Abstract
Biodiversity is of critical value to human societies, but recent evidence that biodiversity may mitigate infectious-disease risk has sparked controversy among researchers. The majority of work on this topic has focused on direct assessments of the relationship between biodiversity and endemic-pathogen prevalence, without disentangling intervening mechanisms; thus study outcomes often differ, fuelling more debate. Here, we suggest two critical changes to the approach researchers take to understanding relationships between infectious disease, both endemic and emerging, and biodiversity that may help clarify sources of controversy. First, the distinct concepts of hazards versus risks need to be separated to determine how biodiversity and its drivers may act differently on each. This distinction is particularly important since it illustrates that disease emergence drivers in humans could be quite different to the general relationship between biodiversity and transmission of endemic pathogens. Second, the interactive relationship among biodiversity, anthropogenic change and zoonotic disease risk, including both direct and indirect effects, needs to be recognized and accounted for. By carefully disentangling these interactions between humans' activities and pathogen circulation in wildlife, we suggest that conservation efforts could mitigate disease risks and hazards in novel ways that complement more typical disease control efforts.
This article is part of the themed issue ‘Conservation, biodiversity and infectious disease: scientific evidence and policy implications’.
Keywords: hazards, risk, biodiversity, zoonotic, emerging infectious disease, pathogen
1. Introduction
Disease outbreaks caused by emerging pathogens such as Influenza/A viruses, severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome coronavirus, Nipah virus and Ebola viruses have focused the attention of the media, the public and policy-makers on the causes of disease emergence [1–4]. Currently, scientific studies consider (i) the role of anthropogenic change, particularly land-use change, on disease emergence [5] or (ii) how biodiversity affects the dynamics of endemic wildlife zoonoses and spillover risk to humans [6,7]. However, no studies, to the best of our knowledge, try to link the two ideas. This failure to recognize the entangled and interactive relationships between biodiversity, anthropogenic change, the circulation level of endemic pathogens and the risk of disease emergence in humans has limited researchers' ability to predict when and at what spatio-temporal scales the emergence or outbreak of zoonotic diseases are most likely to occur [6–8] (see glossary for a definition of terms).
For example, research on specific disease systems like Lyme disease [9], West Nile virus [10] and a few others [11] suggests that more diverse systems often have less pathogen transmission, and thus present a lower hazard, or source of harm to humans, even if the generality of this pattern is still debated [12]. Alternatively, a series of large-scale analyses spanning multiple disease systems suggest that more diverse systems pose a greater hazard [8,13]. However, most of these studies tend to focus on biodiversity as an explanatory variable without accounting for confounding variables such as land-use change. At the extreme, this leads to the short-sighted ideas that the most biodiverse systems will always pose the least risk in terms of disease emergence, or that all risk of emerging disease can be eliminated if biodiversity is eliminated. These views do not consider the links between biodiversity and processes that convert hazards into risks—processes influenced strongly by anthropogenic change.
In order to understand how drivers of disease emergence (e.g. land-use change) and changes in biodiversity are inherently confounded, we need to consider separately how the hazards and the risks of disease emergence relate to biodiversity. As in the field of quantitative risk analysis [14], a hazard is a potential source of harm, while risk is the likelihood of adverse events (e.g. disease outbreaks) caused by exposure to a hazard (e.g. pathogen), potentially weighted by the severity of the adverse event (e.g. expected number of cases). Within this context, the term risk factor can be used to describe those factors that either increase or decrease risk. For example, in the case of Ebola virus disease, Ebola viruses circulating in wildlife are the hazard, an outbreak in the human population is the adverse event, and there are a number of risk factors, bushmeat consumption, hunting in the deep forest and quality of public health infrastructure, that can affect the risk of an outbreak by influencing its probability or severity [15].
To illustrate the different ways in which biodiversity relates to disease emergence, we will review three main issues: (i) how biodiversity relates to the hazard of emergence; (ii) how biodiversity relates to the risk of emergence; and (iii) how the concurrent and confounding effects of anthropogenic change, particularly land-use change, influence the relationship between biodiversity and disease emergence (box 1 provides specific examples). Finally, we explore the utility of developing management strategies [16,17] aimed at mitigating the effects of anthropogenic change to reduce the risk of disease emergence and conserve biodiversity.
Box 1. Relationship between microbial hazard, human exposure, vulnerability and disease severity.
Risk = Hazard × (Vulnerability × Exposure)
Impact = Risk × Severity = Hazard × (Vulnerability × Exposure) × Severity
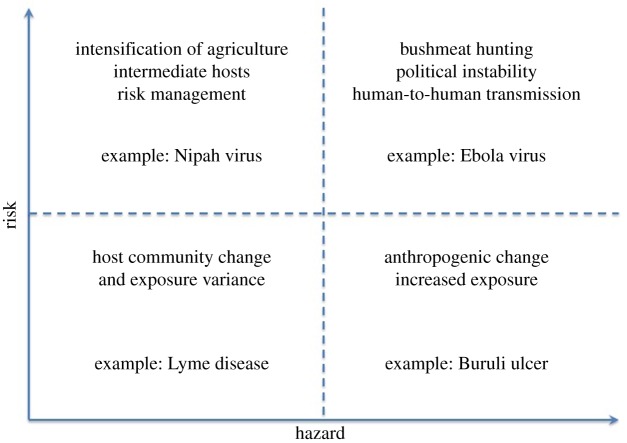
Here, we define risk, hazard, vulnerability, severity and impact to humans from microbial hazards. Hazards are defined as potential sources of harm from microbes, such as viruses, bacteria and other pathogens. Exposure represents the likelihood of contact, including vector-borne transmission, between humans and the hazards, and vulnerability represents the possibility given exposure that the microbial hazard can actually cause harm. Severity is a measure of actual harm done, such as DALY, which includes human mortality and morbidity, and together these variables incorporate the impact of an outbreak. As there is no suggested predictive relationship between biodiversity and severity, we limit our discussion to the distinction between hazard and risk (figure 1).
Buruli ulcer. Buruli ulcer is a devastating emerging disease of the skin caused by the aquatic environmentally persistent pathogen Mycobacterium ulcerans. It mostly affects rural populations in the tropical world and notably the poorest ones of Central and Western Africa. Geographical distribution of both the pathogen and disease cases has been recently associated with aquatic ecosystems especially in areas with slow-flowing and stagnant waters including swamps and flat river floodplains. The distribution of Buruli ulcer is highly focal both at national and local scales, and disease risk increases with human modifications of these natural ecosystems, such as for cultivation and floodplain forest clearing. As human encroachments and new settlements increase in many tropical areas (e.g. for dam construction, aquaculture and agriculture production) human individuals and communities are now more exposed to this aquatic microbial hazard, which may benefit from land-use changes and anthropization to flourish in aquatic ecosystems and exacerbate the risk of skin infection for the most exposed and susceptible human individuals.
Nipah virus. Human Nipah virus infection was first recognized in reported cases with a mortality rate of near 40% in peninsular Malaysia and Singapore in 1998 and 1999. Large fruit bats of the genus Pteropus appear to be the natural reservoir of Nipah virus, and the virus was isolated from urine or saliva specimens. Fruit bats commonly drop partially eaten saliva-laden fruit contaminated with Nipah virus. Because intensive pig farming in Malaysia was combined with fruit orchards, this led to frequent exposure of pigs to Nipah virus. Unfortunately, pigs can transmit the virus to other pigs, particularly efficiently under intensive farming practices, and transmit it to human farm workers, causing a prolonged outbreak. The outbreak led to a reduction in pig farming in Malaysia as well as adoption of risk reduction practices at the remaining pig farms, including separating pigs from fruit trees on which bats could feed, and increases in biosecurity practices.
Lyme disease in fragmented areas. Lyme disease, caused by Borrelia burdorferi and transmitted mostly between Ixodes scapularis ticks and mammals, re-emerged in the northeastern USA several decades ago. While such re-emergence events are multi-factorial, habitat alteration by human activities has been shown to dramatically increase the risk of transmission through the change in host community composition. For instance, in suburban areas with low diversity, the key amplifying reservoir is generally more abundant, thus these areas have the greatest hazard due to increased prevalence in nymphal ticks. These areas also have the highest exposure to humans. Meanwhile, large forest tracts have more diversity, are less dominated by the key amplifying reservoir, and also probably have less human exposure, thus having less risk. However, the densest urban areas of the northeast USA may completely lack reservoir hosts, and have humans who have the least exposure to the nymphal ticks, eliminating the hazard locally. This local elimination reduces risk but does not eliminate it, as people may readily travel to areas where the hazard is present, thus risking exposure.
Ebola viruses. Ebola virus disease outbreaks often occur in areas where humans rely in part on bushmeat hunting for animal protein, either due to culinary preferences or to lack of alternative sources of protein. Even if the bushmeat is hunted with intent to trade to urban areas, the actual hunting often occurs where fairly dense human populations are in proximity to areas of high diversity. However, the very act of bushmeat hunting often reduces the biodiversity of these same areas. The risks are further exacerbated because populations that rely on bushmeat hunting are often economically insecure in areas that have frequent political instability and thus less developed healthcare infrastructure and less trust of these resources. Thus, highly vulnerable populations with high exposure are exposed to a very harmful hazard.
2. Disease hazards related to biodiversity
Around half of all known infectious agents of humans (and the majority of EIDs) are carried by animals (i.e. are zoonoses), and most emerging zoonoses originate in wildlife [18]. Moreover, most EIDs infect multiple host species [8,19]. How then do we predict the risk of disease emergence when the sources of potential EIDs are so diverse? One key predictor of emergence risk should be the hazard presented by the diversity of microparasites (e.g. viruses and bacteria) and macroparasites (e.g. helminths and protozoa) that can be pathogenic to humans [2]. Yet, there are few to no data on the hazard presented by the broad diversity of potentially pathogenic organisms [20]. Instead, scientists have used available data on the biodiversity of mammals or other vertebrates as predictors of the biodiversity of potential infectious agents [8,19], since the majority of pathogens originate in wildlife. Thus, wildlife is often seen as a natural reservoir for the hazard of infectious agents, but it does not automatically follow that biodiversity hotspots (i.e. geographical areas where wildlife biodiversity is high) are necessarily disease emergence hotspots (i.e. geographical areas where the risk of disease emergence is high) [8]. Indeed, biodiversity here relates only to the prediction of the hazard, not the prediction of the risk.
3. Disease risk related to biodiversity
The process of emergence also involves other factors (e.g. human presence and contact with disease reservoirs, high-risk behaviours, pathogen transmissibility) that can convert a hazard into risk of pathogen emergence [1,21]. For many zoonotic pathogens, contact with wildlife reservoirs and vectors is a key risk [22,23]. This is in part because ‘host-jumping’—the process by which a pathogen infects a novel host—among humans, domestic animals and wildlife is more likely when human activities occur in or near wildlife areas, creating opportunities for jumps to occur. For vector-borne diseases, this can include exposure to vectors that can switch between wildlife and humans [24,25]. This includes human activities such as wildlife trade and environmental changes that may favour contacts between susceptible humans and infectious agents and/or their hosts [1,26]. Without a greater understanding of the limits on host jumping, and what makes some types of pathogens more or less likely to jump, it is difficult to predict how any given hazard may convert into a risk.
A beneficial role of biodiversity in limiting or preventing outbreaks of disease has been observed at genetic and species levels [27–29]. In these studies, a reduction in species or genotypic diversity, for instance, is typically correlated with increases in pathogen prevalence in reservoir hosts or vectors. An increasing number of studies also show correlations between levels of species diversity, often measured as host species richness, and a variety of metrics of zoonotic disease occurrence in humans or zoonotic pathogen prevalence in animals or vectors [6,29], with lower species diversity generally correlated with increased infection rates. This has been shown, for example, for Lyme disease in the eastern USA [30], West Nile virus infection [10], hantavirus disease [23], Buruli ulcer [31], leptospirosis on Pacific Islands and other diseases of humans, wildlife and plants [27,32]. These studies suggest that biodiversity could be a factor in mitigating the risk of disease spillover, by reducing the transmission of zoonotic pathogens in their natural reservoirs, and subsequently to humans.
In summary, greater biodiversity is expected to increase the hazard of emerging infectious diseases, because host diversity (e.g. mammalian diversity) is correlated with pathogen diversity, which is often assumed to predict hazard [8] (see figure 1 in box 1). Yet this relationship is sufficiently indirect and it should not be expected that it would be predictive of disease risk at all scales or in all systems [6]. Paradoxically, greater host biodiversity may decrease risk of zoonotic pathogen spillover (for both emerging and established zoonoses) by reducing the prevalence of pathogens among a diversity of host species; and yet this effect may also be inconsistent [12,28,29]. Finally, because human activity often decreases biodiversity, areas with high diversity often are areas with lower human presence, and thus are at lower risk due to lower human exposure to novel pathogenic hazards. If human activity increases—increasing exposure—humans in these areas may experience higher risk due to the high hazards present. This risk could also be increased further by disruption of local ecosystems and reduction of local biodiversity [1,2]. Thus, we have paradoxical effects of biodiversity on disease risk because it has different effects on the risk and the hazards, and these effects are not always consistent (see box 1).
Figure 1.
Examples of the impacts of anthropogenic changes on risk and hazards. (Online version in colour.)
As an example, bats are considered to harbour a high diversity of pathogens, particularly viruses [33,34]. This would suggest that the diversity of bats is a key to understanding the diversity of hazardous viruses [2]. However, in the best-studied cases of disease emergence and spillover from bats, anthropogenic factors have been critical to their emergence in human and livestock populations [5,15,35]. The case of Hendra virus is particularly illustrative, because the anthropogenic impacts include not only reductions of native biodiversity (e.g. native fruit trees with geographically different seasonal availability requiring bat migration), but also introductions of non-native species (e.g. irrigated suburban fruit trees, which allow bats to cease migratory habits) and changes in reservoir host population demography and behaviour, which seem to have increased the prevalence of Hendra virus infection in the bat reservoir [2,5]. Thus, Hendra virus seems to be an example where the pre-disturbance system would have had a pathogen hazard (Hendra virus), at low prevalence in a naturally highly diverse ecosystem. Yet anthropogenic impacts increased the risk by reducing local diversity, altering the dynamics of the pathogen to increase its prevalence in bats, and increasing contact among humans, livestock and bats. Thus, while the hazard of these viruses has probably always been present in Australasia, anthropogenic impacts, including (but not limited to) biodiversity loss, have increased risks to humans.
4. Concurrent human impacts on biodiversity and disease
From the Hendra virus example, we see that anthropogenic impacts are an important driver of disease emergence, which can alter and interact with biodiversity. Human impacts, particularly land-use change, are cited as both a driver of disease emergence and a driver of biodiversity loss [2,28,36–39]. Thus, human impacts have the potential to alter the hazards and risks of disease emergence at the same time as they alter biodiversity, independent of any particular association between biodiversity and infectious disease.
For example, anthropogenic land-use changes cause habitat destruction, fragmentation, local species extinction and habitat homogenization. All of these effects, in turn, contribute to the loss of biodiversity. These human-induced changes also impact disease because homogenization of ecosystems and landscapes may increase similarity among ecological communities favouring generalist host species over habitat and dietary specialists [24,36,40–43]. While habitat fragmentation can disrupt connectivity and gene flow between host populations leading to declines in disease resistance [39], land-use change can also introduce human-associated invasive species (rats, cats, pigs, etc.), altering the ecological community and diversity in ways that affect the pathogen community [36,38,44,45]. Therefore, these human-associated species can act as bridge hosts, increasing zoonotic transmission via contacts with both humans and wildlife. Human-associated species may also act as amplifying hosts, increasing pathogen prevalence [46]. Importantly, humans directly involved in land-use change activities (e.g. deforestation, dam construction, agriculture and mining) generally have higher contact with wildlife and often engage in behaviours that increase their risk of exposure to zoonotic diseases [1,2,25,47–49]. Thus, anthropogenic activities can act as a potent force shaping the ecological dynamics and diversity of both hosts and pathogens.
To reduce the risk of disease emergence using ecological knowledge, we need to understand how land-use change and other anthropogenic impacts, including biodiversity loss, relate to the risks and hazards of disease emergence. Bushmeat hunting, for example, is a major cause of biodiversity loss [50], and has been directly linked to the emergence of infectious diseases, notably AIDS [51,52] and Ebola virus disease [15]. Thus, the process of anthropogenic loss of biodiversity can increase the risk of emergence of novel infectious diseases for reasons that are more directly related to processes that reduce biodiversity (e.g. bushmeat hunting), rather than the numerical or compositional value of biodiversity per se. Further, if areas with high reservoir host diversity support a higher diversity of pathogens, the process of anthropogenic reduction of biodiversity in areas of high biodiversity could lead to a greater risk of EIDs than similar disturbance in areas of low biodiversity (lower hazards with lower initial biodiversity, but similar risk processes). The area with high initial biodiversity would have more pathogen hazards than the area with lower initial biodiversity, but the risk factors involved with anthropogenic change would be similar, even if these activities reduced biodiversity to similar levels. Yet if the high-biodiversity area is left intact, it may present less risk, despite higher pathogen hazards, because the risk factors would be kept low.
However, the relationship between biodiversity protection and disease risk is not always simple either [53,54]. For example, conservation corridors, which can increase movement between patches in a metapopulation, can increase the population of an endangered species and increase the spread of disease throughout the metapopulation. McCallum & Dobson [55] showed that some movement rates decrease an endangered species population size, by enabling the spread of a pathogen. Yet other movement rates can maximize the endangered species population size, and minimize pathogen spread. Likewise, Köndgen et al. [56] demonstrated that ecotourism can help prevent poaching, protecting wildlife on one hand, but increasing the risk of reverse zoonotic spillover from humans to apes on the other. Yet we can still develop epidemiological models based on our ecological understanding of these systems to minimize risk, even if the relationship is nonlinear.
More could be done to understand how to balance and combine the goals of conserving diversity and reducing risk from emerging infectious diseases, and zoonotic diseases, more generally. To this extent, McCarthy et al. [57] used reserve design and cost allocation methods to balance direct costs and benefits of conservation programmes. Their methods suggest ways to more productively allocate resources for conservation of biodiversity hotspots combined with efficient surveillance for influenza in Thailand. While some biologists are working with land-use planners locally to try and incorporate knowledge of disease risk into land-use planning, and the US Environmental Protection Agency [58] has sponsored regional meetings to encourage such activities, we could also use outcomes of experimental and theoretical work to understand how ecological knowledge about disease spillover and risk can be used to minimize and mitigate risks.
5. Conclusion
Human efforts to minimize biodiversity loss could also reduce disease risk, mostly by reducing contact between humans and wildlife and limiting introduction of exotic species, even if these efforts maintain areas of biodiversity of pathogen hazards. Despite the fact that we do not fully understand biodiversity vis-a-vis disease risk in the absence of human impact, anthropogenic changes that reduce biodiversity can increase the risk of disease, either directly or indirectly. We can increase the likelihood that both goals—disease risk reduction and biodiversity conservation—are met if we explicitly address both goals, and measure outcomes of management strategies for both issues. Although understanding the role of biodiversity is important in understanding the risk of disease emergence, more research is needed on how to manage ecosystems to mitigate disease emergence and outbreaks while ensuring other ecosystem functions and services [59].
In this context, deciphering the concept of hazards and risks could shed a new light on the possible best-management options. Given anthropogenic changes, how do we best manage the impacts on biodiversity to reduce the risk of disease emergence? Are particular areas or ecosystems more likely to have greater pathogen hazards, and thus be more likely to generate risk of disease emergence in response to human modification? Are certain anthropogenic impacts more likely to convert hazards into risks for humans and other species? To limit risk, is it better to intensively change a small area in a concentrated way, or to distribute the impact more broadly? Although there are examples where research addresses these issues, typically they are not presented under a management strategies framework [5]. Most studies of ecological disease dynamics, spillover and emergence have been post hoc analyses. In specific cases, it would be useful to develop ecological management strategies to reduce risk, protect biodiversity and maintain ecosystem services. However, this should involve monitoring of changes in land-use planning and review of a plan's successes and failures over time. These reviews can then be used to adapt a management strategy to maximize its success.
In order to use ecological knowledge to reduce the risk of environmentally acquired pathogens and zoonotic diseases, particularly EIDs, we need to focus research on two complementary dimensions of disease dynamics simultaneously. First, we must continue to study the diversity of microbes and parasites, particularly potential pathogens, their vectors, hosts and non-hosts, and their inter- and intra-specific relationships. Second, we must understand how anthropogenic impacts alter ecological processes and convert microbial hazards in naturally occurring pathogen diversity into risks to the health of humans and other species [60]. Given that human impacts will continue, we need to strengthen research on using our understanding of ecological process to limit and mitigate the effects of anthropogenic impacts. Thus, we recommend that disease ecology incorporate more diverse approaches that embrace the confounded nature of the drivers of disease spillover and biodiversity loss, including more applied approaches that focus on restoration of ecosystem function and mitigation of impacts. While biodiversity itself may alter the hazards and risks of disease spillover and emergence, the drivers of biodiversity loss may also be drivers of disease spillover and emergence independently [36,61]. Therefore, we need to conduct research on management strategies in addition to abstract questions. Research on these management strategies will greatly benefit from using co-designed (and co-produced) research involving researchers in both health (medical and veterinary) and ecology disciplines as well as practitioners in ecology, conservation, land-use planning, public health and veterinary sciences in reducing the risk of disease emergence.
Competing interests
We declare we have no competing interests.
Funding
The project received additional financial support from DIVERSITAS and the Fondation Total. This work also benefited from an ‘Investissement d'Avenir’ grant from Agence Nationale de la Recherche (ANR-10-LABX-2501) to J-F Guégan, B. Roche, and G. E. Garcia-Peña; an NSF Research Coordination Network grant ‘EcoHealthNet’ (DEB-0955897) to P. Daszak, and funding from the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT. The BIODIS project is scientifically supported by the French Foundation for Biodiversity Research (FRB; www.fondationbiodiversite.fr) through its Centre for Synthesis and Analysis of Biodiversity (CESAB, www.cesab.org) and the DIVERSITAS-ecoHEALTH project (www.diversitas-international.org).
Disclaimer
The contents are the responsibility of the authors and do not necessarily reflect the views of NSF, USAID or the United States Government.
Glossary
- Biodiversity
the UN Convention on Biological Diversity defines biological diversity as ‘the variability among living organisms from all sources including terrestrial, marine and aquatic ecosystems and the ecological complexes of which they are a part: this includes diversity within species, between species and of ecosystems’
- Community
an ecological unit composed of all the populations of species occurring in a particular area, and usually interacting with each other and their environment
- Connectivity (landscape)
degree to which the landscape facilitates or impedes movement among resource patches. Connectivity includes both structural connectivity (the physical arrangements of patches) and functional connectivity (the movement of individuals among patches)
- EID
emerging infectious disease, usually referring to the spread of a pathogen to novel hosts, particularly humans, novel areas or increasing incidence that qualitatively alters the impact of the disease, from localized to regional or global concern
- Global environmental change
the set of biophysical transformation of states and flows of land, oceans and atmosphere, driven by an interwoven system of human and natural processes; these are intimately connected with processes of socio-economic and cultural globalization
- Hazard
a potential source of harm or adverse effect
- Host switching or host jumping
process by which a parasite which is endemic to one species is transmitted to another host and potentially impacts or spreads in the novel host.
- Parasite
organism benefiting at the expense of another organism, the host
- Pathogen or infectious agent
a microorganism—in the widest sense, such as a virus, bacterium, prion or fungus—that causes disease in its host. The host may be an animal (including humans), a plant or another microorganism
- Risk
the likelihood that harm occurs when in the vicinity of a hazard
- Zoonoses
diseases characterized by their agents being shared between vertebrate animals and humans
References
- 1.Weiss RA, McMichael AJ. 2004. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 10, S70–S76. ( 10.1038/nm1150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray KA, Daszak P. 2013. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Curr. Opin. Virol. 3, 79–83. ( 10.1016/j.coviro.2013.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson PTJ, de Roode JJC, Fenton A. 2015. Why infectious disease research needs community ecology. Science 349, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezenwa VO, et al. 2015. Interdisciplinarity and infectious diseases: an Ebola case study. PLoS Pathog. 11, e1004992 ( 10.1371/journal.ppat.1004992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostfeld RS, Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. ( 10.1146/annurev-ecolsys-102710-145022) [DOI] [Google Scholar]
- 7.Roche B, Dobson AP, Guégan J-F, Rohani P. 2012. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Phil. Trans. R. Soc. B 367, 2807–2813. ( 10.1098/rstb.2011.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–994. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567–571. ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swaddle JP, Calos SE. 2008. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PLoS ONE 3, e2488 ( 10.1371/journal.pone.0002488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Civitello DDJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faust CL, Dobson AP, Gottdenker N, Bloomfield LSP, McCallum HI, Gillespie TR, Diuk-Wasser M, Plowright RK. 2017. Null expectations for disease dynamics in shrinking habitat: dilution or amplification? Phil. Trans. R. Soc. B 372, 20160173 ( 10.1098/rstb.2016.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood C, Lafftery K, DeLeo G, Young H, Hudson P, Kuris A. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 14.Stamatis DH. 2014. Introduction to risk and failures: tools and methodologies. Boca Raton, FL: CRC Press. [Google Scholar]
- 15.Muyembe-Tamfum JJ, Mulangu S, Masumu J, Kayembe JM, Kemp A, Paweska JT. 2012. Ebola virus outbreaks in Africa: past and present. Onderstepoort J. Vet. Res. 79, 451. [DOI] [PubMed] [Google Scholar]
- 16.Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 35, 557–581. ( 10.1146/annurev.ecolsys.35.021103.105711) [DOI] [Google Scholar]
- 17.Langwig KE, et al. 2015. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 13, 195–202. ( 10.1890/140241) [DOI] [Google Scholar]
- 18.Woolhouse MEJ. 2008. Emerging diseases go global. Nature 451, 898–899. ( 10.1038/451898a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KF, Guégan J-F. 2010. Changing geographic distributions of human pathogens. Annu. Rev. Ecol. Evol. Syst. 41, 231–250. ( 10.1146/annurev-ecolsys-102209-144634) [DOI] [Google Scholar]
- 20.Poulin R. 2014. Parasite biodiversity revisited: frontiers and constraints. Int. J. Parasitol. 44, 581–589. ( 10.1016/j.ijpara.2014.02.003) [DOI] [PubMed] [Google Scholar]
- 21.Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, Calisher CH, Laughlin CA, Saif LJ, Daszak P. 2008. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 72, 457–470. ( 10.1128/MMBR.00004-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumilo D, Asokliene L, Bormane A, Vasilenko V, Golovljova I, Randolph SE, Sutherland C. 2007. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS ONE 2, e500 ( 10.1371/journal.pone.0000500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzán G, et al. 2009. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PLoS ONE 4, e5461 ( 10.1371/journal.pone.0005461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allan BF, Keesing F, Ostfeld RS. 2003. Effect of forest fragmentation on lyme disease risk. Conserv. Biol. 17, 267–272. ( 10.1046/j.1523-1739.2003.01260.x) [DOI] [Google Scholar]
- 25.Cox-Singh J, Singh B. 2008. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 24, 406–410. ( 10.1016/j.pt.2008.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolhouse MEJ, Gowtage-Sequeria S. 2005. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842–1847. ( 10.3201/eid1112.050997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. 2000. Genetic diversity and disease control in rice. Nature 406, 718–722. ( 10.1038/35021046) [DOI] [PubMed] [Google Scholar]
- 28.Thomas F, Guégan JF, Renaud F. 2009. Ecology and evolution of parasitism. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkerhoff RJ, Folsom-O'Keefe CM, Tsao K, Diuk-Wasser MA. 2009. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. Front. Ecol. Environ. 9, 103–110. ( 10.1890/090062) [DOI] [Google Scholar]
- 31.García-Peña GE, Garchitorena A, Carolan K, Canard E, Prieur-Richard AH, Suzán G, Mills JN, Roche B, Guégan JF. 2016. Niche-based host extinction increases prevalence of an environmentally-acquired pathogen. Oikos 125, 1508–1515. ( 10.1111/oik.02700) [DOI] [Google Scholar]
- 32.Johnson PTJ, Hartson RB, Larson DJ, Sutherland DR. 2008. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 11, 1017–1026. ( 10.1111/j.1461-0248.2008.01212.x) [DOI] [PubMed] [Google Scholar]
- 33.Anthony S, et al. 2013. A strategy to estimate unknown viral diversity in mammals. MBio. 4, e00598-13. ( 10.1128/mBio.00598-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280, 20122753 ( 10.1098/rspb.2012.2753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulliam JRC, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9, 89–101. ( 10.1098/rsif.2011.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patz JA, et al. 2004. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 112, 1092–1098. ( 10.1289/ehp.6877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills JN, Gage KL, Khan AS. 2010. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 118, 1507–1514. ( 10.1289/ehp.0901389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pysek P, Richardson DM. 2010. Invasive species, environmental change and management, and Health. Annu. Rev. Environ. Resour. 35, 25–55. [Google Scholar]
- 39.Jousimo J, Tack AJM, Ovaskainen O, Mononen T, Susi H, Tollenaere C, Laine A-L. 2014. Ecological and evolutionary effects of fragmentation on infectious disease dynamics. Science 344, 1289–1293. ( 10.1126/science.1253621) [DOI] [PubMed] [Google Scholar]
- 40.Dobson A, Cattadori I, Holt RD, Ostfeld RS, Keesing F, Krichbaum K, Rohr JR, Perkins SE, Hudson PJ. 2006. Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Med. 3, 1–5. ( 10.1371/journal.pmed.0030231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinney ML. 2002. Urbanization, biodiversity, and conservation. Bioscience 52, 883–890. ( 10.1641/0006-3568%282002%29052%5B0883%3AUBAC%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 42.Rubio AV, Ávila-Flores R, Suzán G. 2014. Responses of small mammals to habitat fragmentation: epidemiological considerations for rodent-borne hantaviruses in the Americas. Ecohealth 11, 526–533. ( 10.1007/s10393-014-0944-9) [DOI] [PubMed] [Google Scholar]
- 43.Dar PA, Reshi ZA. 2015. Do alien plant invasions cause biotic homogenization of terrestrial ecosystems in the Kashmir Valley, India? Trop. Ecol. 56, 111–123. [Google Scholar]
- 44.Wolfe LM, Elzinga JA, Biere A. 2004. Increased susceptibility to enemies following introduction in the invasive plant Silene latifolia. Ecol. Lett. 7, 813–820. ( 10.1111/j.1461-0248.2004.00649.x) [DOI] [Google Scholar]
- 45.Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE. 2008. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 6, 238–246. ( 10.1890/070151) [DOI] [Google Scholar]
- 46.Bevins SN, Pedersen K, Lutman MW, Gidlewski T, Deliberto TJ. 2014. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 64, 291–299. ( 10.1093/biosci/biu015) [DOI] [Google Scholar]
- 47.Bausch DG, et al. 2003. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 9, 1531–1537. ( 10.3201/eid0912.030355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, Benbow ME, Boakye DA, Phillips RO. 2010. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl. Trop. Dis. 4, e911 ( 10.1371/journal.pntd.0000911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adjemian J, et al. 2011. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 204, S796–S799. ( 10.1093/infdis/jir312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison RD. 2011. Emptying the forest: hunting and the extirpation of wildlife from tropical nature reserves. Bioscience 61, 919–924. ( 10.1525/bio.2011.61.11.11) [DOI] [Google Scholar]
- 51.Chen Z, Telfier P, Gettie A, Reed P, Zhang L, Ho DD, Marx PA. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70, 3617–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keele BF, et al. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313, 523–526. ( 10.1126/science.1126531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilpatrick AM, Salkeld DJ, Titcomb G, Hahn MB. 2017. Conservation of biodiversity as a strategy for improving human health and well-being. Phil. Trans. R. Soc. B 372, 20160131 ( 10.1098/rstb.2016.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millins C, Gilbert L, Medlock J, Hansford K, Thompson DBA, Biek R. 2017. Effects of conservation management of landscapes and vertebrate communities on Lyme borreliosis risk in the United Kingdom. Phil. Trans. R. Soc. B 372, 20160123 ( 10.1098/rstb.2016.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCallum H, Dobson A. 2002. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. B 269, 2041–2049. ( 10.1098/rspb.2002.2079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Köndgen S, et al. 2008. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. 18, 260–264. ( 10.1016/j.cub.2008.01.012) [DOI] [PubMed] [Google Scholar]
- 57.McCarthy MA, Thompson CJ, Hauser C, Burgman MA, Possingham HP, Moir ML, Tiensin T, Gilbert M. 2010. Resource allocation for efficient environmental management. Ecol. Lett. 13, 1280–1289. ( 10.1111/j.1461-0248.2010.01522.x) [DOI] [PubMed] [Google Scholar]
- 58.US EPA. 2009. Extramural Research - Biodiversity and Human Health.
- 59.Postel SL, Thompson BH. 2005. Watershed protection: capturing the benefits of nature's water supply services. Nat. Resour. Forum. 29, 98–108. ( 10.1111/j.1477-8947.2005.00119.x) [DOI] [Google Scholar]
- 60.Woolhouse MEJ, Haydon DT, Antia R. 2005. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 20, 238–244. ( 10.1016/j.tree.2005.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. 2008. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Front. Ecol. Environ. 6, 420–429. ( 10.1890/070086) [DOI] [Google Scholar]