Abstract
As biodiversity declines with anthropogenic land-use change, it is increasingly important to understand how changing biodiversity affects infectious disease risk. The dilution effect hypothesis, which points to decreases in biodiversity as critical to an increase in infection risk, has received considerable attention due to the allure of a win–win scenario for conservation and human well-being. Yet some empirical data suggest that the dilution effect is not a generalizable phenomenon. We explore the response of pathogen transmission dynamics to changes in biodiversity that are driven by habitat loss using an allometrically scaled multi-host model. With this model, we show that declining habitat, and thus declining biodiversity, can lead to either increasing or decreasing infectious-disease risk, measured as endemic prevalence. Whether larger habitats, and thus greater biodiversity, lead to a decrease (dilution effect) or increase (amplification effect) in infection prevalence depends upon the pathogen transmission mode and how host competence scales with body size. Dilution effects were detected for most frequency-transmitted pathogens and amplification effects were detected for density-dependent pathogens. Amplification effects were also observed over a particular range of habitat loss in frequency-dependent pathogens when we assumed that host competence was greatest in large-bodied species. By contrast, only amplification effects were observed for density-dependent pathogens; host competency only affected the magnitude of the effect. These models can be used to guide future empirical studies of biodiversity–disease relationships across gradients of habitat loss. The type of transmission, the relationship between host competence and community assembly, the identity of hosts contributing to transmission, and how transmission scales with area are essential factors to consider when elucidating the mechanisms driving disease risk in shrinking habitat.
This article is part of the themed issue ‘Conservation, biodiversity and infectious disease: scientific evidence and policy implications'.
Keywords: habitat loss, dilution effect, amplification effect, allometry, multi-host, disease ecology, infectious disease dynamics
1. Introduction
Understanding how habitat loss affects pathogen prevalence and infection risk is a critical challenge in the face of unprecedented rates of anthropogenic landscape transformation [1]. It is clear that habitat loss leads to biodiversity loss [2–4], but it remains uncertain how declining habitat and diversity affect the transmission of infectious diseases [5]. A dominant hypothesis, known as the dilution effect, states that declining biodiversity leads to increased infectious-disease transmission [6,7]. The rationale is that greater host diversity provides a higher proportion of low competent hosts and therefore ‘dilutes’ the transmission chain. This creates a strong incentive to conserve intact ecological communities from the perspective of human health.
Despite the appeal of applying the dilution effect to guide win–win management strategies for conservation and public health, the empirical evidence has been inconsistent [8]. Some studies suggest that increasing host diversity decreases infectious-disease risk (a ‘dilution effect’) [7,9–13], while others suggest increasing diversity increases infectious-disease risk (an ‘amplification effect’) [10,14]. Large-scale surveys of West Nile virus and Lyme disease suggested that communities with higher diversity (measured as species richness) have a lower prevalence of disease [15,16]. By contrast, surveys of trophically transmitted (pathogens transmitted through food webs) and host-specific pathogens have suggested that intact ecosystems have higher pathogen prevalence than less-diverse systems. For example, losses of host populations due to fragmentation in Californian estuarine ecosystems led to a reduction in observed pathogen prevalence [17–20]. These conflicting patterns in empirical data have resulted in considerable academic debate about the generalizability of the dilution effect [10,21–25].
An increasing number of studies have investigated relationships between biodiversity and disease risk, and subsequent meta-analyses have attempted to identify consistent patterns. The majority of empirical studies use prevalence as a proxy for infection risk. By comparing communities with one host species to communities with at least two host species, Civitello and co-workers [26] found support for lower disease risk in multi-species communities compared with single-species populations across a variety of pathogen systems. By contrast, Salkeld and co-workers [24] found minimal support for the dilution effect among zoonotic pathogens. Salkeld et al. also highlighted a potential publication bias for studies supporting the dilution effect [24]—small studies that do not support the dilution effect have not been published. The allure of the dilution effect may be influencing the publication rates and overselling it as a generalizable phenomenon.
In parallel to empirical studies, models of disease transmission in multi-host parasite systems have been developed to understand when species diversity and community structure impact parasite transmission [27–30]. Theory suggests that the type of pathogen transmission determines the outcome of diversity–disease relationships. Multi-host pathogens with frequency-dependent transmission are expected to decrease in prevalence as biodiversity increases, i.e. the dilution effect [29,30]. Vector-borne pathogen transmission is typically frequency-dependent and dilution effects are predicted [29,30]; however, vector preference and variation in host competence can complicate the outcome of these diversity–disease relationships [31–33]. Pathogens that follow density-dependent transmission are expected to increase as host diversity increases [29]. While amplification effects are predicted for most density-dependent pathogens, when total abundance saturates well before species richness, both amplification and dilution effects can be observed [34].
Most theoretical studies of disease and diversity rely on measuring changes in community R0. Community R0 describes the number of secondary cases caused by the introduction of a single infectious individual into a completely naive community of multiple-host species. Community R0 is a proxy for infection risk—it indicates whether or not an epidemic will occur (R0 ≥ 1) and is linked to peak prevalence of an epidemic. In simple cases, R0 can be used to infer endemic equilibrium, but numerical simulations are required to understand more complex multi-host pathogens [35]. Methods have been developed to use equilibrium prevalence of a disease to infer community R0 (see [36]), but oftentimes there is a disconnect between model predictions and available empirical data.
While habitat loss is a major driver of declining biodiversity [37], this process has not yet been linked explicitly to models of disease. Landscapes undergoing habitat loss often have lower biodiversity, both in number of species and evenness of communities. Empirical studies of mammalian disease burdens often report higher parasite prevalence in fragmented landscapes [38–46]. By contrast, a positive relationship between parasite prevalence and patch size has been observed in several wild-bird populations [47–49]. Relationships between habitat patch size, host diversity and pathogen transmission within a habitat remain relatively unexplored (although see [5]). While most studies of parasites across a gradient of habitat loss do not directly measure biodiversity, they offer the opportunity to understand how real-world processes affecting community composition impact disease transmission.
Here, we develop null expectations for disease transmission in a single habitat patch undergoing loss of area and host diversity. We explore how a directly transmitted pathogen responds to changing habitat size in a multi-host community. Specifically, we ask: (i) How does habitat patch size affect the dynamics and prevalence of a generalist pathogen with density-dependent transmission? (ii) How does patch size affect a generalist pathogen with frequency-dependent transmission? (iii) How does the order of community disassembly affect these results? (iv) What assumptions about host competence are necessary to observe dilution versus amplification effects in an area undergoing habitat loss? and (v) How does changing assumptions of host range affect transmission of density-dependent pathogens?
2. Material and methods
(a). Definitions of biodiversity and disease risk
An array of terminology and measurements are used in the dilution and amplification effect literature to describe the relationships between host biodiversity and disease (reviewed in [10,25]). To keep the results of simulations consistent with empirical observations, we used species richness and equilibrium disease prevalence to measure biodiversity and disease, respectively. Unless indicated otherwise, we used the terminology and metrics interchangeably.
(b). Model assumptions and formulation
(i). Disease-free equilibrium
We examined community composition and resultant disease dynamics across a gradient of habitat size, from 0.1 km2 (10 hectares) to 100 km2 (10 000 hectares). We assumed there were up to 10 host species that contributed to pathogen transmission in these simulations. The average body mass (m) of individuals from each of the 10 host species was randomly selected from a skewed normal distribution fit to observed distributions of terrestrial mammalian body sizes [50,51] (electronic supplementary material, figure S1).
The carrying capacity (Kia for each species (i) was determined by its body-mass–determined density (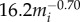 per km2) and a given habitat size (a). These assumptions gave us reasonable species accumulation curves at disease-free equilibrium: increasing habitat size increased the number of individuals in a given patch and the number of species, realistically mirroring patterns observed in natural systems (electronic supplementary material, figures S2–S4). We did not take into account interspecies interactions such as competition, as other authors have explored the impacts of these interactions on disease dynamics [52].
per km2) and a given habitat size (a). These assumptions gave us reasonable species accumulation curves at disease-free equilibrium: increasing habitat size increased the number of individuals in a given patch and the number of species, realistically mirroring patterns observed in natural systems (electronic supplementary material, figures S2–S4). We did not take into account interspecies interactions such as competition, as other authors have explored the impacts of these interactions on disease dynamics [52].
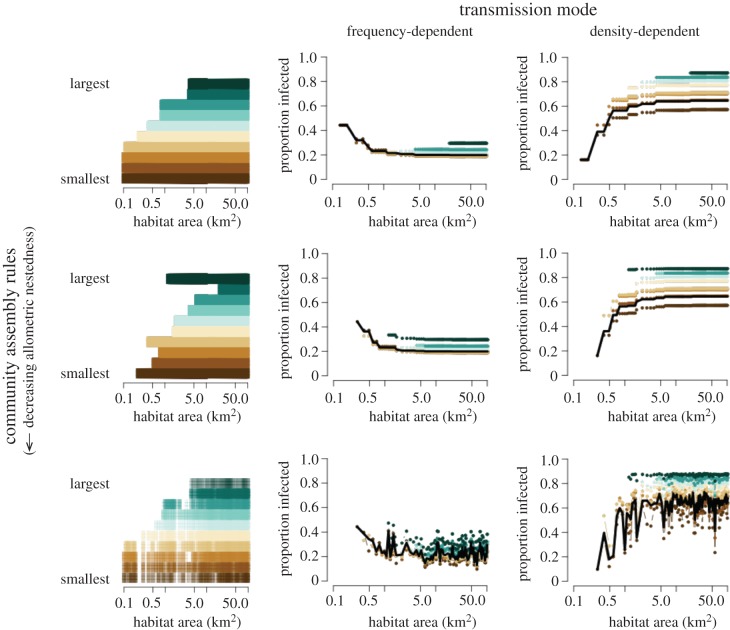
The community of hosts at a given habitat size was determined following one of three community assembly rules: (i) a species was resident in a patch if the carrying capacity was above a fixed threshold of 10 individuals, (ii) a species was considered resident if the carrying capacity was above the species-specific threshold, which was randomly selected from a uniform distribution (1–100), or (iii) a unique habitat density modifier (ɛia) was randomly selected from a uniform distribution (0,2) for each species (i), and thus density of each host species varies at each habitat area (figure 1, column 1). In the first two scenarios, species are perfectly nested—i.e. once they are extinct in a habitat patch, they never recolonize as the habitat continues to shrink. This constant density across habitat size is supported by empirical data [27]. The second community assembly rule is akin to some species being more tolerant to habitat loss (low population threshold) compared with other species that may be highly sensitive (high population threshold). The third assembly rule allows variation in species responses to habitat size—this can be due to variations in resource requirements, sensitivity to edge effects or another confounding factor.
Figure 1.
Community disease prevalence in a shrinking habitat. Each row represents a unique community assemblage assumption—figures in column 1 indicate when a species is present in a habitat patch of a given size, and the shade indicates modifications to density (lighter = less dense/lower ɛia; see methods and SI for details). The prevalence of infection in individual species and the overall community at equilibrium (t = 150 years) in both frequency-dependent (column 2) and density-dependent (column 3) transmission simulations are shown. There was no variation in host competence or between-species contact (within-species R0 = 2.0; ψ = 0.5). Simulations for a single community, shown in colour, represent a community with species that have an average mass of 0.011, 0.030, 0.065, 0.075, 0.23, 0.537, 1.505, 1.515, 13.333, 14.201 kg. For all simulations, the prevalence for each species is shown with colours representing their size, from brown (smallest host) to aquamarine (largest host). The black line indicates the prevalence of disease across the community and is most similar to the intermediate body classes. Simulations with 100 random communities are shown in electronic supplementary material, figure S7. A pathogen with frequency-dependent transmission declines in prevalence as habitat area increases (from left to right) and species richness increases, thus leading to a dilution effect, although the strength of this declines with increasing randomness in the community structure. Pathogen prevalence within each species and across the entire population increased as habitat size increased for density-dependent pathogens, demonstrating an amplification effect; however, this asymptotes when species were no longer added to the community. Community assembly only affects the rate of increase or decrease in prevalence when species and abundance are nested, but not the directionality of diversity–disease relationships. When species presence and density are not directly related to area, then the relationship between diversity and disease becomes less predictable.
(ii). Disease model and assumptions
The deterministic multi-host model was modified from an allometrically scaled S-I-R model for microparasites [53]. For each species i, susceptible (Si), infected (Ii) and recovered (Ri) numbers of hosts were modelled (density-dependent transmission shown in equations ((2.1)–(2.3)), frequency-dependent transmission in electronic supplementary material, equations S1–S3):
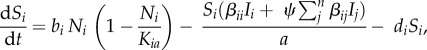 |
2.1 |
| 2.2 |
| 2.3 |
In these models, we are assuming microparasites are transmitted through direct contact with conspecifics and heterospecifics. For density-dependent pathogens, transmission rates are dependent on the baseline force of infection and the density of infected and uninfected hosts (SI/a, where a is the total area of the patch). By contrast, frequency-dependent pathogen transmission is related to the proportion of infected individuals in the population (SI/N, where N is the total number of individuals in a patch). Conceptualizing transmission in this way focuses on two ends of what is probably a continuum [54], but it is a convenient way to understand the spectrum of transmission modes.
Demographic and disease parameters were determined by allometric scaling (following [53] detailed in electronic supplementary material, table S1). For baseline models, it was assumed that R0 within each species was constant (electronic supplementary material, equations S4 and S5)—an isolated, closed, non-breeding population of each species would experience similar disease epidemics and host competence did not vary between species. R0 is often thought of as a static parameter, but it is dependent on the population into which it is introduced. R0 for a single species is different than the community R0. For multi-host pathogens, the number of secondary cases depends not only on characteristics of a single-host species, but also of all species capable of transmission in the community. In the methods, we will use R0 to talk mainly about the species-specific parameter, but will specify when we are referring to R0 for the whole community.
Within-species transmission rates (βii) were calculated using species-specific recovery, mortality and disease-induced mortality rates (electronic supplementary material, table S1). We assumed that between-species transmission rates were the product of the between-species transmission rate (βij), which was the geometric mean of the donor (i) and recipient (j) within-species transmission rates 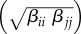 , and a between-species contact rate, which was a proportion of within-species contact (ψ). The between-species contact allowed us to vary interactions between species from zero (non-overlapping contact patterns; ψ = 0), to one (between-species contact was nearly equivalent to within-species contacts; ψ = 1). Simulations were run to equilibrium at a given patch size (t = 150 years) and resultant prevalences in each species and across the community were calculated. In the above model set-up, we assumed that host competence, defined as within-species R0 (equations S4 and S5), was the same for each species.
, and a between-species contact rate, which was a proportion of within-species contact (ψ). The between-species contact allowed us to vary interactions between species from zero (non-overlapping contact patterns; ψ = 0), to one (between-species contact was nearly equivalent to within-species contacts; ψ = 1). Simulations were run to equilibrium at a given patch size (t = 150 years) and resultant prevalences in each species and across the community were calculated. In the above model set-up, we assumed that host competence, defined as within-species R0 (equations S4 and S5), was the same for each species.
(c). Variation in host competence
An alternative scenario is that host competence varies between species, and therefore within-species R0 is not constant across body size for a given pathogen. Some authors argue that life-history trade-offs dictate a negative relationship between R0 and body size [55], meaning smaller species are more competent hosts. Species with faster life histories, indicating smaller body sizes, have been shown to acquire and transmit infections better than slow-lived species [56,57]. This variation in competence could be related to lower investment in adaptive immunity in fast-living species [58,59]. We scaled βii as a function of body size, ensuring that the smallest-bodied species' R0 was the largest (electronic supplementary material, figure S5).
Conversely, if behavioural allometry is taken into account, R0 is expected to increase with body size [60]. This increase in R0 is mediated by increasing group size and social contacts in larger-bodied animals and is supported by prevalence data for ungulates and primates from the Global Mammalian Database [60]. In some simulations, we included conditions of behavioural allometry, scaling βii so that the largest R0 was found in the largest host species (electronic supplementary material, figure S5).
Lastly, we examined the effect of variation in host competence by assembling a community that included incompetent hosts unrelated to body size (and thus unrelated to community assemblage in this model). Incompetent hosts could get infected but could not transmit the pathogen onwards (βii = βij = 0; within– and between–incompetent-host transmission rates were set to zero).
(d). Variation in evenly mixed populations
Finally, we considered a null model of community pathogen dynamics with allometric scaling of home ranges. In the baseline model, we considered infection risk of a density-dependent pathogen to scale with individuals per square kilometre. However, individual species may not come into contact with all individuals in a square kilometre. To account for heterogeneity in between-species contact, we used approximate home ranges as the area in which contacts occur. The minimum within-species transmission rate, 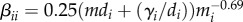 , is similar to density-dependent transmission calculated previously [53] but includes the assumption that home range scales with body mass. In addition to a different transmission parameter, the system of ODEs was adapted so that the force of infection was dependent on the number of individuals in the home range of the recipient species, rather than the density of hosts in a square kilometre.
, is similar to density-dependent transmission calculated previously [53] but includes the assumption that home range scales with body mass. In addition to a different transmission parameter, the system of ODEs was adapted so that the force of infection was dependent on the number of individuals in the home range of the recipient species, rather than the density of hosts in a square kilometre.
3. Results
(a). Null disease model results (R0 constant across all species)
Assuming that host competence does not vary between species, we simulated how changing the size of a habitat patch, thus changing the diversity of hosts, would affect pathogen prevalence. For frequency-dependent transmitted pathogens, (βSI/N), additional species decreased prevalence because contacts were divided between all hosts (figure 1, column 2) and the addition of larger-bodied species meant that contacts were likely to be with individuals that had a lower force of infection in the community (electronic supplementary material, figure S5c). By contrast, for pathogens with density-dependent transmission (βSI/A), additional species increased prevalence within each species and across the community, irrespective of the new species' body size relative to the existing community (figure 1, column 3). The largest changes in equilibrium prevalence of a disease occurred when subsequent species were lost in small and intermediate habitat patches, mainly because these species changed the density and number of individuals in the habitat patch to a greater degree than large-bodied species. This was especially true when between-species transmission was very small (i.e. 10%) compared with within-species transmission (electronic supplementary material, figure S6). These outcomes assumed that R0, and therefore host competence, did not depend on host body size. In these simulations, we assumed that the disease does not cause mortality (α = 0).
Changing assumptions regarding the order of community disassembly did not affect the general relationship between diversity and disease—as long as species abundance and presence were nested. Although the general trend did not change for nested community assemblies, the rate at which prevalence within each species and in the overall community increased (DD) or decreased (FD) with habitat size and as host density increased (figure 1, row 2). When the densities were not constant across habitat size, the relationships between prevalence, species richness and habitat size were less predictable (figure 1, row 3). Although on average the relationships between diversity and disease were consistent among pathogen transmission types, there is more variation in prevalence (electronic supplementary material, figure S7).
(b). When host competence was dependent on body size (R0 varies among species)
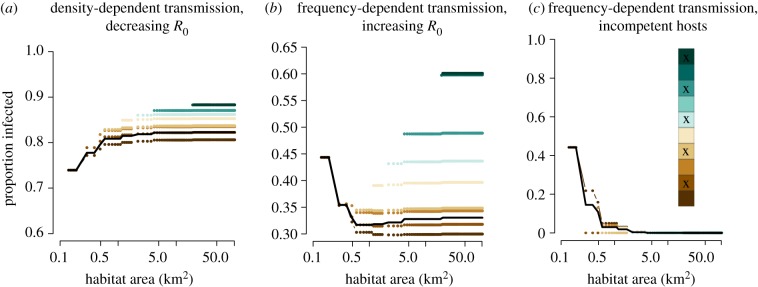
If R0 within populations of smaller species was larger than R0 within populations of larger-bodied species [55], then the difference in prevalence of a frequency-dependent pathogen between the smallest and largest habitat was even greater than observed for null simulations (electronic supplementary material, figure S8). This means when smaller hosts are more competent at transmitting frequency-dependent pathogens, the dilution effect should be easier to observe. Yet when the same assumptions are applied to density-dependent pathogens (figure 2a), there is still an amplification effect. Even though a higher proportion of competent hosts occupy smaller habitats, larger habitats have a higher host density, and thus drive higher pathogen prevalence, at least within the range of R0 used in these scenarios.
Figure 2.
Variation in host competence and underlying assumptions impact diversity–disease relationships. (a) Even if the smallest hosts are the most competent [61] and there are extreme differences in R0 between body sizes (electronic supplementary material, figure S5), only the amplification effect is observed for density-dependent pathogens. (b) If behavioural allometry leads to an increase in R0 across body size, this can lead to an amplification effect for frequency-dependent pathogens at larger patch sizes, but a dilution effect for small to intermediate patches. (c) When species that can become infected but are in turn not infectious (incompetent hosts, denoted by x) are randomly assembled along the distribution of body sizes, then dilution effects can be exacerbated for frequency-dependent pathogens, but this depends on the order of community introduction of these incompetent hosts.
Under the assumption that behavioural traits vary with body size and influence R0 for a given pathogen [60], biodiversity can increase disease risk for frequency-dependent pathogens at larger more diverse patch sizes, despite a dilution effect between small and intermediate patches (figure 2b). The assemblage of species and a significant change in R0 by body size (electronic supplementary material, figure S5) can reverse the relationship between biodiversity and prevalence observed for null models.
When we assumed that host competence was unrelated to body size and dead-end hosts were randomly assigned to species, there were communities that produced non-monotonic changes in prevalence across habitat loss. For each community simulation, 50% of species in the global community were assigned dead-end host status. These dead-end hosts could become infected but were not infectious. When the smallest host was incompetent, the pathogen invaded only when competent hosts were present in habitat patches. Incompetent hosts always decreased the prevalence of disease when they were added to a community with frequency-dependent pathogens (figure 2c). For frequency-dependent pathogens, the distribution of body sizes of the incompetent species determined the degree of this decline, but decreases could be ‘rescued’ by the addition of competent host species (electronic supplementary material, figure S9).
(c). Heterogeneous mixing of populations
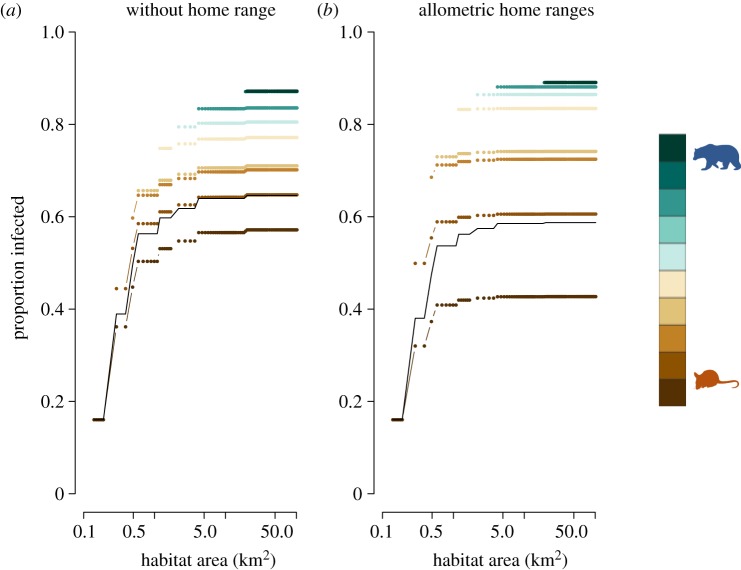
If we assumed host contacts scaled with home range size, then the direction of diversity–disease relationships remained unchanged. However, when new host species entered habitat patches, the within-species prevalence increased more than in the baseline assumptions (figure 3b versus figure 3a). The greater infection prevalence in these hosts was driven by a larger home range, and thus more potential infectious contacts over the lifetime of an individual. While individual species had greater infection prevalence, the community infection prevalence was lower than the baseline model (figure 3a). As body size increased, transmission rate declined for a density-dependent model including home ranges of species. Prevalence increased because the overall density of hosts was greater in species-rich patches, but the larger-bodied species were less efficient at transmitting pathogens and thus the increase in prevalence was less steep than in the baseline simulations.
Figure 3.
Impact of heterogeneous contacts on density-dependent pathogen transmission. Using identical disease parameters, endemic prevalence was observed for a host system that assumed contacts were determined by density of hosts (a) and compared with a system in which home range determined the average contacts of an individual from a given species (b). When home range is not taken into account, overall prevalence is higher, but when home range is considered, larger-bodied species that have larger home ranges have higher within-species infection prevalence.
4. Discussion
We simulated null expectations of changing disease prevalence in a host community in shrinking habitat. Using an allometric multi-host model, we showed that declining biodiversity can either amplify or dilute pathogen prevalence. The direction of the effect depended upon the transmission mode and how relative host competence scaled with body size. Amplification effects occurred when we increased host diversity for pathogens with density-dependent transmission and in some scenarios involving changes of host competence with frequency-dependent pathogens. Dilution effects were observed for simulations when host diversity increased for pathogens with frequency-dependent transmission. By contrast, dilution effects were never observed for density-dependent pathogens when host competence varied among species. Incorporating assumptions of home ranges and community composition did not affect diversity–disease relationships for a given pathogen transmission mode, but did change the magnitude of increases in prevalence observed over the community. This framework we have developed can be tailored to specific systems and host assemblages to predict disease prevalence in habitats of varying size. It can also be used to determine the assumptions necessary for observing specific diversity–disease relationships.
Allometric scaling provides an appropriate tool for understanding null expectations for diversity and pathogen transmission across a fragmenting landscape. Allometric disease models can recreate epidemic cycles observed in nature [62,63], and empirical evidence supports scaling of disease parameters by body size [64]. Although another study also suggested host competence affects the relationship between diversity and disease [55], we show that these differences are significant (up to a 40% change in prevalence) and show that this can be detected in field situations. Furthermore, the proposed allometric model across declining habitat produced results that are consistent with empirical observations of pathogens in hosts experiencing habitat loss. For example, the prevalence of New World Trypansoma species (vector-borne protozoans) in primate [39] and bat [42] populations in habitat patches is higher than the prevalence in continuous habitats where host diversity and average biomass is higher. The presumed density-dependent fungal pathogen, Batrachochytrium dendrobatidis, the cause of chytridiomycosis in amphibians, has a lower prevalence in hosts that reside in small habitat patches with lower amphibian diversity than in hosts that reside in contiguous habitat [65]. The models we presented simulated prevalence across a range of habitat sizes, but the largest differences were between patches where one to three host species were present, compared with the full host community (10 host species). In field studies, differences in prevalence are likely to be observed, but significant differences may only be detectable between communities that have a large difference in species richness.
Confirming results of other theoretical work, we found that transmission mode was consistently the best predictor of diversity–disease relationships. We recognize that these transmission processes are an over simplification of actual dynamics [54], but they provide an important benchmark and models with these frameworks can be used to explain empirical data. Density-dependent multi-host pathogens may best capture dynamics of foot-and-mouth disease ([66]). Frequency-dependent transmission has better represented dynamics of Lyme disease (Borellia burgdorferi), sarcoptic mange and Mycoplasma ovipnuemonae [15,67,68]. By contrast, work on other wildlife pathogens shows that empirical data are best represented by models that incorporate transmission that lies in between frequency- and density-dependent transmission [69,70]). The framework presented here can be adapted to incorporate the complexities of transmission observed in natural systems.
The patterns of community assembly and disassembly are important for disentangling diversity–disease relationships. Previous work assembled communities from a global species pool [34,71,72], using Fisher's Law and Preston's Law for determining the number of vector and host species, respectively. In our models, we are considering a community within shrinking habitat with predictable community disassembly patterns. Although fragmentation can have complicated effects on local species abundance and persistence, overall habitat loss consistently excludes larger-bodied species from landscapes. Our model is consistent with empirical observations: larger habitats support a greater number of species than smaller habitats; larger habitats support increasing numbers of larger-bodied species [73,74], all at higher net density. Nestedness of host communities has been recorded in several systems, suggesting that the hosts found in the least-biodiverse communities are predictable [61,75].
A key research focus should be how to quantify host competence across body size and determine if predictable changes occur across multiple taxonomic groups [61]. If we are also able to quantify how host competence varies across the habitat range, then we will improve predictions of how diseases respond to changes in habitat. Changes in host competence may be even more important than variations in host density [61], which are often highlighted as important factors driving disease dynamics. Additionally, we show that these differences in competence can lead to dilution effects and amplification effects, depending on the details of habitat loss and community assembly.
Host competence can be described as the product of the probability of getting infected (susceptibility), the duration of infectious period and the probability of infecting another host. Host competence varies in natural systems [76–78], and this variation has been highlighted as an important mechanism driving the dilution effect in natural systems [55,56,61,75,79,80]. Our simulations confirm the importance of host competence—although we did not explicitly include each component of host competence; we varied host competence by changing species-specific R0, resulting in a change in transmission rate (βii) but not infectious period (1/γ). By linking host competence to body size, we demonstrated that the amplification effect could be observed for frequency-dependent pathogens—reversing the effect observed when host competence is assumed to be constant across species. Fast-lived species (i.e. smaller body size) are often thought to have higher host competence than larger-bodied hosts. For host competence to affect transmission of density-dependent pathogens, additional incompetent hosts must also be competitive and suppress or indirectly compete with the competent hosts. Unless the density of competent hosts changes, the addition of species will not affect dynamics even if they are incompetent. Competition may explain why the dilution effect has been observed in density-dependent pathogens such as Sin Nombre virus [11,81]. Although the dilution effect was observed for frequency-dependent pathogens using null assumptions, decreasing host competence with larger body size increased the dilution effect observed. Therefore, incompetent hosts (also called incidental hosts), facilitated a dilution effect (i.e. [82]), but their inclusion was a sufficient, but not necessary, condition to explain the dilution effect for frequency-dependent pathogens.
Alternatively, it has also been proposed that R0 is greater in larger-bodied species. This general trend is driven by increases in average group size as hosts get larger. If larger hosts are actually more competent, this exacerbates the amplification effect observed for null expectations of density-dependent pathogens. It can also flip the null observations of frequency-dependent pathogens, so that in extreme cases the amplification effect can be observed. Although we do not know of any empirical examples to support this, the lack of published results may be due to publication biases [24] rather than the absence of this phenomena in natural systems. Variation in host competence may arise from vector preference for particular hosts [83], rather than innate host biology [79], which in turn may be related to allometry. Understanding how host species contribute to infection dynamics [84] and how this changes with body size, and thus community assemblage, should be a priority for future empirical studies.
Most of our simulations indicated a monotonic change in prevalence and diversity—either increasing (amplification) or decreasing (dilution) prevalence as patch size and diversity increased. Yet some field studies show a concave relationship between prevalence and diversity, so that the highest observed prevalence is found in intermediate fragments and the lowest prevalence is found in the smallest and largest habitat fragments [39,43]. These situations may arise when the community abundance peaks prior to biodiversity as habitats get larger—these conditions were simulated using similar sets of differential equations [35]. These patterns could also be observed in our scenarios when larger hosts are more competent (figure 2b), mirroring trait-based amplification effects observed for frequency-dependent pathogens by O'Regan and colleagues [52]. Competent hosts that enter intermediate habitat patches could bolster infection prevalence, and subsequent addition of incompetent hosts in larger habitats could decrease prevalence, but this is complicated by the size distribution of hosts and their relative densities. Host and pathogen life history could also play a role in individual parasite species' responses to habitat fragmentation [45,46], particularly for pathogens with complex life cycles. These complexities underscore the importance of understanding how species assemble and how host competence changes with increases in diversity.
We did not include explicit models of vector populations, which are expected to undergo frequency-dependent pathogen transmission. Including non-competent hosts into vector-borne disease models reduces disease prevalence as biodiversity increases [25], yet observed dilution effects in populations that undergo frequency-dependent transmission do not necessarily require the addition of incompetent hosts, as it is the null expectation for this type of transmission. Temporal and spatial heterogeneity within a habitat patch [85], host distributions [86] and immunological responses to prior infection [87] will also impact transmission dynamics of vector-borne pathogens. Stage-specific transmission dynamics has also been shown to impact disease dynamics [88]. Incorporating vector populations into these null models and how these respond to changes in habitat area and host availability in these models will be an essential next step [89,90].
Although habitat loss often results in a reduction in overall diversity, effects on individual species abundance and distribution often vary [91]. Patterns of infection in wildlife populations will be influenced by host ranging patterns, density, predation, intraspecific and interspecific contact rates and diet [92], and these characteristics can be affected by changes in habitat structure [93,94], edge effects [95] and synergistic effects of habitat loss and disturbances such as fire [96]. The presence of competitors and/or predators has been suggested as a mechanism for reducing infection prevalence in communities with high levels of biodiversity [25,97], and is supported by a model with varying interspecific contact rates [52]. These variations in species-specific responses to habitat loss will be important to consider for certain disease systems and will probably influence changes in prevalence depending on the host competence and sensitivity to habitat loss. Here, we highlight that host communities decline in a predictable manner, emphasizing a nested host species community that is dominated by smaller-bodied species in low-diversity, small habitats. While this produces the dilution effect for frequency-dependent pathogens and the amplification effect for density-dependent pathogens, changes in how host competence scales with body size can alter these expectations.
Our multi-host model focuses on a single habitat patch and describes infection dynamics that vary with patch size and community composition. In natural populations, habitat patches are rarely isolated to the extent that immigration and emigration are non-existent. Habitat patches exist in meta-populations with varying levels of connectivity and models emphasize that connectivity and host dispersal are important characteristics determining system dynamics [5,98–100]. Larger habitat patches may support higher incidences of density-dependent pathogens and serve as sources of pathogen pollution for small habitat fragments in the landscape. By contrast, if movement only occurs towards larger, higher-quality habitat, the average prevalence in large patches could decline if uninfected individuals emigrate, or increase if the density of susceptible hosts increases. The framework we present here could be extended to incorporate spatial structure so that the influence of allometric home ranges and dispersal on prevalence can be explored in each sub-population within a meta-population/meta-community context [60].
Critically, the temporal and spatial scale at which observations are made will affect the infectious-disease dynamics [27]. Moving forward, some of the biggest challenges will be to determine at which spatial and temporal scale empirical measurements would be useful for testing these null predictions. Disease prevalence should be measured in fragmented long-term ecological study sites, such as Stability of Altered Forest Ecosystems (S.A.F.E.) in Borneo [101] or Biological Dynamics of Forest Fragments Project (B.D.F.F.P.) in Brazil [102]. These systems have existing data on host demographics, population densities, community compositions and environmental changes, which would complement disease-dynamic investigations. Changes in pathogen prevalence can be observed with respect to transmission mode, pathogen characteristics and host competence in order to disentangle mechanisms driving transmission dynamics.
5. Conclusion
Using realistic allometric models of a multi-host pathogen, we showed that amplification and dilution effects can be observed in a shrinking habitat. The observed change in equilibrium prevalence with declining habitat and decreasing biodiversity depends on how communities disassemble, how competence scales with body size, the likelihood of a given species' residence in a patch and the transmission mode. The infectious-disease–habitat loss model developed in this study provides a useful template for the design of longitudinal empirical studies of multi-host pathogens in shrinking habitat. It can be adapted to study-specific host species, transmission mode and habitat loss responses to direct sampling effort across a landscape.
Understanding how habitat loss, biodiversity and disease are related is an essential challenge in natural area management. In cases where habitat loss correlates with emerging diseases of humans [103–105], management of disease systems will benefit from evaluating whether biodiversity is the underlying mechanism of pathogen emergence, or if it is a combination of changing contact patterns, environmental conditions and complex species interactions driving emergence. While we often focus on human health, habitat loss and subsequent changes in disease incidence also affect disease risk in animal and plant populations [5,106].
Supplementary Material
Acknowledgements
We are grateful to the editor and two anonymous reviewers for their thoughtful comments that helped to significantly improve the manuscript. We would like to thank other members of the SESYNC/NCEAS Land Use Change and Infectious Diseases Working Group who gave feedback and provided stimulating discussions throughout the workshop series. In particular, we would like to acknowledge A. Baeza Castro, N. Bharti, M. Bonds, G. De Leo, M. Levy, C. Ngonghala, M. Pascual and M. Santos Vega.
Data accessibility
The data-gathered model parametrization was gathered from the primary literature. All data have been included as tables or figures in the electronic supplementary material.
Authors' contributions
L.S.P.B., A.P.D., C.L.F., T.R.G., N.G., H.I.M. and R.K.P. conceived the study. C.L.F., A.P.D., R.K.P. and N.G. designed the study and analysed the results. C.L.F., N.G., L.S.P.B. and T.R.G. acquired empirical data for model conceptualization. C.L.F., R.K.P., N.G., A.P.D., L.S.P.B., M.D.-W., H.I.M. and T.R.G. wrote the manuscript and all contributed to the editing and approval of the final draft.
Competing interests
We have no competing interests.
Funding
This work was supported by the National Socio-Environmental Synthesis Center (SESYNC) and the National Center for Ecological Analysis and Synthesis (NCEAS) under funding received from the National Science Foundation DBI-1052875 and DEB-94–21535, respectively. C.L.F. was funded by the National Defence Science and Engineering Graduate Fellowship and the Truman Foundation. M.D.-W. was supported by the National Institutes of Health, Ecology and Evolution of Infectious Disease Program (5R01GM105246). R.K.P. was supported by National Institutes of Health IDeA Program grants P20GM103474 and P30GM110732, P. Thye and Montana University System Research Initiative: 51040-MUSRI2015-03.
References
- 1.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. ( 10.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 2.Haddad NM, et al. 2015. Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv. 1, e1500052 ( 10.1126/sciadv.1500052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle MJW, et al. 2015. Logging cuts the functional importance of invertebrates in tropical rainforest. Nat. Commun. 6, 6836 ( 10.1038/ncomms7836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newbold T, et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. ( 10.1038/nature14324) [DOI] [PubMed] [Google Scholar]
- 5.McCallum H, Dobson A. 2002. Disease, habitat fragmentation and conservation. Proc. R. Soc. Lond. B 269, 2041–2049. ( 10.1098/rspb.2002.2079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman R, Bowers RG, Begon M, Hudson PJ. 1999. Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. J. Theor. Biol. 200, 111–118. ( 10.1006/jtbi.1999.0982) [DOI] [PubMed] [Google Scholar]
- 7.Schmidt KA, Ostfeld RS. 2001. Biodiversity and the dilution effect in disease ecology. Ecology 82, 609–619. ( 10.1890/0012-9658%282001%29082%5B0609%3ABATDEI%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 8.Kilpatrick AM, Salkeld DJ, Titcomb G, Hahn MB. 2017. Conservation of biodiversity as a strategy for improving human health and well-being. Phil. Trans. R. Soc. B 372, 20160131 ( 10.1098/rstb.2016.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell CE, Tilman D, Groth JV. 2002. Effects of grassland plant species diversity, abundance, and composition on disease. Ecology 83, 1713–1726. ( 10.1890/0012-9658%282002%29083%5B1713%3AEOGPSD%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 10.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 11.Clay CA, Lehmer EM, Jeor SS, Dearing MD. 2009. Sin nombre virus and rodent species diversity: a test of the dilution and amplification hypotheses. PLoS ONE 4, e6467 ( 10.1371/journal.pone.0006467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas SE, Hooten MB, Rizzo DM, Meentemeyer RK. 2011. Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecol. Lett. 14, 1108–1116. ( 10.1111/j.1461-0248.2011.01679.x) [DOI] [PubMed] [Google Scholar]
- 14.Zolnik CP, Falco RC, Kolokotronis S-O, Daniels TJ. 2015. No observed effect of landscape fragmentation on pathogen infection prevalence in blacklegged ticks (Ixodes scapularis) in the Northeastern United States. PLoS ONE 10, e0139473 ( 10.1371/journal.pone.0139473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567 ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezenwa VO, Godsey MS, King RJ, Guptill SC. 2006. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B 273, 109–117. ( 10.1098/rspb.2005.3284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. 2004. Species coextinctions and the biodiversity crisis. Science 305, 1632–1634. ( 10.1126/science.1101101) [DOI] [PubMed] [Google Scholar]
- 18.Hechinger RF, Lafferty KD. 2005. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc. R. Soc. B 272, 1059–1066. ( 10.1098/rspb.2005.3070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altizer S, Nunn CL, Lindenfors P. 2007. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 76, 304–314. ( 10.1111/j.1365-2656.2007.01214.x) [DOI] [PubMed] [Google Scholar]
- 20.Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS. 2009. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. B 276, 3037–3045. ( 10.1098/rspb.2009.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randolph SE, Dobson ADM. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. ( 10.1017/S0031182012000200) [DOI] [PubMed] [Google Scholar]
- 22.Ostfeld RS, Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. ( 10.1146/annurev-ecolsys-102710-145022) [DOI] [Google Scholar]
- 23.Wood CL, Lafferty KD. 2012. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol. 28, 1–9. ( 10.1016/j.tree.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 24.Salkeld DJ, Padgett KA, Jones JH. 2013. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 16, 679–686. ( 10.1111/ele.12101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 26.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowers MA, Matter SF. 1997. Landscape ecology of mammals: relationships between density and patch size. J. Mammal. 78, 999 ( 10.2307/1383044) [DOI] [Google Scholar]
- 28.Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM. 2003. Parasite establishment in host communities. Ecol. Lett. 6, 837–842. ( 10.1046/j.1461-0248.2003.00501.x) [DOI] [Google Scholar]
- 29.Dobson A. 2004. Population dynamics of pathogens with multiple host species. Am. Nat. 164, 64 ( 10.1086/424681) [DOI] [PubMed] [Google Scholar]
- 30.Rudolf VHW, Antonovics J. 2005. Species Coexistence and Pathogens with Frequency-Dependent Transmission. Am. Nat. 166, 112–118. ( 10.1086/430674) [DOI] [PubMed] [Google Scholar]
- 31.Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Duik-Wasser MA. 2012. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B 279, 925–933. ( 10.1098/rspb.2011.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller E, Huppert A. 2013. The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLoS ONE 8, e80279 ( 10.1371/journal.pone.0080279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molaei G, Thomas MC, Muller T, Medlock J, Shepard JJ, Armstrong PM, Andreadis TG. 2016. Dynamics of vector-host interactions in avian communities in four Eastern Equine encephalitis virus foci in the Northeastern U.S. PLoS Negl. Trop. Dis. 10, e0004347 ( 10.1371/journal.pntd.0004347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihaljevic JR, Joseph MB, Orlofske SA, Paull SH. 2014. The scaling of host density with richness affects the direction, shape, and detectability of diversity-disease relationships. PLoS ONE 9, e97812 ( 10.1371/journal.pone.0097812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeling MJ, Pejman R. 2008. Modeling infectious diseases in humans and animals. Princeton, NJ, USA: Princeton University Press. [Google Scholar]
- 36.Funk S, Nishiura H, Heesterbeek H, Edmunds WJ, Checchi F. 2013. Identifying transmission cycles at the human-animal interface: the role of animal reservoirs in maintaining Gambiense Human African trypanosomiasis. PLoS Comput. Biol. 9, e1002855 ( 10.1371/journal.pcbi.1002855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tittensor DP, et al. 2014. A mid-term analysis of progress toward international biodiversity targets. Science 346, 241–244. ( 10.1126/science.1257484) [DOI] [PubMed] [Google Scholar]
- 38.Gillespie TR, Chapman CA, Greiner EC. 2005. Effects of logging on gastrointestinal parasite infections and infection risk in African primates. J. Appl. Ecol. 42, 699–707. ( 10.1111/j.1365-2664.2005.01049.x) [DOI] [Google Scholar]
- 39.Vaz VC, D'Andrea PS, Jansen AM. 2007. Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi. Parasitology 134, 1785–1793. ( 10.1017/S003118200700323X) [DOI] [PubMed] [Google Scholar]
- 40.Trejo-Macías G, Estrada A, Mosqueda Cabrera MÁ. 2007. Survey of Helminth Parasites in Populations of Alouatta palliata mexicana and A. pigra in Continuous and in Fragmented Habitat in Southern Mexico. Int. J. Primatol. 28, 931–945. ( 10.1007/s10764-007-9137-5) [DOI] [Google Scholar]
- 41.Püttker T, Meyer-Lucht Y, Sommer S. 2007. Effects of fragmentation on parasite burden (nematodes) of generalist and specialist small mammal species in secondary forest fragments of the coastal Atlantic Forest, Brazil. Ecol. Res. 23, 207–215. ( 10.1007/s11284-007-0366-z) [DOI] [Google Scholar]
- 42.Cottontail VM, Wellinghausen N, Kalko EKV. 2009. Habitat fragmentation and haemoparasites in the common fruit bat, Artibeus jamaicensis (Phyllostomidae) in a tropical lowland forest in Panamá. Parasitology 136, 1133–1145. ( 10.1017/S0031182009990485) [DOI] [PubMed] [Google Scholar]
- 43.Raharivolona BM, Ganzhorn JU. 2009. Gastrointestinal parasite infection of the Gray mouse lemur (Microcebus murinus) in the littoral forest of Mandena, Madagascar: Effects of forest fragmentation and degradation. Madagascar Conserv. Dev. 4, 1–11. ( 10.4314/mcd.v4i2.48650) [DOI] [Google Scholar]
- 44.Zommers Z, Macdonald DW, Johnson PJ, Gillespie TR. 2012. Impact of human activities on chimpanzee ground use and parasitism (Pan troglodytes). Conserv. Lett. 6, 264–273. ( 10.1111/j.1755-263X.2012.00288.x) [DOI] [Google Scholar]
- 45.Froeschke G, van der Mescht L, McGeoch M, Matthee S. 2013. Life history strategy influences parasite responses to habitat fragmentation. Int. J. Parasitol. 43, 1109–1118. ( 10.1016/j.ijpara.2013.07.003) [DOI] [PubMed] [Google Scholar]
- 46.Sá RM, Petrášová J, Pomajbíková K, Profousová I, Petrželková KJ, Sousa C, Cable J, Bruford MW, Modrý D. 2013. Gastrointestinal symbionts of chimpanzees in Cantanhez National Park, Guinea-Bissau with respect to habitat fragmentation. Am. J. Primatol. 75, 1032–1041. ( 10.1002/ajp.22170) [DOI] [PubMed] [Google Scholar]
- 47.Bonneaud C, et al. 2009. The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. J. Trop. Ecol. 25, 439–447. ( 10.1017/S0266467409006178) [DOI] [Google Scholar]
- 48.Spurgin LG, Illera JC, Padilla DP, Richardson DS. 2011. Biogeographical patterns and co-occurrence of pathogenic infection across island populations of Berthelot's pipit (Anthus berthelotii). Oecologia 168, 691–701. ( 10.1007/s00442-011-2149-z) [DOI] [PubMed] [Google Scholar]
- 49.Laurance SGW, Jones D, Westcott D, Mckeown A, Harrington G, Hilbert DW. 2013. Habitat fragmentation and ecological traits influence the prevalence of avian blood parasites in a tropical rainforest landscape. PLoS ONE 8, e76227–e76235. ( 10.1371/journal.pone.0076227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchinson GE, Macarthur RH. 1959. A theoretical ecological model of size distributions among species of animals. Am. Nat. 93, 117–125. ( 10.1086/282063) [DOI] [Google Scholar]
- 51.Gardezi T, da Silva J. 1999. Diversity in relation to body size in mammals: a comparative study. Am. Nat. 53, 110–123. ( 10.1086/303150) [DOI] [PubMed] [Google Scholar]
- 52.O'Regan SM, Vinson JE, Park AW. 2015. Interspecific contact and competition may affect the strength and direction of disease-diversity relationships for directly transmitted microparasites. Am. Nat. 186, 480–494. ( 10.1086/682721) [DOI] [PubMed] [Google Scholar]
- 53.De Leo GA, Dobson AP. 1996. Allometry and simple epidemic models for microparasites. Nature 379, 720–722. ( 10.1038/379720a0) [DOI] [PubMed] [Google Scholar]
- 54.McCallum H, Barlow N, Hone J. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16, 295–300. ( 10.1016/S0169-5347(01)02144-9) [DOI] [PubMed] [Google Scholar]
- 55.Joseph MB, Mihaljevic JR, Orlofske SA, Paull SH. 2013. Does life history mediate changing disease risk when communities disassemble? Ecol. Lett. 16, 1405–1412. ( 10.1111/ele.12180) [DOI] [PubMed] [Google Scholar]
- 56.Cronin JP, Welsh ME, Dekkers MG, Abercrombie ST, Mitchell CE. 2010. Host physiological phenotype explains pathogen reservoir potential. Ecol. Lett. 13, 1221–1232. ( 10.1111/j.1461-0248.2010.01513.x) [DOI] [PubMed] [Google Scholar]
- 57.Huang S, Bininda-Emonds ORP, Stephens PR, Gittleman JL, Altizer S. 2013. Phylogenetically related and ecologically similar carnivores harbour similar parasite assemblages. J. Anim. Ecol. 83, 671–680. ( 10.1111/1365-2656.12160) [DOI] [PubMed] [Google Scholar]
- 58.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. ( 10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 59.Lee KA, Wikelski M, Robinson WD, Robinson TR, Klasing KC. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363. ( 10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 60.Han BA, Park AW, Jolles AE, Altizer S. 2015. Infectious disease transmission and behavioural allometry in wild mammals. J. Anim. Ecol. 84, 637–646. ( 10.1111/1365-2656.12336) [DOI] [PubMed] [Google Scholar]
- 61.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. 2014. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233. ( 10.1038/nature11883) [DOI] [PubMed] [Google Scholar]
- 62.Bolzoni L, De Leo GA, Gatto M, Dobson AP. 2008. Body-size scaling in an SEI model of wildlife diseases. Theor. Popul. Biol. 73, 374–382. ( 10.1016/j.tpb.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 63.Bolzoni L, Dobson AP, Gatto M, De Leo GA. 2008. Allometric scaling and seasonality in the epidemics of wildlife diseases. Am. Nat. 172, 818–828. ( 10.1086/593000) [DOI] [PubMed] [Google Scholar]
- 64.Cable JM, Enquist BJ, Moses ME. 2007. The allometry of host-pathogen interactions. PLoS ONE 2, e1130 ( 10.1371/journal.pone.0001130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker CG, Zamudio KR. 2011. Tropical amphibian populations experience higher disease risk in natural habitats. Proc. Natl Acad. Sci. USA 108, 9893–9898. ( 10.1073/pnas.1014497108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tildesley MJ, Savill NJ, Shaw DJ, Deardon R, Brooks SP, Woolhouse ME, Grenfell BT, Keeling MJ. 2006. Optimal reactive vaccination strategies for a foot-and-mouth outbreak in the UK. Nature 440, 83–86. ( 10.1038/nature04324) [DOI] [PubMed] [Google Scholar]
- 67.Manlove KR, Cassirer EF, Cross PC, Plowright RK, Hudson PJ. 2014. Costs and benefits of group living with disease: a case study of pneumonia in bighorn lambs (Ovis canadensis). Proc. R. Soc. B 281, 20142331 ( 10.1098/rspb.2014.2331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devenish-Nelson ES, Richards SA, Harris S, Soulsbury C, Stephens PA. 2014. Demonstrating frequency-dependent transmission of sarcoptic mange in red foxes. Biol. Lett. 10, 20140524 ( 10.1098/rsbl.2014.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith MJ, Telfer S, Kallio ER, Burthe S, Cook AR, Lambin X, Begon M. 2009. Host-pathogen time series data in wildlife support a transmission function between density and frequency dependence. Proc. Natl Acad. Sci. USA 106, 7905–7909. ( 10.1073/pnas.0809145106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cross PC, Creech TG, Ebinger MR, Manlove K, Irvine K, Henningsen J, Rogerson J, Scurlock BM, Creel S. 2013. Female elk contacts are neither frequency nor density dependent. Ecology 94, 2076–2086. ( 10.1890/12-2086.1) [DOI] [PubMed] [Google Scholar]
- 71.Roche B, Rohani P, Dobson AP, Guégan J-F. 2013. The impact of community organization on vector-borne pathogens. Am. Nat. 181, 1–11. ( 10.1086/668591) [DOI] [PubMed] [Google Scholar]
- 72.Roche B, Dobson AP, Guegan J-F, Rohani P. 2012. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Phil. Trans. R. Soc. B 367, 2807–2813. ( 10.1098/rstb.2011.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKinney ML. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516. ( 10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 74.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lacroix C, Jolles A, Seabloom EW, Power AG, Mitchell CE, Borer ET. 2013. Non-random biodiversity loss underlies predictable increases in viral disease prevalence. J. R. Soc. Interface 11, 20130947 ( 10.1098/rsif.2013.0947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levin ML, Nicholson WL, Massung RF. 2002. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector-Borne Zoonotic Dis. 2, 125–136. ( 10.1089/15303660260613693) [DOI] [PubMed] [Google Scholar]
- 77.Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT. 2013. Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8, e54341 ( 10.1371/journal.pone.0054341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbour AG, Bunikis J, Fish D, Hanincová K. 2015. Association between body size and reservoir competence of mammals bearing Borrelia burgdorferi at an endemic site in the northeastern United States. Parasites Vectors 8, 299 ( 10.1186/s13071-015-0903-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 273, 2327–2333. ( 10.1098/rspb.2006.3575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venesky MD, Liu X, Sauer EL, Rohr JR. 2013. Linking manipulative experiments to field data to test the dilution effect. J. Anim. Ecol. 83, 557–565. ( 10.1111/1365-2656.12159) [DOI] [PubMed] [Google Scholar]
- 81.Dizney LJ, Ruedas LA. 2009. Increased host species diversity and decreased prevalence of Sin Nombre Virus. Emerg. Infect. Dis. 15, 1012–1018. ( 10.3201/eid1507.081083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaves LF, Hernandez M-J, Dobson AP, Pascual M. 2007. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 23, 311–316. ( 10.1016/j.pt.2007.05.003) [DOI] [PubMed] [Google Scholar]
- 83.Lyimo IN, Ferguson HM. 2009. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 25, 189–196. ( 10.1016/j.pt.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 84.Fenton A, Streicker DG, Petchey OL, Pedersen AB. 2015. Are all hosts created equal? Partitioning host species contributions to parasite persistence in multihost communities. Am. Nat. 186, 610–622. ( 10.1086/683173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith DL, Dushoff J, McKenzie FE. 2004. The risk of a mosquito-borne infectionin a heterogeneous environment. PLoS Biol. 2, e368 ( 10.1371/journal.pbio.0020368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burkett-Cadena ND, McClure CJW, Estep LK, Eubanks MD. 2013. Hosts or habitats: what drives the spatial distribution of mosquitoes? Ecosphere 4, art30. ( 10.1890/ES13-00009.1) [DOI] [Google Scholar]
- 87.Pollitt LC, Bram JT, Blanford S, Jones MJ, Read AF. 2015. Existing infection facilitates establishment and density of malaria parasites in their mosquito vector. PLoS Pathog. 11, e1005003 ( 10.1371/journal.ppat.1005003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caraco T, Glavanakov S, Chen G, Flaherty JE, Ohsumi TK, Szymanski BK. 2002. Stage-structured infection transmission and a spatial epidemic: a model for lyme disease. Am. Nat. 160, 348–359. [DOI] [PubMed] [Google Scholar]
- 89.Kilpatrick AM, et al. 2017. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Phil. Trans. R. Soc. B 372, 20160117 ( 10.1098/rstb.2016.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Millins C, Gilbert L, Medlock J, Hansford K, Thompson DBA, Biek R. 2017. Effects of conservation management of landscapes and vertebrate communities on Lyme borreliosis risk in the United Kingdom. Phil. Trans. R. Soc. B 372, 20160123 ( 10.1098/rstb.2016.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laurance WF, Bierregaard RO. 1997. Tropical forest remnants: ecology, management, and conversation of fragmented communities. Chicago, IL: University of Chicago Press. [Google Scholar]
- 92.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. 2002. The ecology of wildlife diseases. Oxford, UK: University Press Oxford. [Google Scholar]
- 93.DeWalt SJ, Maliakal SK, Denslow JS. 2003. Changes in vegetation structure and composition along a tropical forest chronosequence: implications for wildlife. Forest Ecol. Manage. 182, 139–151. ( 10.1016/S0378-1127(03)00029-X) [DOI] [Google Scholar]
- 94.Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F. 2003. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography 31, 79–92. ( 10.1046/j.0305-0270.2003.00994.x) [DOI] [Google Scholar]
- 95.Malcolm JR. 1994. Edge effects in central Amazonian forest fragments. Ecology 75, 2438–2445. ( 10.2307/1940897) [DOI] [Google Scholar]
- 96.Cochrane MA. 2001. Synergistic interactions between habitat fragmentation and fire in evergreen tropical forests. Conserv. Biol. 15, 1515–1521. ( 10.1046/j.1523-1739.2001.01091.x) [DOI] [Google Scholar]
- 97.Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. 2003. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecol. Lett. 6, 797–802. ( 10.1046/j.1461-0248.2003.00500.x) [DOI] [Google Scholar]
- 98.Swinton H, Grenfell G. 1998. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. J. Anim. Ecol. 67, 54–68. ( 10.1046/j.1365-2656.1998.00176.x) [DOI] [Google Scholar]
- 99.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jesse M, Heesterbeek H. 2011. Divide and conquer? Persistence of infectious agents in spatial metapopulations of hosts. J. Theor. Biol. 275, 12–20. ( 10.1016/j.jtbi.2011.01.032) [DOI] [PubMed] [Google Scholar]
- 101.Ewers RM, et al. 2011. A large-scale forest fragmentation experiment: the stability of altered forest ecosystems project. Phil. Trans. R. Soc. B 366, 3292–3302. ( 10.1098/rstb.2011.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laurance WF, et al. 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618. ( 10.1046/j.1523-1739.2002.01025.x) [DOI] [Google Scholar]
- 103.Brownstein JS, Skelly DK, Holford TR, Fish D. 2005. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 146, 469–475. ( 10.1007/s00442-005-0251-9) [DOI] [PubMed] [Google Scholar]
- 104.Goldberg TL. 2008. Forest fragmentation as cause of bacterial transmission among nonhuman primates, humans, and livestock, Uganda. Emerg. Infect. Dis. 14, 1375–1382. ( 10.3201/eid1409.071196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fornace KM, et al. 2016. Association between landscape factors and spatial patterns of plasmodium knowlesi infections in Sabah, Malaysia. Emerg. Infect. Dis. 22, 201–208. ( 10.3201/eid2202.150656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvignac-Spencer S, Leendertz SAJ, Gillespie TR, Leendertz FH. 2014. Wild great apes as sentinels and sources of infectious disease. Clin. Microbiol. Infect. 18, 521–527. ( 10.1111/j.1469-0691.2012.03816.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data-gathered model parametrization was gathered from the primary literature. All data have been included as tables or figures in the electronic supplementary material.