratory infections (tuberculosis) or sexually transmitted diseases (HIV and syphilis). By contrast, for most pathogens responsible for NTDs, environmental transmission plays an important role (ɛ → 1). The general model described above, although difficult to parametrize in its simplest form [19,22], can be used to explore conceptual differences in dynamics and control strategies for a wide variety of pathogens and transmission strategies. A heuristic illustration of such insights is provided in figure 3, where we contrast the impact of common strategies of disease control for directly transmitted diseases (ɛ = 0, φ = 0) and ETDs (ɛ = 1, φ → 1).
Figure 2.
A conceptual framework to account for multiple pathways of disease transmission in coupled economic–epidemiological systems. (a) Qualitative classification of relevant infectious and parasitic diseases in developing countries according to two key parameters that regulate environmental transmission in this conceptual framework (ɛ and φ). Ellipsoids represent educated guesses about the parameter space occupied by each disease, including major NTD groups. (b) Graphical representation of the three main groups of diseases according to parameters ɛ and φ: directly transmitted (ɛ = 0; φ = 0), environmentally transmitted, with focal transmission dependent on human prevalence (ɛ = 1; φ = 0) and environmentally transmitted, driven purely by exogenous environmental factors (ɛ = 1; φ = 1).
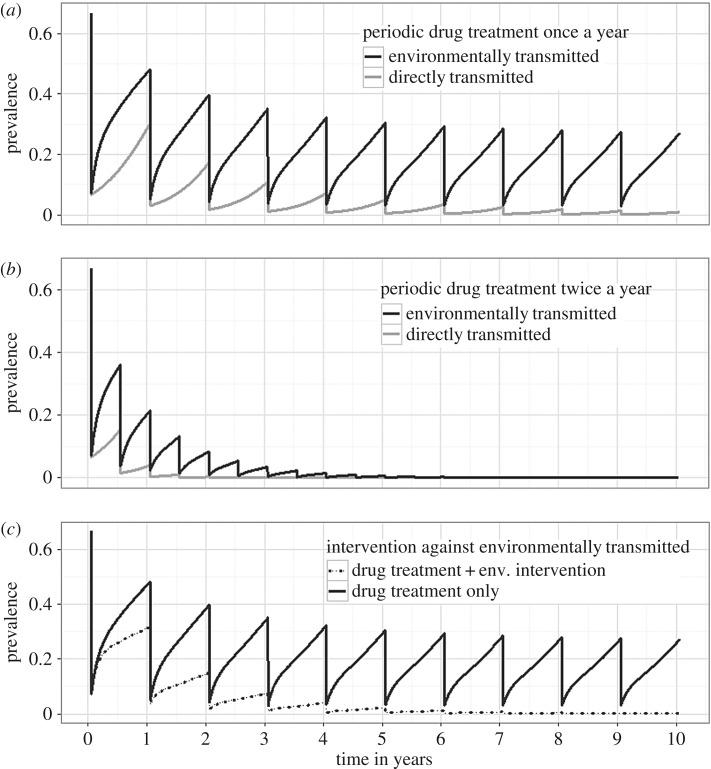
Figure 3.
Effectiveness of periodic drug treatment and environmental interventions for the control of diseases with different transmission pathways. Population prevalence dynamics over 10 years for a directly transmitted disease (grey lines) and an ETD (black lines) with human drug treatment dispensed once a year (a) or twice a year (b). We considered a 90% drop in prevalence after each treatment and R0 = 3 for both the directly transmitted and the ETD (see the electronic supplementary material, Appendix S1 for further parameter values). In (c), only the dynamics of an ETD are displayed, with model parameters set as in (a) and two interventions considered: only drug treatment administered once a year (solid line), and the same drug administration complemented with an environmental intervention (e.g. water sanitation, use of insecticide or biological control) that reduces life expectancy of the pathogen environmental stages by one-third (dashed line).
We show that for direct and ETDs with comparable basic reproduction numbers R0 (i.e. number of secondary infections caused by an infected individual introduced in a susceptible population), control can be effectively achieved for directly transmitted disease through periodic medical treatment. All else being equal (i.e. in terms of the R0 of the disease and the frequency and intensity of intervention), the same drug-focused strategies can have a reduced efficacy for diseases where the environmental reservoir plays an important role (figure 3a,b). In the latter case, more effective control can be achieved when classic treatment strategies are complemented with interventions that act on the environmental reservoir of the pathogen or reduce exposure (figure 3c). A detailed analysis of this system of equations, the parametrization for the presented simulations, and further explanations are provided in the electronic supplementary material, Appendix S1.
(b). Feedbacks between poverty and disease in coupled environment–human systems
Global patterns of poverty and disease have a strong geographic signature, indicative of underlying environmental determinants (figure 4) [26,27]. Building on recent advances in the modelling of coupled economic–epidemiological systems [11,28], we explore how the framework presented in §2a can be integrated with existing models of disease-driven poverty traps to understand the potential role of environmental drivers. This integrated framework is based on the intersection of the following empirical facts: (i) economic and environmental conditions are co-determinants of NTDs, and (ii) NTDs have well-documented negative effects on economic productivity, primarily through child development, educational attainment and labour productivity [1,27,29–31].
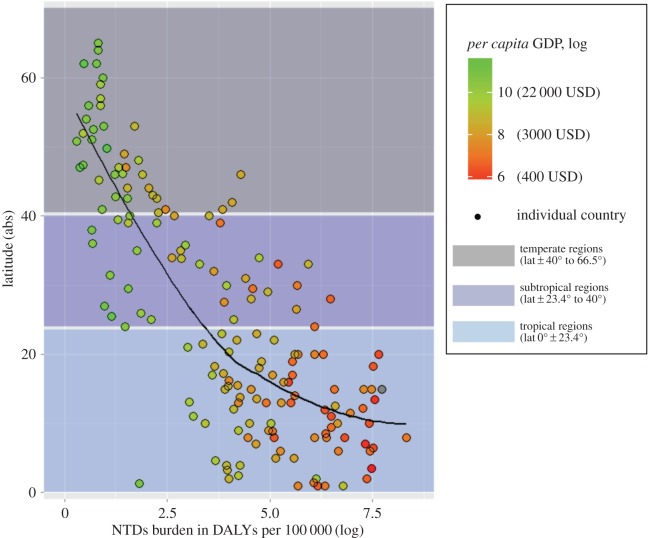
Figure 4.
Latitudinal gradient in NTDs burden and poverty. Tropical and subtropical regions (blue and light blue shade) bear an overwhelming burden of NTDs, represented in the x-axis as DALY per 100 000 population (logarithmic scale). These regions accumulate 88.0% and 11.8% of the global number of NTD-related DALYs, respectively, which together represents more than 99% of the world burden. Concurrently, tropical and subtropical regions present some of the lowest levels of per capita GDP in the world (dot colour gradient from red for lowest GDP to green for highest GDP). The black solid line in the graph shows the relationship between latitude and NTD-related DALYs (loess nonlinear smoother). Figure was created with R statistical software (package ggplot2) with country data obtained from [23–25].
The effect of economic drivers on disease dynamics in a particular system can be captured by transforming some parameters in equations (2.1a,b) to be functions of per capita income or wealth: i.e. βD = βD(k), βE = βE(k), Ω = Ω(k), λ = λ(k); where k is capital, which we define broadly as a stock of economic resources used to produce income. For instance, transmission rates (βD, βE) and environmental recruitment of propagules (σ and Ω) are generally decreasing functions of capital k, because capital is necessary for prevention measures that reduce disease transmission, such as systems for water and sanitation, improved housing conditions or protective clothing [4,5,7]. In addition, the recovery rate γ = γ(k) is generally an increasing function of capital because capital (or income generated from capital) is used for better nutrition and accessing healthcare [32,33]. The relationship of vectors V = V(k) and the mortality rate of infective propagules δ = δ(k) with capital (k, or economic development) are more context- and disease-specific [28].
The effects of disease on income can be captured by adding capital as a third state variable, in the tradition of economic growth theory, to the infectious disease model [34,35]:
| 2.3 |
where k is per capita physical or human capital; d is capital depreciation rate; r is capital accumulation or savings rate, which is negatively affected by disease I, and y = y(I,k) is per capita income, an increasing function of capital and a decreasing function of disease prevalence in the population.
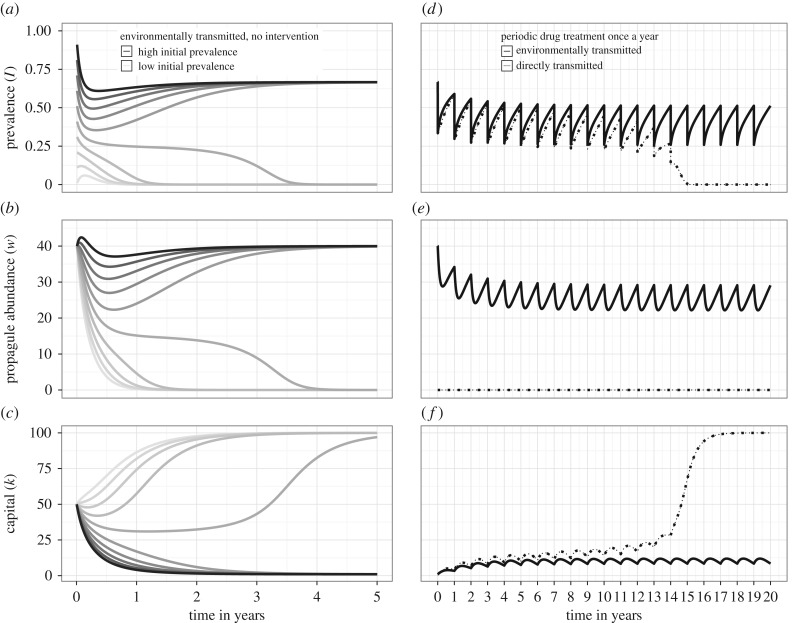
In coupled disease–economic systems [11,36,37], an increase in prevalence of either directly transmitted (electronic supplementary material, Appendix S1; ɛ = 0) or ETD (figure 5a–c; ɛ = 1) can trigger a sequence of cascading effects that reinforce conditions of poverty. Lower capital in turn fosters disease transmission (high β) and reduces recovery (low γ), ultimately creating a negative reinforcing cycle of poverty and disease. A substantial reduction in disease prevalence can trigger the opposite sequence of cascading effects, leading to a reinforcing cycle of better health, increased labour productivity and capital accumulation. Therefore, disease–economic systems can, under some circumstances, be characterized by bistability [11,36,37], where one locally stable equilibrium is associated with low income and high disease prevalence (i.e. a ‘poverty trap’) and the other equilibrium is characterized by high income and little or no disease (figure 5). The equilibrium outcome of either a poverty trap or a virtuous cycle towards health and productivity, is contingent on the history of the system and on specific ‘shocks’ in economic and/or healthcare investments that might push the disease–economic system from one basin of attraction to the other [36]. In the case of ETDs, such as most NTDs, disease–economic dynamics are highly influenced by the dynamics of the pathogen in the environment (figure 5a–c). As a result, the same strategies that could help break a disease-driven poverty trap for directly transmitted diseases, might not be sufficient for ETDs (figure 5d–f). Indeed, failure to act on the environmental reservoir, despite administration of periodic drug treatment, may prevent a sustained prevalence reduction under the critical threshold that switches the basin of attraction for ETDs, therefore perpetuating the poverty trap (figure 5d–f).
Figure 5.
Dynamics of coupled economic–epidemiological systems and impact of periodic drug treatment for directly and environmentally transmitted diseases. Graphs are based on the coupled system described in the main text, and parameter values described in the electronic supplementary material, Appendix S1. (a–c) Dynamics over 5 years in a coupled system for the case of an ETD with R0 = 3. Graphs display dynamics of human prevalence (I), pathogen environmental abundance (W), and per capita capital (k), starting from a range of initial conditions for prevalence I, with same k and W. Shades are increasingly dark for increasing values of initial prevalence. Bistability emerges from this system, with two equilibria depending on initial conditions: a high prevalence low capital equilibrium, and a low prevalence high capital equilibrium. (d–f) Impact of human drug treatment dispensed once a year on the dynamics over 20 years for a directly transmitted disease (dotted lines) and an ETD (solid lines) in a coupled economic–epidemiological system. Initial conditions were set as in the high-prevalence–low-capital equilibrium in the left panel, to assess the effectiveness of this intervention to break a theoretical disease-driven poverty trap. R0 = 3 for both types of disease.
These models, which have not previously incorporated an environmental component of disease transmission, have been extended to include within-human population dynamics, where bistability can occur on coupled epidemiological/economic networks [34]. Even if diseases have low prevalence, and no bistability, disease–economic feedbacks can lead to inequalities in cases of NTDs that cause lifelong disability [38]. Equations ((2.1)–(2.3)) therefore represent a simple modelling framework capable of accounting for a continuum of alternative infectious disease transmission pathways and socio-economic drivers that can be extended to account for system-specific contexts. To illustrate the concepts introduced in our framework for specific systems, we present in §§3 and 4, as case studies, recent evidence on socio-economic impacts, environmental drivers and ecological solutions for two NTDs with environmental transmission (ɛ = 1) at opposite extremes of the local-to-exogenous transmission (φ) axis in figure 2a. Buruli ulcer is a debilitating disease caused by a waterborne mycobacterium whose transmission to people depends on exogenous environmental reservoirs (φ = 1). Schistosomiasis is caused by a blood fluke, whose life cycle depends strongly on a transmission loop from infected people to the local aquatic environment (snails) and back to people (φ = 0 or close to zero).
3. Case study 1. Buruli ulcer: the need for ecological and socio-economic insights for disease control
Buruli ulcer, a devastating skin disease caused by the environmental pathogen Mycobacterium ulcerans, is probably one of the least-studied of all recognized NTDs. Discovered in the 1960s, it has rapidly emerged in tropical and subtropical countries and currently infects 5000 new people every year [39]. Most of the disease burden concentrates in rural areas of central and western Africa, where it presents a highly focal distribution, even within endemic regions [39]. Although initial stages of the disease (i.e. nodules, plaques and small ulcers) can be treated with an eight-week antibiotic regimen, poor access to healthcare in these areas frequently leads to catastrophic disease progression [40,41]. One in four cases experience functional limitations for life as a consequence of complications associated with the later stages of the disease (i.e. extensive ulcers, osteomyelitis) that require invasive surgery and lengthy hospital stays [40,41]. These pathological problems have socio-economic causes and consequences. Besides the enormous cost of the disease episode itself, estimated at 25% of household yearly income in Cameroon [42], handicap and deformity are associated with significant stigma, social isolation, job loss and school dropout [41,42]. Early detection and treatment of cases can avert much of the unnecessary suffering [3]. Yet, for ETDs like Buruli ulcer whose dynamics are driven by extrinsic environmental factors (ɛ → 1, φ → 1, figure 2), our poor understanding of the environmental mechanisms that drive pathogen ecological dynamics and its transmission to human populations undermines our capacity to prevent the disease.
Buruli ulcer is associated with aquatic ecosystems, and M. ulcerans is found in water, mud, aquatic plants and freshwater insects from representative taxa of the whole aquatic community [43]. The ubiquity of this multi-host environmentally persistent pathogen is exacerbated by the lack of safe water in endemic rural areas, where daily activities, such as bathing or washing, repeatedly expose people to infection [44,45]. Prevalence of the disease is concentrated near swamps, flooded areas and along the basin of slow-flowing rivers at low elevation. Land-use change due to agricultural practices, deforestation, or the construction of dams and roads have been correlated with the emergence of Buruli ulcer endemic areas [43]. Recent advances in disease ecology are shedding light on the conditions that favour environmental persistence and growth of M. ulcerans, and drive its spatio-temporal dynamics (W in equation (2.1b)). M. ulcerans thrives in periods of intensive rainfall, and certain physicochemical conditions of lentic ecosystems (i.e. low water flow, low oxygen, mildly acidic pH, high temperature) may allow persistence as free-living stages [46,47] (figure 6a). Under unfavourable water conditions, trophic transmission between aquatic hosts seems to ensure the presence of M. ulcerans in the environment, independently of the infections in people, which may explain the ubiquity of the pathogen in endemic regions [46,47].
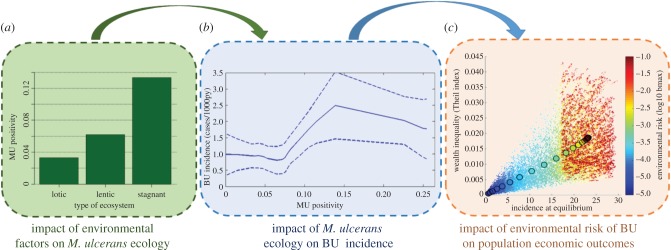
Figure 6.
Understanding feedbacks between environment, Buruli ulcer and economic development. A recent research initiative in Cameroon sought to characterize the environmental dynamics of M. ulcerans through frequent sampling of multiple aquatic ecosystems and molecular analyses, in order to gain new insights on the ecology and epidemiology of the disease. (a) The prevalence of the bacteria in aquatic sites (MU positivity) appeared to be favoured by specific conditions in stagnant and slow-flowing (lentic) ecosystems [46]. (b) Comparisons of M. ulcerans ecological dynamics with patterns of Buruli ulcer incidence from the same areas in mathematical and statistical models, showed that spatial and temporal patterns of Buruli ulcer (y-axis) were influenced by the environmental prevalence of M. ulcerans (x-axis) [48]. (c) Individual-based model simulations of a coupled economic–epidemiological system adapted to Buruli ulcer revealed that the disease can be responsible of economic inequalities in endemic populations, with increases in Theil index (y-axis) as a function of BU incidence (x-axis) and M. ulcerans environmental load (colour gradient) [38]. Figures are adapted from Garchitorena et al. [38,46,48].
An ecological perspective can also provide new insights on Buruli ulcer transmission. It is unclear whether direct entry into the skin [43] or vector transmission through the bites of water bugs [49] are major contributors to transmission. Ecological studies recently provided the first field-based evidence that environmental transmission to humans could play a greater role than vector transmission (figure 6b) and suggested that M. ulcerans' environmental load could predict the temporal and spatial patterns of Buruli ulcer incidence [48]. Integration of this ecological understanding with known socio-economic features of the disease in coupled economic–epidemiological models (as shown in equations (2.1)–(2.3)) is helping to clarify the economic consequences of land-use change through their effects on Buruli ulcer [38]. These models show that in contexts of high environmental risk, Buruli ulcer can cause economic inequalities at the population level (figure 6c), with disproportionate impacts on the poorest socio-economic groups due to disparities in vulnerability and healthcare access [38]. Thus, the negative consequences of land-use change could fall disproportionally on the poor. These studies represent an initial step towards a more comprehensive understanding of Buruli ulcer environmental risk, transmission and economics that could lead to the implementation of novel control strategies.
4. Case study 2. Schistosomiasis: an ecological solution for disease control and economic development
Schistosomiasis is a debilitating disease caused by freshwater trematodes of the genus Schistosoma and one of the most important NTDs based on number of people at risk (almost 800 million) and number infected (approximately 250 million) [3,4,50,51]. More than 90% of infections occur in sub-Saharan Africa, the vast majority of which occur in school-aged children between 5 and 15 years old [5]. Infection occurs through exposure to natural freshwater ecosystems in tropical areas that are inhabited by obligate intermediate hosts of the parasites—snails of the genus Bulinus and Biomphalaria. The snails become infected when people (or, in some case other vertebrate hosts such as rodents or cattle) shed parasite eggs into the environment through urine or faeces (ɛ → 1, φ → 0, figure 2). People (or other vertebrate hosts) are infected when they come into contact with waters containing infectious larvae shed from snails. As with Buruli ulcer, exposure commonly occurs through bathing, washing dishes, fishing or other water-based daily activities. Infection with Schistosoma spp. can trigger acute and chronic inflammatory processes such as haematuria, scarring, calcification, squamous cell carcinoma and occasional embolic egg granulomas in brain or spinal cord (S. japonicum) as well as fever, hepatic egg granulomas, fibrosis and portal hypertension (S. mansonii) [52]. Inflammatory processes lead to debilitation, stunted growth and cognitive impairment in children, increased chances of acquiring HIV in the case of urogenital schistosomiasis in women, and may eventually lead to liver, lung or kidney failure. The drug of choice for schistosomiasis, praziquantel, is cheap (US $0.32 to treat a child [53]) and effective in removing parasites from infected people [52,53]. Yet, despite more than US $1.4 billion spent in the past two decades on MDA programmes in developing countries, prevalence and intensity of infection have changed little compared with the pre-praziquantel era [54]. This is because, after treatment, rural villagers have few alternatives but to return to the parasite-contaminated waters (figure 3), and parasitic loads in people return to pre-treatment levels in as little as six months [55,56].
In Africa, the development of irrigation systems and the construction of dams often lead to a further expansion of the habitat of snails (an increase in parameter V in equation (2.1b)), and hence new potential transmission sites for schistosomiasis [57,58]. The construction of the Diama Dam, built at the mouth of the Senegal River in 1986, is a textbook case. Urinary schistosomiasis was endemic at very low levels before the construction of the dam and intestinal schistosomiasis was absent. The first new cases of both intestinal and urinary schistosomiasis were reported about 1 year after the completion of the Diama Dam [59]. Following two decades of exponential growth in the number of infected people, the lower basin of the Senegal River has now become a hyper-endemic region for schistosomiasis [59]. The construction of the Diama Dam not only led to an expansion of good snail habitat—i.e. a year-around stable water body at low salinity—but also decimated populations of the migratory prawn, Macrobrachium vollenhovenii, a voracious predator of snails [58,60,61]. The combination of increased habitat for the snail population with the removal of a very effective predator provided ideal environmental conditions for Bulinus and Biomphalaria snails to thrive and amplify schistosomiasis transmission.
A pilot study carried out in 2011–2014 provided supportive evidence that the reintroduction of the freshwater prawn can reduce infected snails and significantly lower reinfection rates in the human population [56]. Computer simulations showed that the combination of MDA and prawn reintroduction can achieve results that neither of these approaches can attain independently [56]. As prawns are a delicious food commodity with market value, the development of prawn production through aquaculture could also alleviate hunger and provide a source of revenue for local villagers, so that prawn restoration could become an actionable ecological solution leading to a mutually beneficial outcome for biodiversity, schistosomiasis reduction, food production and income [56]. An extended analysis of the geographical distribution of dams and 24 marketable prawn species around the world in areas where schistosomiasis is endemic [58] shows that the removal of natural predators of schistosomiasis-bearing snails might have occurred in many other places in addition to Senegal. The reintroduction of these predators, either through ecological restoration or aquaculture, might protect people from the risk of contracting schistosomiasis and could complement drug-distribution programmes [58].
5. Discussion and Conclusion
While poverty, infectious disease, and environmental change have been the subject of much independent investigation, they are interlinked in complex ways that require integrated study in order to meet key targets in the Sustainable Development Goals [9]. Poverty and weak health systems arguably pose the most important risk factors for acquiring and succumbing to infectious diseases. Less recognized is the fact that pathogens and parasites responsible for most tropical diseases of poverty are predominantly transmitted through environmental pathways and highly influenced by ecological factors. Here, we describe a simple framework for studying the complex relationships between poverty, infectious diseases, and the environment. The aim of this framework is to help investigate environmental drivers of joint poverty–disease dynamics, with the ultimate goal of identifying sustainable solutions for human and planetary health. Preliminary analyses illustrate the limited effectiveness of human drug treatment for the control of ETDs, when compared with directly transmitted diseases. Although the model cannot be realistically parametrized for specific diseases in its simplest form, this key conceptual difference highlights opportunities for environmental interventions for disease control. By integrating ecological dynamics into coupled disease–economic systems, we show that the presence of persistent environmental reservoirs of pathogens may affect both NTD prevalence and poverty. Future research is needed to investigate how interrupting environmental transmission cycles [3,7] can complement current efforts to expand preventive and curative services for the poorest groups [62,63] to break cycles of poverty and disease.
Building on current ecological knowledge, cost-effective interventions for disease prevention can be designed for many infectious diseases of poverty [64–66]. Mosquito control strategies are among the most widely recognized and extensively deployed of these interventions [67]. In the absence of an effective vaccine for malaria and caution against drug resistance, the recent reduction in disease burden in sub-Saharan Africa has been largely driven by the massive scale-up of indoor residual spraying and long-lasting insecticidal nets [67]. Other examples can be found for zoonotic diseases such as Nipah virus, where the use of bamboo skirts provides a physical barrier that prevents bats (the natural reservoir of NiV) from accessing date palm sap, subsequently reducing transmission to human populations [68,69]. The integration of drug administration with basic infrastructure investments in water, sanitation and hygiene (WASH) has been recognized as a priority in the prevention and care for multiple NTDs, along with improved living conditions, integrated vector control, health education and stronger health systems in endemic areas [3,70]. Yet, the immediate investment costs of infrastructure development hinder the implementation of WASH and similar interventions, leaving drug treatment as the main control strategy for most NTDs. For sapronoses, whose presence in the environment is independent of human infections, such as fungal and waterborne bacterial infections (e.g. Buruli ulcer), more research on environmental drivers will help identify transmission hot spots, contribute to early detection of cases, and inform prevention strategies [46,48]. For ETDs with well-known ecological drivers, such as trematode and vector-borne infections (e.g. schistosomiasis), control strategies based on natural enemies of pathogens or their wildlife hosts/vectors could represent sustainable ecological solutions. A recent analysis of a century of schistosomiasis control programmes [54] showed that, before the availability of the anti-parasitic drug praziquantel, successful elimination was achieved via interventions that directly targeted the snail intermediate host [54,71]. Supportive evidence [56,58,60] suggests that freshwater prawns may reduce disease transmission through predation on schistosomiasis-bearing snails. More research is needed to identify what other natural enemies of parasites or of their wildlife hosts could be successfully harnessed to achieve a better control of disease transmission [72,73]. While broadly used for integrated pest control in agriculture, such ecological strategies have received little attention in the literature of human infectious diseases. Despite showing promising results in the fight against malaria and other vector-borne diseases, environmental strategies to complement medical approaches still remain poorly developed and under recognized [74].
The many dimensions of the Sustainable Development Goals and an increased attention to the interdependence of human wellbeing and the Earth's provisioning of ecosystem services, have recently expanded the focus of the global health community into the new scientific movement of Planetary Health [9]. While this movement reflects a wide consensus on the threats and challenges currently faced by human populations and natural systems, we still lack actionable solutions that cost-effectively leverage our understanding of the ecology of disease transmission to break the reinforcing cycles of poverty, environmental change and disease. To make the Planetary Health concept actionable, research efforts can help identify specific keystone systems for developing and deploying ecological interventions that influence both poverty and disease.
Supplementary Material
Acknowledgements
We are grateful to Alain Rasolomampiandra for creating the artwork in figures 1 and 2.
Authors' contributions
A.G., S.H.S., M.H.B. and G.D.L. conceived the study. G.D.L. analysed the model. All authors helped draft the manuscript and gave final approval for publication.
Competing interests
The authors declare no competing financial interests.
Funding
S.H.S. and G.D.L. have been supported by NSF CNH grant no. 1414102, the Bill and Melinda Gates Foundation, NIH grant no. 1R01TW010286-01, Stanford GDP SEED grant no. 1183573-100-GDPAO, the SNAP-NCEAS-supported working group ‘Ecological levers for health: Advancing a priority agenda for Disease Ecology and Planetary Health in the 21st century’ and the NIMBioS-supported working group on the Optimal Control of ETDs. A.G., C.N.N. and M.H.B. were funded by a James McDonnell Foundation grant (no. 220020322) and NIH grant (no. K01TW008773) from the Fogarty International Center. This work was partly supported by the National Center for Ecological Analysis and Synthesis (NCEAS) and the National Socio-Environmental Synthesis Center (SESYNC) with funds from the NSF. J.F.G. and B.R. were supported by a French grant from the Agence Nationale de la Recherche (CEBA ANR-LABX-10-2501) and J.F.G. received support from the UN programme FutureEarth. E.A.M. was supported by grants from the NSF (DEB-1518681 and DEB-1640780), a Stanford Center for Innovation in Global Health seed grant, and a Stanford Woods Institute Environmental Ventures Program grant.
References
- 1.Sachs J. 2001. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva, Switzerland: WHO.
- 2.Lozano R, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. ( 10.1016/S0140-6736(12)61728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization 2015. Investing to overcome the global impact of neglected tropical diseases: Third WHO report on neglected tropical diseases. Geneva, Switzerland: WHO.
- 4.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. 2009. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373, 1570–1575. ( 10.1016/S0140-6736(09)60233-6) [DOI] [PubMed] [Google Scholar]
- 5.Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 3, e412 ( 10.1371/journal.pntd.0000412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenwick A. 2012. The global burden of neglected tropical diseases. Public Health 126, 233–236. ( 10.1016/j.puhe.2011.11.015) [DOI] [PubMed] [Google Scholar]
- 7.IOM (Institute of Medicine) 2011. The causes and impacts of neglected tropical and zoonotic diseases: opportunities for integrated intervention strategies. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 8.Webster JP, Molyneux DH, Hotez PJ, Fenwick A. 2014. The contribution of mass drug administration to global health: past, present and future. Phil. Trans. R. Soc. B 369, 1–12. ( 10.1098/rstb.2013.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmee S, et al. 2015. Safeguarding human health in the anthropocene epoch: report of the Rockefeller Foundation-Lancet commission on planetary health. Lancet 386, 1973–2028. ( 10.1016/S0140-6736(15)60901-1) [DOI] [PubMed] [Google Scholar]
- 10.Kuris AM, Lafferty KD, Sokolow SH. 2014. Sapronosis: a distinctive type of infectious agent. Trends Parasitol. 30, 386–393. ( 10.1016/j.pt.2014.06.006) [DOI] [PubMed] [Google Scholar]
- 11.Ngonghala CN, Pluciński MM, Murray MB, Farmer PE, Barrett CB, Keenan DC, Bonds MH. 2014. Poverty, disease, and the ecology of complex systems. PLoS Biol. 12, e1001827 ( 10.1371/journal.pbio.1001827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett CB, Travis AJ, Dasgupta P. 2011. On biodiversity conservation and poverty traps. Proc. Natl Acad. Sci. USA 108, 13 907–13 912. ( 10.1073/pnas.1011521108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guernier V, Hochberg ME, Guégan J-F. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141 ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn RR, Davies TJ, Harris NC, Gavin MC. 2010. Global drivers of human pathogen richness and prevalence. Proc. R. Soc. B 277, 2587–2595. ( 10.1098/rspb.2010.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostfeld RS, Keesing F, Eviner VT. 2008. Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. 2000. Climate change and vector-borne diseases: a regional analysis. Bull. World Health Organ. 78, 1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 17.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg MC, Robertson SL, Tien JH. 2013. Identifiability and estimation of multiple transmission pathways in cholera and waterborne disease. J. Theor. Biol. 324, 84–102. ( 10.1016/j.jtbi.2012.12.021) [DOI] [PubMed] [Google Scholar]
- 20.Codeço CT. 2001. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect. Dis. 1, 1 ( 10.1186/1471-2334-1-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tien JH, Earn DJD. 2010. Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bull. Math. Biol. 72, 1506–1533. ( 10.1007/s11538-010-9507-6) [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg JNS, Desai MA, Levy K, Bates SJ, Liang S, Naumoff K, Scott JC. 2007. Environmental determinants of infectious disease: a framework for tracking causal links and guiding public health research. Environ. Health Perspect. 115, 1216–1223. ( 10.1289/ehp.9806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Health Metrics and Evaluation 2016. GDB results tool. Seattle, WA: IHME, University of Washington. See http://ghdx.healthdata.org/gbd-results-tool.
- 24.World Bank 2015. World Bank Open Data. See http://data.worldbank.org/.
- 25.US Central Intelligence Agency 2016. The world factbook. See https://www.cia.gov/library/publications/the-world-factbook.
- 26.Sachs JD. 2001. Tropical underdevelopment. NBER Work. Pap. 8119 Cambridge, MA: National Bureau of Economic Research ( 10.3386/w8119) [DOI] [Google Scholar]
- 27.Bonds MH, Dobson AP, Keenan DC. 2012. Disease ecology, biodiversity, and the latitudinal gradient in income. PLoS Biol. 10, e1001456 ( 10.1371/journal.pbio.1001456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeza A, Bouma MJ, Dhiman RC, Baskerville EB, Ceccato P, Yadav RS, Pascual M. 2013. Long-lasting transition toward sustainable elimination of desert malaria under irrigation development. Proc. Natl Acad. Sci. USA 110, 15 157–15 162. ( 10.1073/pnas.1305728110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miguel E, Kremer M. 2004. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 72, 159–217. ( 10.1111/j.1468-0262.2004.00481.x) [DOI] [Google Scholar]
- 30.Sachs J, Malaney P. 2002. The economic and social burden of malaria. Nature 415, 680–685. ( 10.1038/415680a) [DOI] [PubMed] [Google Scholar]
- 31.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. 1992. Parasitic helminth infection and cognitive function in school children. Proc. R. Soc. Lond. B 247, 77–81. ( 10.1098/rspb.1992.0011) [DOI] [PubMed] [Google Scholar]
- 32.Makinen M, et al. 2000. Inequalities in health care use and expenditures: empirical data from eight developing countries and countries in transition. Bull. World Health Organ. 78, 55–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead M, Dahlgren G, Evans T. 2001. Equity and health sector reforms: can low-income countries escape the medical poverty trap? Lancet 358, 833–836. ( 10.1016/S0140-6736(01)05975-X) [DOI] [PubMed] [Google Scholar]
- 34.Solow RM. 1956. A. contribution to the theory of economic growth. Q. J. Econ. 70, 65–94. ( 10.2307/1884513) [DOI] [Google Scholar]
- 35.Barro RJ, Sala-i-Martin X. 2004. Economic growth. Cambridge, MA: MIT Press. [Google Scholar]
- 36.Bonds MH, Keenan DC, Rohani P, Sachs JD. 2010. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B 277, 1185–1192. ( 10.1098/rspb.2009.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluciński MM, Ngonghala CN, Getz WM, Bonds MH. 2013. Clusters of poverty and disease emerge from feedbacks on an epidemiological network. J. R. Soc. Interface 10, 20120656 ( 10.1098/rsif.2012.0656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garchitorena A, Ngonghala CN, Guegan J-F, Texier G, Bellanger M, Bonds M, Roche B. 2015. Economic inequality caused by feedbacks between poverty and the dynamics of a rare tropical disease: the case of Buruli ulcer in sub-Saharan Africa. Proc. R. Soc. B 282, 20151426 ( 10.1098/rspb.2015.1426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson PDR, Stinear T, Small PLC, Pluschke G, Merritt RW, Portaels F, Huygen K, Hayman JA, Asiedu K. 2005. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med. 2, e108 ( 10.1371/journal.pmed.0020108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barogui Y, Johnson RC, van der Werf TS, Sopoh G, Dossou A, Dijkstra PU, Stienstra Y. 2009. Functional limitations after surgical or antibiotic treatment for Buruli ulcer in Benin. Am. J. Trop. Med. Hyg. 81, 82–87. [PubMed] [Google Scholar]
- 41.Stienstra Y, et al. 2005. Factors associated with functional limitations and subsequent employment or schooling in Buruli ulcer patients. Trop. Med. Int. Heal. 10, 1251–1257. ( 10.1111/j.1365-3156.2005.01519.x) [DOI] [PubMed] [Google Scholar]
- 42.Grietens KP, Boock AU, Peeters H, Hausmann-Muela S, Toomer E, Ribera JM. 2008. ‘It is me who endures but my family that suffers’: social isolation as a consequence of the household cost burden of Buruli ulcer free of charge hospital treatment. PLoS Negl. Trop. Dis. 2, e321 ( 10.1371/journal.pntd.0000321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, Boakye DA. 2010. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl. Trop. Dis. 4, e911 ( 10.1371/journal.pntd.0000911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debacker M, Portaels F, Aguiar J, Steunou C, Zinsou C, Meyers W, Dramaix M. 2006. Risk factors for Buruli ulcer, Benin. Emerg. Infect. Dis. 12, 1325–1331. ( 10.3201/eid1209.050598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nackers F, Johnson RC, Glynn JR, Zinsou C, Tonglet R, Portaels F. 2007. Environmental and health-related risk factors for Mycobacterium ulcerans disease (Buruli ulcer) in Benin. Am. J. Trop. Med. Hyg. 77, 834–836. [PubMed] [Google Scholar]
- 46.Garchitorena A, Guégan J-F, Léger L, Eyangoh S, Marsollier L, Roche B. 2015. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. Elife 4, 1–19. ( 10.7554/eLife.07616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garchitorena A, et al. 2014. Mycobacterium ulcerans ecological dynamics and its association with freshwater ecosystems and aquatic communities: results from a 12-month environmental survey in Cameroon. PLoS Negl. Trop. Dis. 8, e2879 ( 10.1371/journal.pntd.0002879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garchitorena A, Ngonghala CN, Texier G, Landier J, Eyangoh S, Bonds MH, Guégan J-F, Roche B. 2015. Environmental transmission of Mycobacterium ulcerans drives dynamics of Buruli ulcer in endemic regions of Cameroon. Nat. Sci. Reports 5, 18055 ( 10.1038/srep18055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsollier L, et al. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68, 4623–4628. ( 10.1128/AEM.68.9.4623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai Y, et al. 2015. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet. Infect. Dis. 3099, 1–14. ( 10.1016/S1473-3099(15)00066-3) [DOI] [PubMed] [Google Scholar]
- 51.Hotez PJ, et al. 2014. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 8, e0002865 ( 10.1371/journal.pntd.0002865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross AGP, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. 2002. Schistosomiasis. N. Engl. J. Med. 346, 1212–1220. ( 10.1056/NEJMra012396) [DOI] [PubMed] [Google Scholar]
- 53.Hotez PJ, Fenwick A, Kjetland EF. 2009. Africa's 32 cents solution for HIV/AIDS. PLoS Negl. Trop. Dis. 3, e430 ( 10.1371/journal.pntd.0000430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokolow S, et al. 2016. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 7, e0004794 ( 10.1371/journal.pntd.0004794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC, Rollinson D. 2013. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop. 128, 292–302. ( 10.1016/j.actatropica.2012.09.010) [DOI] [PubMed] [Google Scholar]
- 56.Sokolow SH, et al. 2015. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl Acad. Sci. USA 112, 9650–9655. ( 10.1073/pnas.1502651112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6, 411–425. ( 10.1016/S1473-3099(06)70521-7) [DOI] [PubMed] [Google Scholar]
- 58.Sokolow SH, et al. 2017. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Phil. Trans. R. Soc. B 372, 20160127 ( 10.1098/rstb.2016.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sow S, de Vlas SJ, Engels D, Gryseels B. 2002. Water-related disease patterns before and after the construction of the Diama dam in northern Senegal. Ann. Trop. Med. Parasitol. 96, 575–586. ( 10.1179/000349802125001636) [DOI] [PubMed] [Google Scholar]
- 60.Sokolow SH, Lafferty KD, Kuris AM. 2014. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop. 132, 64–74. ( 10.1016/j.actatropica.2013.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swartz SJ, De Leo GA, Wood CL, Sokolow SH. 2015. Infection with schistosome parasites in snails leads to increased predation by prawns: implications for human schistosomiasis control. J. Exp. Biol. 218, 3962–3967. ( 10.1242/jeb.129221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JY, Farmer PE, Porter ME. 2013. Redefining global health-care delivery. Lancet 382, 1060–1069. ( 10.1016/S0140-6736(13)61047-8) [DOI] [PubMed] [Google Scholar]
- 63.Sachs JD. 2012. Achieving universal health coverage in low-income settings. Lancet 380, 944–947. ( 10.1016/S0140-6736(12)61149-0) [DOI] [PubMed] [Google Scholar]
- 64.Prüss-Üstün A, Corvalán C. 2007. How much disease burden can be prevented by environmental interventions? Epidemiology 18, 167–178. ( 10.1097/01.ede.0000239647.26389.80) [DOI] [PubMed] [Google Scholar]
- 65.Kilpatrick AM, Salkeld DJ, Titcomb G, Hahn MB. 2017. Conservation of biodiversity as a strategy for improving human health and well-being. Phil. Trans. R. Soc. B 372, 20160131 ( 10.1098/rstb.2016.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pattanayak SK, Kramer RA, Vincent JR. 2017. Ecosystem change and human health: implementation economics and policy. Phil. Trans. R. Soc. B 372, 20160130 ( 10.1098/rstb.2016.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization 2014. World malaria report 2013. Geneva, Switzerland: WHO.
- 68.Khan SU, Gurley ES, Hossain MJ, Nahar N, Sharker MAY, Luby SP. 2012. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS ONE 7, 1–7. ( 10.1371/journal.pone.0042689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Islam MS, et al. 2016. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 22, 664–670. ( 10.3201/eid2204.151747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organisation 2015. Water sanitation & hygiene for accelerating and sustaining progress on neglected tropical diseases 2015–2020. Geneva, Switzerland: WHO. [Google Scholar]
- 71.Pointier JP, Theron A, Imbert-Establet D. 1989. Decline of a sylvatic focus of Schistosoma mansoni in Guadeloupe (French West Indies) following the competitive displacement of the snail host Biomphalaria glabrata by Ampullaria glauca. Oecologia 75, 38–43. ( 10.1007/BF00378811) [DOI] [PubMed] [Google Scholar]
- 72.Wu T, Perrings C. 2017. Conservation, development and the management of infectious disease: avian influenza in China, 2004–2012. Phil. Trans. R. Soc. B 372, 20160126 ( 10.1098/rstb.2016.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young HS, et al. 2017. Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Phil. Trans. R. Soc. B 372, 20160116 ( 10.1098/rstb.2016.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamareddine L. 2012. The biological control of the malaria vector. Toxins (Basel) 4, 748–767. ( 10.3390/toxins4090748) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.