Abstract
Multiple scattering of light on coral skeleton enhances light absorption efficiency of coral symbionts and plays a key role in the regulation of their internal diffuse light field. To understand the dependence of this enhancement on skeleton meso- and macrostructure, we analysed the scattering abilities of naked coral skeletons for 74 Indo-Pacific species. Sensitive morphotypes to thermal and light stress, flat-extraplanate and branching corals, showed the most efficient structures, while massive-robust species were less efficient. The lowest light-enhancing scattering abilities were found for the most primitive colonial growth form: phaceloid. Accordingly, the development of highly efficient light-collecting structures versus the selection of less efficient but more robust holobionts to cope with light stress may constitute a trade-off in the evolution of modern symbiotic scleractinian corals, characterizing two successful adaptive solutions. The coincidence of the most important structural modifications with epitheca decline supports the importance of the enhancement of light transmission across coral skeleton in modern scleractinian diversification, and the central role of these symbioses in the design and optimization of coral skeleton. Furthermore, the same ability that lies at the heart of the success of symbiotic corals as coral-reef-builders can also explain the ‘Achilles's heel’ of these symbioses in a warming ocean.
Keywords: multiple scattering, coral skeleton, coral morphology, colonial growth form, holobiont emergent traits
1. Background
Scleractinian corals are responsible for building one of the most diverse and productive marine ecosystems on Earth, the habitat for over 1 million species. Coral-reef survival in tropical oceans has relied for the last 200 Myr on the mutualistic endosymbioses established between simple animals and unicellular photosynthetic dinoflagellates in the genus Symbiodinium [1,2]. Symbiodinium photosynthesis significantly enhances coral calcification and growth [2–4], which is critical for the maintenance of the primary reef framework [5].
The success of scleractinian corals in oligotrophic tropical environments has been attributed to their extraordinary efficiency in collecting [6] and processing [7] light for carbon fixation. Multiple light scattering within coral skeleton explains the twofold to fivefold enhancement of light absorption efficiency documented for Symbiodinium in hospite relative to freshly isolated and/or cultured cells [6]. An eightfold variation in the ability of the skeleton nano–micro-scale structure to enhance local illumination has been also documented [8]. Such amplification in the local light field of Symbiodinium allows enhancement of photosynthesis production of the host-symbiont unit (holobiont), and subsequently coral calcification and growth [2–4]. Micro-scale characterizations of holobiont photosynthesis have established the stimulation of oxygen evolution rates and algal photochemical activity away from the area directly illuminated by a laser beam [9]. These micro-scale descriptions have also documented large heterogeneity within coral tissues in the light field of Symbiodinium [10]. Thus, the optical properties of coral skeletons have a direct impact on holobiont photosynthetic performance up to now ignored, and may play an important role in coral ecology and the diversification of modern symbiotic scleractinian. To understand the variation in the absorption/scattering properties of stony corals, the evaluation of the contribution of macro-structural elements such as septa, columella, coenosteum and chalices, and the void space between them to modulate diffuse and bulk scattering, is fundamental.
In this study, we examined the variability of coral skeleton capacity to enhance light scattering across a broad range of colonial morphologies. We used a coral collection maintained by the Museum of Tropical Queensland, and used a simple descriptor, the light enhancement factor (LEF), to carry out relative comparisons of the maximum ability to enhance the local light field by naked coral skeletons. We analysed this variation in relation to: (i) the colonial growth form (e.g. phaceloid, massive, flat and branching morphs); (ii) the presence/absence of coenosteum and other skeleton macro-components such as chalices and septa; and (iii) the type of polyp budding. The variability found highlighted a possible mechanism for the coordinated evolution between coral hosts and Symbiodinium, which we believe can describe a key driving force within the evolution of coral skeleton diversity and holobiont specificity. This mechanism might explain differences in coral performance associated with particular structural characteristics of the species [11], but also differences in coral sensitivity-robustness to natural perturbations, in particular those related to light stress [12].
2. Material and methods
The quantification of the relative changes in local illumination intensification on a naked coral skeleton because of multiple light scattering was performed using a simple descriptor: LEF. As variation in coral pigmentation was excluded, these measurements reflect relative changes in the maximum ability of coral skeleton to enhance the light field perceived by the symbiotic algae. For a given directional illumination, the fluence rate received by an omnidirectional optical sensor was estimated and compared with that received by the same sensor in the absence of the skeleton. A black fabric was placed behind the probe to maintain a constant reference (fluence rate with no skeleton effect) among determinations. Small and fairly simple isotropic probes were fabricated using optical fibres. The probes were fabricated with standard fused silica step-index optical fibres (numerical aperture = 0.39 and 200 µm diameter core) whose ends were covered with a drop of white ceramic repair liquid. Tests were conducted to improve the isotropy of the response with the direction of incidence. Deviations less than 15% are not difficult to reach with such a procedure, except for directions close to the fibre axis. This level of uniformity was considered sufficient. The near spherical drop of diffusing material that covered the end of the probe had a diameter of approximately 1 mm. This type of sensor has previously been used for dosimetry studies [13–15], and it is interesting to note that the results of Lilge et al. [14] also presented an enhanced optical signal near the boundary between a transparent and a scattering medium (see their fig. 4).
To facilitate the visualization of light scattering abilities of the skeleton structure, as well as the alignment of the optical system, we performed the measurements using the miniature isotropic probe as a light source rather than as a detector. The probe was placed in the proximity or in the cavities of the skeleton, and the scattered light was collected, fairly directionally, in the far field of the sample using a silicon detector (model 201/579–7227, Thorlabs Inc., Newton, NJ, USA). A schematic diagram of the optical set-up is shown in the electronic supplementary material, figure S1. Reversing source and detector facilitated the alignment of the system and allowed visualization of the area contributing to the LEF. This ‘reverse-ray’ experimental design relies on a well-established principle in electromagnetism and optics, the Helmholtz's reciprocity theorem [16,17], which has been used, among other things, to demonstrate the equivalence between different kinds of microscopes or any other optical system in which rays are reversed (exchange of the source and detector). In our case, the illumination was provided by a linearly polarized continuous wave laser diode (655 nm wavelength) whose output was coupled to the optical fibre with the help of a lens. A standard silicon optical detector was used for the detection.
Bearing in mind that in normal circumstances, the whole of the skeleton is illuminated, to obtain an unbiased estimation of the LEF with our equivalent system, one must ensure that the detector receives light from the whole of the illuminated area of the skeleton. This condition was not satisfied with most of the branching corals and, for this reason, measurements with such morphologies are not reported.
It is also worth mentioning that LEF determinations were obtained with dry skeletons, as the use of wet conditions was not possible in this study. The transport mean free path of the light in the skeleton increases when the sample is immersed in water, because of the reduction in the dielectric contrast between the embedding medium (water) and the calcium carbonate inhomogeneities. For a medium with low absorption (which is a reasonable assumption for a naked skeleton), the size of the illuminated area of the skeleton owing to point source on its surface is expected to increase. In the case of a flat coral with no absorption, this is of no consequence, as the diffuse reflectance is always one, but the LEF of a concave surface can increase with respect to that of a flat one because of the spatial redistribution of the scattered light (the scattering is no longer Lambertian). So, we can assume that the measurements obtained with dry skeletons tend to underestimate the real amplification factors by a small amount. However, the aim of this study was not the characterization of the maximum amplification factor of different coral skeleton morphologies, but the quantification of the magnitude of the relative changes in this important optical property, among different coral skeletons. The estimated LEF values generated in this study provide, thus, a reasonable first comparative basis for relative changes between coral species.
The ratio between the signal reaching the detector in the presence of the skeleton and the signal obtained with the isolated probe was taken as the estimation of LEF. A black fabric was used to eliminate reflections from nearby objects. The reported LEF value for a given sample represents the average of measurements taken in three equivalent regions of the skeleton. The species mean value was determined from measurements made with three different skeletons, which means that the value given for each organism was determined from a total of nine measurements per species (see the electronic supplementary material, table S1). All coral skeletons belonged to the Museum of Tropical Queensland collection (Townsville, Australia). Branching corals were excluded from this comparison, as the illuminated areas observed in these cases were larger than the field of view of the detector.
3. Results
The LEF descriptor quantifies relative changes in the intensification of local illumination on a naked coral skeleton. As the variation in coral pigmentation was excluded from the analysis, LEF does not constitute a descriptor of what happens in a pigmented coral. It reflects, however, the maximum potential ability of a particular coral skeleton to enhance the light field of the symbiotic algae in the animal tissue. In this comparison, LEF varied by a factor of up to three across the wide diversity of coral skeletons examined: 49 species of massive scleractinian corals belonging to the Faviidae family and 25 species belonging to eight other families according to Veron [18] but currently considered distributed across 11 families [19] (see the electronic supplementary material, table S1). The lowest and the highest mean values were found for two species belonging to the former Faviidae family (sensu Veron): Caulastrea curvata (LEF = 2.99) and Echinopora lamellosa (LEF = 10.2), which have been placed by Budd et al. [19] in the family Merulinidae (clade XVII). The variation observed within Faviidae (sensu Veron classification) or within Merulinidae (sensu Budd et al. classification) was as large (coefficient of variation (CV) > 25%) as the variation determined for the whole dataset. The intra-specific variation also changed broadly, owing in part to differences in skeleton pigmentation (i.e. skeleton light absorption). Despite the fact that we selected only clean specimens to perform this comparison in an attempt to minimize the effect of dirt on the ‘apparent’ condition of the skeletons, the intra-specific variation may have been slightly affected by the degree of dirt present on the aragonite skeleton. Platygyra pini showed the lowest intra-specific variability (CV = 3.7%), whereas Pocillopora damicornis (CV = 30%) and E. lamellosa (CV > 30%) presented the largest. Part of this large intra-specific variation was related to the morphological plasticity of the coral skeleton, which was particularly large for the species Poc. damicornis.
Only three families showed LEF means below the average value of 5.5 ± 0.15 determined in this study: Siderastreae (mean ± s.e. = 4.1 ± 0.04; no. spp = 2; no. obs = 18); Fungiidae (5 ± 0.44; no. spp = 5; no. obs = 45); and Poritidae (4.88 ± 0.3; no. spp = 3; no. obs = 27). The values estimated for the three Indo-Pacific species of the genus Porites (table 1) showed similar magnitude to the maximum enhancement factor of approximately 5 estimated by Enríquez et al. [6] for bleached organisms of the Caribbean species Porites branneri, despite the fact that these authors used other descriptor. Enríquez et al. [6] compared the variation of the chlorophyll a-specific absorption coefficient (a*, m2 mg chla−1) of the intact coral structure against suspensions of freshly isolated cells of Symbiodinium (i.e. blastates) with similar chlorophyll a cross section (pigment content per projected area) to the intact coral surfaces.
Table 1.
Summary of the most important conclusions from this comparative analysis. (Online version in colour.)
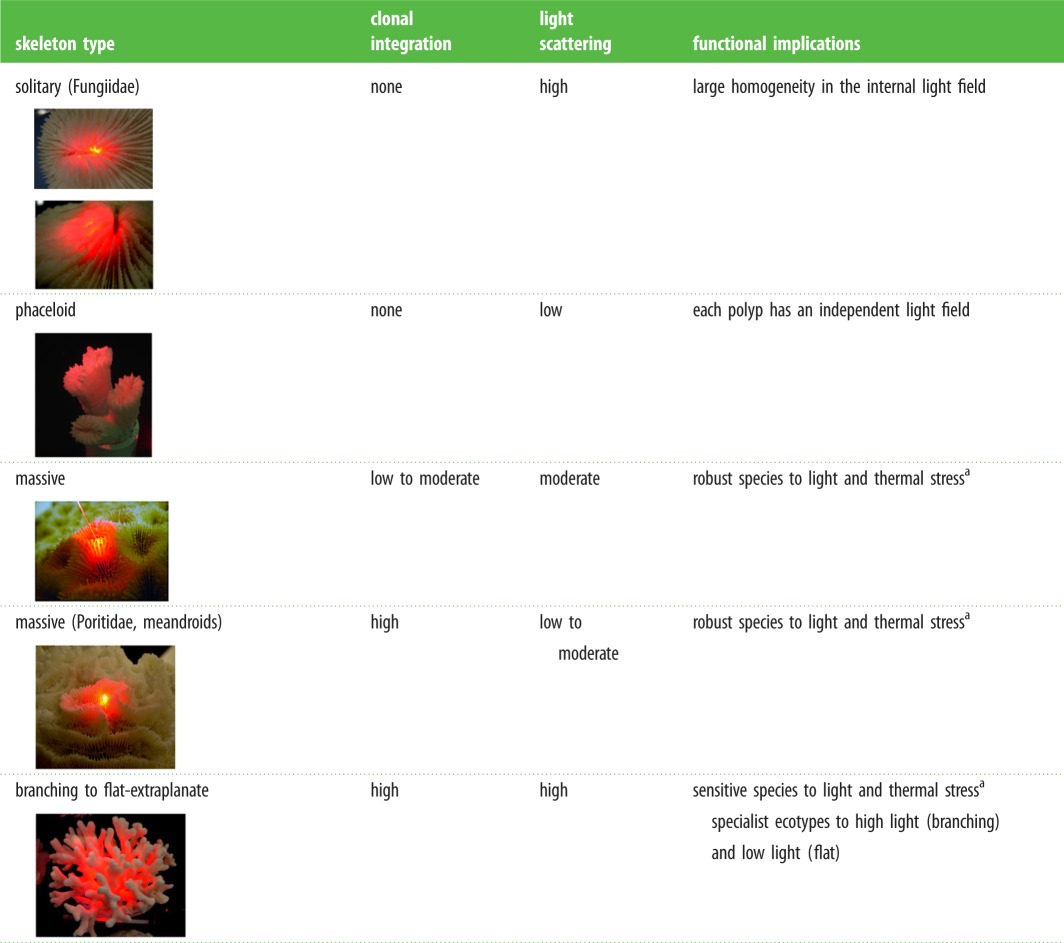 |
aCoral reef-builders are probably more common within these two categories.
In our analysis, only one family, Faviidae sensu [18], or Merulinidae sensu [19], can be considered to be well represented (n = 47 or 45 species, respectively; electronic supplementary material, table S1). This implies that the average LEF estimations for the other families have to be considered preliminary values, although no significant changes should be expected for the taxa that present low variation in coral skeleton morphology. As the light detector was unable to follow the large fluctuations produced by most of the branching morphologies (electronic supplementary material, figure S1), we focused our attention on the characterization of massive to flat colonial morphs. We interpreted this observation as evidence that branching corals have developed a very efficient morphology to enhance the local light environment, and may produce LEF values with a much greater average than the maximum of 10 estimated in this study for flat-extraplanate skeletons. These large LEF values are owing to the large area of light collection associated with this morphology. An illustration of this is provided in figure 1 where we show the skeleton of a branching coral illuminated by a directional red laser beam, whose size is revealed by the bright spot on the lower right of the photograph (this bright spot represents the light scattered by one of the walls of the transparent acrylic box protecting the skeleton). By reversing light rays from the camera to the source, one can conclude that a particle placed in the spot where the laser hits the skeleton would receive light from the whole of the illuminated area.
Figure 1.
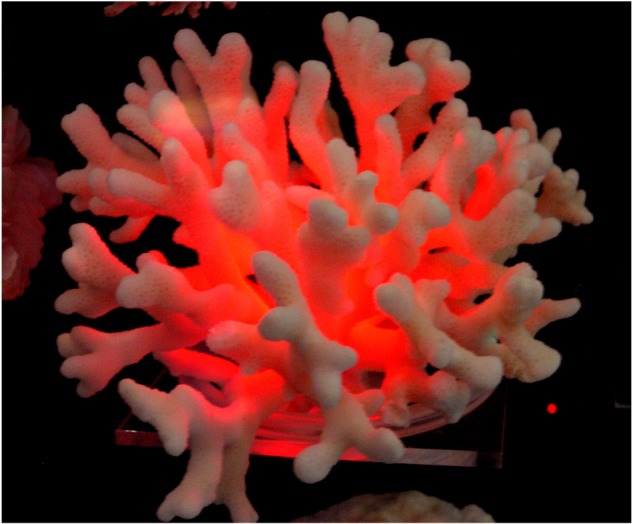
Light scattered by a branching coral illuminated with a laser beam. Reflected on an acrylic structure, the size of the laser beam can be seen in the lower and right side of the image. (Online version in colour.)
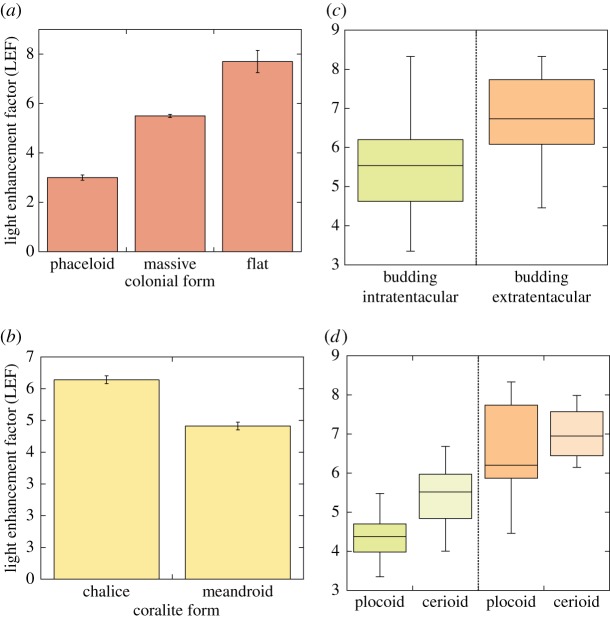
For the analysis of LEF variation as a function of changes in massive skeleton morphology, we exploited the large structural diversity displayed by the 49 species formerly considered to belong to the Faviidae family (sensu [18]), although it should be mentioned that this large and complex taxonomic group is currently the subject of significant taxonomic modifications [19]. Phaceloid, massive and flat morphologies, which describe the main colonial growth forms of this group, showed significant variation: (ANOVA, F2,487 = 71.3; p < 0.0001). Flat to extraplanate colonies (n = 40) showed the highest LEF values, ranging from a minimum of 5.6 to a maximum of 10.2 (electronic supplementary material, table S1; figure 2a). Massive corals (n = 432) showed intermediate scattering abilities (3.3 < LEF > 8.3, figure 2a), and phaceloids were the least efficient coral reflectors (3 < LEF > 3.3, n = 18; figure 2a).
Figure 2.
(a) Mean ± s.e. of the three colonial growth forms analysed in the Faviidae family; (b) mean ± s.e. of two coral skeleton morphologies, chalice and meandroid morphs, within the Faviidae family; (c) box plot describing the distribution of the variability displayed by the type of budding (intra- versus extra-calicular); and (d) box plot describing the importance of coenosteum (plocoid versus cerioid) within each type of budding. Boxes encompass the 25% and 75% quartiles of all the data, the central line represents the median, bars extend to the 95% and 5% of confidence limits. No values beyond those confidence limits were observed. (Online version in colour.)
Significant differences associated with the corallite form were also found: chalice morphologies showed higher LEF values than those of meandroid structures (ANOVA, F1,422 = 32.1; p < 0.0001; figure 2b). To determine the significance of the presence/absence of coenosteum on the skeleton light scattering abilities, we compared plocoid and ceriod morphs, as corallites in the plocoid morphs are always separated by coenosteum, whereas in the cerioid type, polyps are simply juxtaposed (coenosteum is absent) or coenosteum is largely reduced. No significant LEF variation was detected between plocoid and ceriod morphs (F1,488 = 0.70, p = 0.4). This finding could be interpreted that the coenosteum does not contribute or modify the ability of coral skeleton to scatter light. However, while comparing coenosteoid and non-coenosteoid skeletons in relation to the type of budding (intracalicular or extracalicular sensu [19]), we observed that the significant effect of coenosteum on LEF was dominated by a stronger effect of the type of budding on LEF (figure 2c). Organisms with extracalicular budding presented significantly higher scattering abilities (higher LEF values) than organisms with intracalicular budding (F1,488 = 142.4; p < 0.0001; figure 2c). However, within each budding type, cerioid non-coenosteoid skeletons showed higher LEF values than plocoid organisms (F1,281 = 89; p < 0.0001; for the intracalicular budding group, and F1,147 = 13, p < 0.0001; for the extracalicular budding group, figure 2d). We interpreted these results as indication that the presence of coenosteum in the plocoid structures could have represented a reduction in the ability of coral skeleton to scatter light, and that the selection of extracalicular budding may have helped to offset this effect in the coenosteoid organisms.
To analyse the effect of macro-structural elements, such as concavities (chalices) or parallel macro-structures (septa) on light scattering, we selected particular species and performed a more detailed analysis on them. The species Moseleya latistellata was selected because of its large polyps to explore the effect of concavities on LEF, which was determined along the central axes of the polyp. The results revealed a linear increase in LEF along the chalice axes, from a value of 1.97 ± 0.16 at the most external part of the polyp to a maximum of 5.46 ± 0.46 at the deepest point at the centre of the polyp (figure 3a). The genus Fungia was selected for the analysis of the effect of septa on LEF because of their large septa. In contrast with M. latistellata, no light gradients were found for both within the mouth or between Fungia sp. septa, only homogeneous light fields (figure 3b). However, we observed highly enhanced LEF values between septa (LEF > 6; figure 3b).
Figure 3.
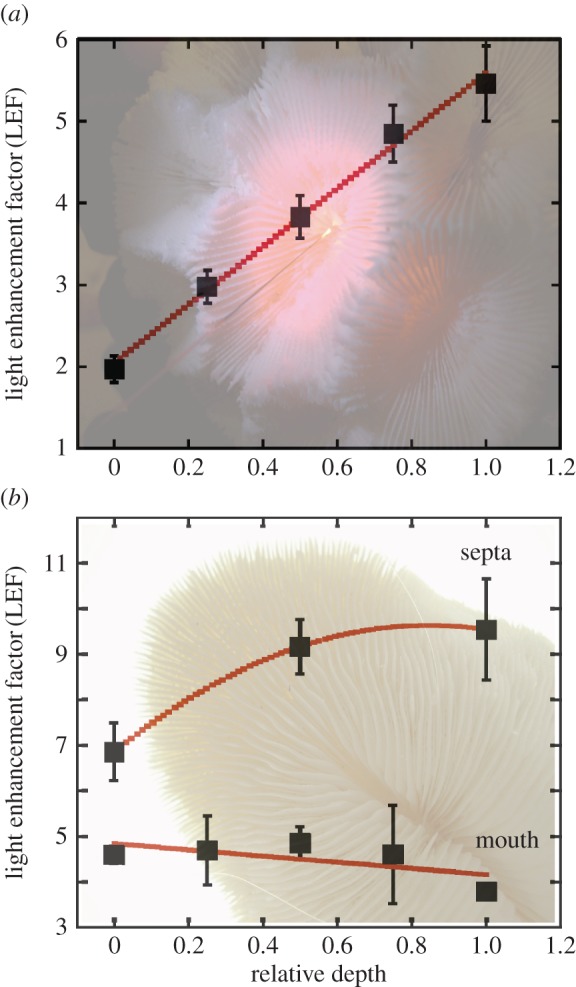
Mean ± s.e. of the LEF measured in (a) Moseleya latistellata along a perpendicular axes at the centre of the polyp; 0 is the shallowest area and 1 refers to the deepest centre of the polyp; and (b) Fungia sp. along two axes, one at the mouth of the organism (solid line) and the second within septa (dashed line). (Online version in colour.)
4. Discussion
The estimated LEF values for coral skeletons of different morphologies presented in the previous section reveal that the skeleton macrostructure plays a significant role in the shaping of the algal light environment. Although limited to naked skeletons, the data presented here represent, to our knowledge, the first comparative study of this parameter for realistic skeletal morphologies. Previous evaluations have focused on the effects of the nanostructure and microstructure of the skeleton considering a ‘flat coral’ model [8].
In our study, the lowest LEF values were found for two species that represent the most primitive colonial growth form: phaceloid (Caulastrea spp.). This growth form is not considered to be branching or even colonial, as polyps are organically independent and remain linked only through a coral skeleton that has not completed its separation. On the other hand, the species with the largest LEF values, E. lamellose, forms a highly integrated flat colony with abundant presence of micro–nanostructure on its coenosteum (electronic supplementary material, table S1). The presence of coenosteum is considered a fundamental acquisition for the progression of colonial integration [20]. Polyps in coenosteoid organisms have a continuum gastrovascular cavity throughout the colony and grow as a communal project in a coordinated pattern. These organisms have achieved one of the highest levels of colonial integration. As the coenosteum of E. lamellose is abundant in micro–nanostructures, the largest LEF values estimate for this species can be explained by the documented contribution of micro–nanostructure to the enhancement of light scattering [8]. However, it may seem paradoxical that coenosteum has showed in this study a negative effect in the enhancement of LEF (figure 2c). Considering that the development of coenosteum may have resulted in an initial structural simplification of the coral skeleton, with the consequent loss of scattering abilities, the acquisition of the ability to generate structural complexity on its surface (micro–nanostructure) through the selection of extracalicular budding, may have contributed to resolve this initial constraint. Therefore, we propose that the selection of extracalicular budding in massive coenosteoid organisms may have been fundamental for the generation of structural complexity on an otherwise smooth coenosteum surface, thanks to its capacity to favour the expansion of the skeletogenic surface of the coenosarc. The coenosarc is the part of the living coral tissue that forms the coenosteum. This implies that the generation of complex coenosteum micro–nanostructures may have been linked in the evolution of modern symbiotic scleractinian, to the selection of extracalicular budding. This interpretation could also explain why the largest presence of micro–nanostructure occurs in highly integrated coenosteoid species that have developed a flat-extraplanate skeleton shape [8].
It should be mentioned that micro–nanostructure is not exclusive of the coenosteum but that it can be developed on septa and on any other macro-structural element of the coral skeleton [19]. Moreover, our study supports the role of these macro-structural elements in the determination of the final light scattering pattern [21]. We observed that parallel septa allow large enhancements of the local illumination of Symbiodinium and produce more homogeneous light fields, while chalices favour the generation of light gradients. This implies that the particular shape of coral skeleton has a significant impact on holobiont optical properties. In addition to it, if considering that the LEF values of the branching morphologies, not measured for this study, can be significantly higher than those reported here, the shape of the branching colony may also play an important role in the regulation of holobiont optics.
(a). Coral skeleton and emergent holobiont functional traits
Micro-scale three-dimensional descriptions of the distribution of solar radiation within living coral tissues are useful tools to describe the variation of the light field of Symbiodinium in hospite and its possible dependence on different holobiont characteristics, such as polyp size, tissue thickness and pigment distribution. This type of analyses has already documented the presence of more complex vertical light gradients for polyps over coenosarcs, in accordance with our results, and also lateral light transfer [10]. Some of these descriptions have concluded that the coral living tissue presents similar scattering abilities to aragonite crystals [6,21]. However, the analysis reported by Enríquez et al. [6] for 10 coral species has shown that the light scattering properties of living coral tissues are similar to those reported for unicells [22], and far less important than the values estimated for the intact coral structure (see fig. 2c in [6]).
Allometric analyses can be also very useful to identify key morpho-functional traits for the holobiont, similar to those documented by comparative plant ecology between basic structural descriptors (i.e. tissue pigmentation, nutrient content and size) and key functional properties such as light absorption efficiency [23,24], metabolic rates [25] and specific growth rates [23,26], but also decomposition, nutrient uptake and grazing rates [27]. This morpho-functional approach is fundamental to understand why just a few trait combinations can explain the large diversity of plant forms and life histories [28]. Applied to coral research, this approach may facilitate the understanding of interspecific differences in coral competitive ability, the role that each species plays in the ecosystem, and the differential habitat impact derived from shifts in the dominant species [29]. The present comparison offers a hypothesis to explain why skeletons with high ability to scatter light can support more productive holobionts, with higher growth rates and, ultimately, with increasing competitive ability for space. By contrast, organisms with less efficient light scattering structures may develop robust holobionts with the caveat of reduced productivity and growth rates.
Two potential emergent optical properties in the holobiont derived from the capacity of coral skeleton to enhance multiple scattering deserve special attention: (i) the reduction in the structural costs of its light-harvesting system; and (ii) the progression of its capacity to offset pigment self-shading. The first acquisition could have played a central role in supporting holobiont production and growth under conditions of nutrient limitation. The second represents a key acquisition for a photosynthetic organism to minimize the most important constraint of photosynthetic metabolism: pigment self-shading [25]. The steep light gradients that are often formed within photosynthetic tissues and canopies [30] cause important carbon losses when the lower layers are below the compensation irradiance for too long. Thus, the acquisition of the ability to enhance the local light field of Symbiodinium and reduce the internal light gradients may have been critical for the development of holobionts with enhanced photosynthesis and calcification rates. In accordance with this interpretation, the emergence of these new functional properties of the holobiont could also explain the origin of modern Scleractinia reef-builders, with relevant implications for habitat formation and coral diversification.
(b). Coral ecotypes
The documented light-induced plasticity of corallite architecture [11] could be understood within this context. For example, the higher presence of coenosteum relative to corallites documented for deep-growing colonies may be the holobiont response to minimize Symbiodinium light limitation. This response may reduce the higher pigment packaging within polyps over coenosarcs, as polyps concentrate the largest pigment cross section when all tentacles are retracted [10]. Additionally, the colony growth form is also affected by this functional constraint, as branching structures produce highly heterogeneous light fields [31] with reduced tissue illumination in certain areas owing to branch shading. The branching growth form is the most common morphology in shallow environments, whereas flat-extraplanate colonies increase in abundance with depth. Both growth forms showed in this study the largest skeletal abilities to enhance light scattering. This condition is clearly beneficial for colonizing deep areas (shade ecotype), but the advantages of the large scattering abilities of branching colonies for shallow environments (sun ecotype) are less obvious. However, when considering the integrated colony production, branching colonies can be more productive and exploit their larger photosynthetic surface only when all the photosynthetic tissue is sufficiently illuminated. Thus, the ability to enhance light scattering and increase photosynthesis rates at the more shaded levels may significantly improve colony production and fitness. Furthermore, the branching colonial growth form would be equivalent to a multi-layered plant canopy, and the flat-extraplanate morphology would represent a monolayer canopy. Plant ecology has developed a quantitative descriptor, the leaf area index (LAI, total one-sided leaf area per ground area; [32]), where LAI ≤ 1 describes monolayer and LAI > 1 multi-layered canopies. This interpretation provides an explanation for the higher growth rates of branching colonies, their competitive ability under high light conditions, and ultimately, their ecological success in shallower high-illuminated areas. Furthermore, it provides functional significance for the capacity of a descriptor of reef surface complexity, rugosity [33], to explain key processes in coral-reef habitats [29,34–36]. This descriptor is calculated similarly to LAI but considering the whole area generated by coral colonies, including surfaces without a living tissue. Correcting this descriptor for the quantification of the total area expanded by the living tissue of the colony in relation to the substrate area occupied, the larger the LAI-rugosity value of the colony (LAI-rugosity>>1), the greater assimilation could be achieved under sufficient illumination [29,37]. In addition to this, branching colonies can also provide shade (photoprotection) for a significant fraction of the holobiont living tissue, while flat-extraplanate morphs (LAI ≤ 1) constitute the most efficient colonial morphotype to colonize homogeneous low-light environments [30]. Yet, the emergent optical properties of the holobiont derived from the particular scattering abilities of coral skeleton also provide functional significance to a large body of documented changes in coral skeletal morphology [11,38]. Understanding these morpho-functional traits may also contribute to explain morphological convergences in skeletal characteristics between distant taxa and/or divergences of closely related species, helping to resolve some of the discrepancies already recognized between conventional coral taxonomy and genetic analyses [19,39].
(c). Evolutionary implications
From the evolutionary perspective, we know that modern scleractinian corals first appear in the fossil record in the middle Triassic (approx. 240 Ma), long after the Precambrian explosion of ocean biodiversity and after the Permian mass extinction [40]. The first fossil record appeared after a 14 Myr period characterized by a global suppression of carbonate deposition [40] and were already highly diverse morphologically [41]. The sudden appearance of a highly diverse fauna has been explained by the loss and re-acquisition of the ability to form skeletons by an earlier diverse group of ‘naked’, ‘anemone-like’ ancestors [40] in the early-to-mid Triassic. Evidence for the ‘naked coral’ hypothesis and the polyphyletic origin of modern corals is supported by molecular phylogenetic analyses based on mitochondrial sequence data [42] and amino acid sequences of proteins encoded by the mitochondrial genome [43]. Calibrated molecular clock analyses of mitochondrial rRNA and tRNA sequences support, however, a monophyletic origin of modern Scleractinia [43,44]. According to these studies, modern Scleractinia are descendants of a basal clade that diverged in the Palaeozoic (approx. 425 Ma) prior the Complexa/Robusta split, and which survived the Permian/Triassic boundary mass extinction. These corallimorpharian-like ancestors may have diverged close to the point where scleractinian corals and corallimorpharian lineages split or when the carbonate skeleton was lost (soft-bodied ancestor; [45]).
None of these primitive organisms, however, were reef-builders. The origin of modern coral reefs is still unknown, although it is widely accepted that it relates to the evolution of the coral-dinoflagellate symbiosis despite no explicit evidence for this relationship being influenced by the evolution of scleractinian corals, be that biochemical or structural. Nevertheless, it is also widely accepted that modern scleractinian corals have been symbiotic with dinoflagellates for over 200 Myr [46]. The primitive fauna was mainly formed by solitary and phaceloid morphs, which were predominant between Late Triassic and Late Jurassic [47]. Phaceloids were particularly abundant in the Jurassic (approx. 50 genera). These organisms had a thick outer wall, termed epitheca [47], which protected the isolated polyp and strengthened coral skeleton, but strongly limited light transmission. Epitheca was the prevailing wall in Triassic and Jurassic corals [47]. Species with higher levels of colonial integration such as ceriod, thamnasterioid, meandroid and plocoid, already present in the Triassic at low abundance [48] were also epitechate morphs. The presence of epithecal walls during adult stages has significantly decreased in extant scleractinian since the Late Cretaceous [47]. Solitary and phaceloid morphs still develop thick external walls, which offer mechanical protection to the polyps, and that could explain the low-light scattering abilities determined in our comparison for the phaceloids. This finding suggests similar low scattering abilities for the primitive fauna.
The decline in the epitheca wall may have been fundamental to enhance light transmission across polyps with the consequent benefits for holobiont photosynthesis and growth. The ecological advantages acquired by organisms with reduced epitheca may have contributed to the epitheca evolutionary decline, which occurred immediately after the mid-Cretaceous faunistic crisis (120–80 Ma), when the Mesozoic coral diversity developed during the Triassic and Jurassic (250–145 Ma) was extensively reduced [47]. This period was characterized by an exceptionally warm climate with maximum sea surface temperatures 3–5°C warmer than today and low species competition [49].
The palaeontological record indicates that concomitant with the decline in epitheca, the edge-zone was developed, in what is probably one of the most important steps in the evolution of modern Scleractinia [48,49]. This trend is particularly well represented in the evolutionary history of caryophylliine corals [47]. The edge-zone refers to the part of the organic mass of the polyp that lies outside of the skeletal wall over the free portions of the corallite [50]. The development of the edge-zone and the continuation-expansion of the living tissue beyond the polyp wall (the coenosarc) have been fundamental for the enlargement of the skeletogenic surface of the polyp [47], and for the origin of coenosteum and the progression of Scleractinia colonial integration [20]. Coenosteum development is considered the main responsible for the explosion of species diversity [51]. It occurred in parallel with key changes in polyp anatomy and in the growth pattern of the coral skeleton [47]. The coincidence of all these structural modifications with epitheca decline supports our interpretation that the enhancement of light transmission across coral skeleton may have played a central role in coral diversification. Our findings provide a mechanistic explanation for the explosion of species diversity in modern symbiotic corals [47], to be considered in addition to the proposed hypothesis that increasing bioerosion in the Mesozoic shallow-water environments would explain the development of the edge-zone [47]. Our results also indicate that the skeleton morphologies that showed the largest ability to scatter light, flat-extraplanate and branching morphs, are those that have achieved the highest degree of colonial integration [50]. Massive coral morphs, which present a large variety of intermediate steps in colonial integration, displayed a wide range of intermediate capabilities for enhancing the light environment. Accordingly, a potential association between the evolution of polyp integration and the progression of coral skeleton ability to scatter light could also be inferred from this analysis. The fact that colony-forming corals are more common and complex in symbiotic organisms [51,52], and that facultative symbiotic species also present simple integration of corallites [53] significantly strengthens this interpretation.
Not all evolutionary lines that have led to the progression of colonial integration may have favoured the development of efficient structures for scattering light. Meandroids and Poritidae, which represent highly integrated colonies, were categorized in this comparison as less efficient structures for enhancing the light environment. The meandroid morph, considered a successful adaptation for a large number of genera, is a robust morphotype under thermal stress [12]. Families such as Fungiidae, Siderastreae and Poritidae, also categorized in our study as less efficient structures to scatter light, have been identified as coral families that present a robust response against coral bleaching [12]. In contrast with these robust taxa, branching corals and thin-tissue flat-extraplanate colonies are reported as the more sensitive morphs to thermal stress [12]. As light stress is the main driver of the adverse effects of thermal stress on this symbiosis [54], we can conclude that the more sensitive organisms to thermal and light stress are those that have developed the most efficient light collectors, whereas the more robust species produce less efficient structures for scattering light. Therefore, the progression of highly efficient organisms for absorbing light (coenosteoid flat-extraplanate and branching corals) versus the selection of less efficient but more robust structures to cope with light stress (e.g. non-coenosteoid massive meandroids) may constitute a trade-off between two successful adaptive solutions in the evolution of symbiotic Scleractinia.
(d). Contribution of Symbiodinium to coral diversification
Our study does not overlook the significant role played by Symbiodinium in scleractinian coral evolution. On the contrary, we postulate that the diversification of coral skeleton and the emergence of new optical properties in the holobiont may have opened new spaces of opportunity (ecological niches) for the coevolution and genetic diversification of the animal and Symbiodinium. These particular coevolutionary opportunities may have favoured algal niche diversification and the selection of the most efficient/robust holobionts.
5. Conclusion
The intimate association between the skeleton builder, simple marine animals of the phylum Cnidaria and the sink of solar energy, Symbiodinium, may have been fundamental for the design and optimization of the most extraordinary light collector [6] and user [7] developed by natural selection. This interpretation offers a solid explanation for the explosive diversification of symbiotic scleractinian corals, providing a mechanism for the origin of modern coral-reef-builders. This mechanism also explains the fragility of these symbioses to light and thermal stress. Thus, the same ability explaining the ecological and evolutionary success of symbiotic corals in oligotrophic tropical areas that lies at the heart of the success of modern scleractinian as coral-reef-builders may set the physiological ‘tipping point’ of this symbiosis, determining the ‘Achilles’ heel’ of one of the most ancient, diverse and valuable ecosystems on Earth.
Supplementary Material
Supplementary Material
Acknowledgements
We acknowledge the kind access to the coral collection provided by the Museum of Tropical Queensland.
Data accessibility
Data can be obtained by mailing at enriquez@cmarl.unam.mx.
Authors' contributions
S.E., E.R.M., O.H.-G. and R.I.-P. designed research; S.E., E.R.M., O.H.-G. and R.I.-P. performed research; S.E. analysed data and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
The study was funded by a CONACYT 129880 project and the EU-PFP7UE-244161 project to S.E., and by the GEF project (UNESCO-IOC, the World Bank-Block-B funds) to R.I.-P. and O.H.-G.
References
- 1.Muscatine L, Porter JW. 1977. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. BioScience 27, 454–460. ( 10.2307/1297526) [DOI] [Google Scholar]
- 2.Muscatine L, Weis V. 1992. Productivity of zooxanthellae and biogeochemical cycles. In Primary productivity and biogeochemical cycles in the sea (eds Falkowski PG, Woodhead AD), pp. 257–272. New York, NY: Plenum. [Google Scholar]
- 3.Goreau TF, Goreau NI. 1959. The physiology of skeleton formation in corals II. Calcium deposition by hermatypic corals under various conditions in the reef. Biol. Bull. 117, 239–250. ( 10.2307/1538903) [DOI] [Google Scholar]
- 4.Colombo-Pallota MF, Rodríguez-Román A, Iglesias-Prieto R. 2010. Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29, 899–907. ( 10.1007/s00338-010-0638-x) [DOI] [Google Scholar]
- 5.Perry CT, Spencer T, Kench PS. 2008. Carbonate budgets and reef production states: a geomorphic perspective on the ecological phase-shift concept. Coral Reefs 27, 853–866. ( 10.1007/s00338-008-0418-z) [DOI] [Google Scholar]
- 6.Enríquez S, Méndez ER, Iglesias-Prieto R. 2005. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 50, 1025–1032. ( 10.4319/lo.2005.50.4.1025) [DOI] [Google Scholar]
- 7.Rodríguez-Román A, Hernández-Pech X, Tome P, Enríquez S, Iglesias-Prieto R. 2006. Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnol. Oceanogr. 51, 2702–2710. ( 10.4319/lo.2006.51.6.2702) [DOI] [Google Scholar]
- 8.Marcelino LA, et al. 2013. Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLoS ONE 8, e61492 ( 10.1371/journal.pone.0061492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wangpraseurt D, Larkum AWD, Franklin J, Szabó M, Ralph PJ, Khül M. 2014. Lateral light transfer ensures efficient resource distribution in symbiont-bearing corals. J. Exp. Biol. 217, 489–498. ( 10.1242/jeb.091116) [DOI] [PubMed] [Google Scholar]
- 10.Wangpraseurt D, Larkum AWD, Ralph PJ, Khül M. 2012. Light gradients and optical microniches in coral tissues. Front. Microbiol. 3, 00316 ( 10.3389/fmicb.2012.00316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ow YX, Todd PA. 2010. Light-induced morphological plasticity in the scleractinian coral Goniastrea pectinata and its functional significance. Coral Reefs 29, 797–808. ( 10.1007/s00338-010-0631-4) [DOI] [Google Scholar]
- 12.Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R. 2001. Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131. ( 10.1046/j.1461-0248.2001.00203.x) [DOI] [Google Scholar]
- 13.Star WM, Marijnissen JPA. 1989. Calculating the response of isotropic light dosimetry probes as a function of the tissue refractive index. Appl. Opt. 28, 2288–2291. ( 10.1364/AO.28.002288) [DOI] [PubMed] [Google Scholar]
- 14.Lilge L, Haw T, Wilson BC. 1993. Miniature isotropic optical fibre probes for quantitative light dosimetry in tissue. Phys. Med. Biol. 38, 215–230. ( 10.1088/0031-9155/38/2/001) [DOI] [PubMed] [Google Scholar]
- 15.van Staveren HJ, Marijnissen HPA, Aalders MCG, Star WM. 1995. Construction, quality assurance and calibration of spherical isotropic fibre optic light diffusers. Lasers Med. Sci. 10, 137–147. ( 10.1007/BF02150852) [DOI] [Google Scholar]
- 16.Saxon DS. 1955. Tensor scattering matrix for the electromagnetic Field. Phys. Rev. 100, 1771–1775. ( 10.1103/PhysRev.100.1771) [DOI] [Google Scholar]
- 17.Méndez ER, Greffet JJ, Carminati R. 1997. On the equivalence between the illumination and collection modes of the scanning near-field optical microscope. Opt. Commun. 142, 7–13. ( 10.1016/S0030-4018(97)00268-X) [DOI] [Google Scholar]
- 18.Veron JEN. 2000. Corals of the world. Townsville, Australia: Australian Institute of Marine Science. [Google Scholar]
- 19.Budd AF, Fukami H, Smith ND, Knowlton N. 2012. Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool. J. Linn. Soc. 166, 465–529. ( 10.1111/j.1096-3642.2012.00855.x) [DOI] [Google Scholar]
- 20.Beklemishev VN. 1964. Principles of comparative anatomy of invertebrates. Edinburgh, UK: Oliver & Boyd; (Engl. Translation 1970, Edinburgh: Chicago Press.) [Google Scholar]
- 21.Terán E, Méndez ER, Enríquez S, Iglesias-Prieto R. 2010. Multiple light scattering and absorption in reef-building corals. Appl. Optics 49, 5032–5042. ( 10.1364/AO.49.005032) [DOI] [PubMed] [Google Scholar]
- 22.Morel A, Bricaud A. 1981. Theoretical results concerning light absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep-Sea Res. 28, 1375–1393. ( 10.1016/0198-0149(81)90039-X) [DOI] [Google Scholar]
- 23.Enríquez S, Sand-Jensen K. 2003. Variation in light absorption properties of Mentha aquatica L. as a function of leaf form. Implications for plant growth. Int. J. Plant Sci. 164, 125–136. ( 10.1086/344759) [DOI] [Google Scholar]
- 24.Enríquez S, Agustí S, Duarte CM. 1994. Light absorption by marine macrophytes. Oecologia 98, 121–129. ( 10.1007/BF00341462) [DOI] [PubMed] [Google Scholar]
- 25.Enríquez S, Duarte CM, Sand-Jensen K, Nielsen S. 1996. Broad-scale comparison of photosynthetic rates across phototrophic organisms. Oecologia 108, 197–206. ( 10.1007/BF00334642) [DOI] [PubMed] [Google Scholar]
- 26.Nielsen SL, Enríquez S, Duarte CM, Sand-Jensen K. 1996. Scaling maximum growth rates across photosynthetic organisms. Funct. Ecol. 10, 167–175. ( 10.2307/2389840) [DOI] [Google Scholar]
- 27.Duarte CM, Sand-Jensen K, Nielsen SL, Enríquez S, Agustí S.. 1995. Comparative functional plant ecology: rational and potentials. Trends Ecol. Evol. 10, 418–421. ( 10.1016/S0169-5347(00)89163-6) [DOI] [PubMed] [Google Scholar]
- 28.Díaz S, et al. 2016. The global spectrum of plant form and function. Nature 529, 167–171. ( 10.1038/nature16489) [DOI] [PubMed] [Google Scholar]
- 29.Álvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R. 2013. Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 3, 3486 ( 10.1038/srep03486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björkman O. 1981. Responses to different quantum flux densities. In Physiological plant ecology I. Responses to the physical environment. Encyclopedia of plant physiology (eds Lange OL, Nobel PS, Osmond CB, Ziegler H), (eds A Pirson, MH Zimmermann), pp. 57–107. Berlin, Germany: Springer. [Google Scholar]
- 31.Kaniewska P, Anthony KRN, Hoegh-Guldberg O. 2008. Variation in colony geometry modulates internal light levels in branching corals, Acropora humilis and Stylophora pistillata. Mar. Biol. 155, 649–660. ( 10.1007/s00227-008-1061-5) [DOI] [Google Scholar]
- 32.Watson DJ. 1947. Comparative physiological studies on the growth of field crops. I. Variations in net assimilation rate and leaf area between species and varieties and within and between years. Ann. Bot. NS 11, 41–76. ( 10.1093/oxfordjournals.aob.a083148) [DOI] [Google Scholar]
- 33.Álvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR. 2009. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. B 276, 3019–3025. ( 10.1098/rspb.2009.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Álvarez-Filip L, Gill JA, Dulvy NK. 2011. Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2, 1–17. ( 10.1890/ES11-00185.1) [DOI] [Google Scholar]
- 35.Álvarez-Filip L, Côté IM, Gill JA, Watkinson AR, Dulvy NK. 2011. Region-wide temporal and spatial variation in Caribbean reef architecture: is coral cover the whole story? Glob. Change Biol. 17, 2470–2477. ( 10.1111/j.1365-2486.2010.02385.x) [DOI] [Google Scholar]
- 36.Graham NAJ, Nash KL. 2013. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. ( 10.1007/s00338-012-0984-y) [DOI] [Google Scholar]
- 37.Scurlock JMO, Cramer W, Olson RJ, Parton WJ, Prince SD. 1999. Terrestrial NPP: towards a consistent data set for global model evaluation. Ecol. Appl. 9, 913–919. ( 10.2307/2641338) [DOI] [Google Scholar]
- 38.Todd PA. 2008. Morphological plasticity in scleractinian corals. Biol. Rev. 83, 315–337. ( 10.1111/j.1469-185X.2008.00045.x) [DOI] [PubMed] [Google Scholar]
- 39.Fukami H, Budd AF, Paulay G, Solé-Cava A, Chen CA, Iwao K, Knowlton N. 2004. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427, 832–835. ( 10.1038/nature02339) [DOI] [PubMed] [Google Scholar]
- 40.Stanley GD Jr, Fautin DG. 2001. The origins of modern corals. Science 291, 1913–1914. ( 10.1126/science.1056632) [DOI] [PubMed] [Google Scholar]
- 41.Romano SL, Palumbi SR. 1996. Evolution of scleractinian corals inferred from molecular systematics. Science 271, 640–642. ( 10.1126/science.271.5249.640) [DOI] [Google Scholar]
- 42.Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL. 2006. Naked corals: skeleton loss in scleractinia. Proc. Natl Acad. Sci. USA 103, 9096–9100. ( 10.1073/pnas.0602444103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitahara MV, Lin M-F, Forêt S, Huttley G, Miller DJ, Chen CA. 2014. The ‘Naked Coral’ hypothesis revisited—evidence for and against scleractinian monophyly. PLoS ONE 9, e94774 ( 10.1371/journal.pone.0094774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stolarski J, Kitahara MV, Miller DJ, Cairns SD, Mazur M, Meibom A. 2011. The ancient evolutionary origins of Scleractinia revealed by azooxanthellate corals. BMC Evol. Biol. 11, 2–15. ( 10.1186/1471-2148-11-316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin M-F, Kitahara MV, Luo H, Tracey D, Geller J, Fukami H, Miller DJ, Chen CA. 2014. Mitochondrial genome rearrangements in the Scleractinia/Corallimorpharia complex: implications for coral phylogeny. Genome Biol. Evol. 6, 1086–1095. ( 10.1093/gbe/evu084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muscatine L, Goiran C, Land L, Jaubert J, Cuif J-P, Allemand D. 2005. Stable isotopes (δ13C and δ15N) of organic matrix from coral skeleton. Proc. Natl Acad. Sci. USA 102, 1525–1530. ( 10.1073/pnas.0408921102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roniewicz E, Stolarski J.. 1999. Evolutionary trends in the epithecate scleractinian corals. Acta Paleontol. Pol. 44, 131–166. [Google Scholar]
- 48.Vaughan TW, Wells JW. 1943. Revision of the suborders, families and genera of the Scleractinia. Special papers of the Geological Society of America, no 44, pp. 1–363, pl. 1–51. Geological Society of America. [Google Scholar]
- 49.Wilson PA, Norris RD. 2001. Warm tropical ocean surface and global anoxia during the mid-Cretaceous period. Nature 412, 425–429. ( 10.1038/35086553) [DOI] [PubMed] [Google Scholar]
- 50.Coates AG, Olivier WA Jr. 1973. Coloniality in Zoantharian corals. In Animal colonies. Development and function through time (eds Boardman RS, Cheetham AH, Olivier WA Jr), pp. 3–27. Pennsylvania, PA: Dowden Hutchinson and Ross Inc. [Google Scholar]
- 51.Coates AG, Jackson JBC. 1987. Clonal growth, algal symbiosis and reef formation by corals. Paleobiology 13, 363–378. ( 10.1017/S0094837300008988) [DOI] [Google Scholar]
- 52.Rosen BR. 1986. Modular growth and form of corals: a matter of metamers? Phil. Trans. R. Soc. Lond. B 313, 115–142. ( 10.1098/rstb.1986.0029) [DOI] [Google Scholar]
- 53.Stanley GD Jr, Cairns SD. 1988. Constructional azooxanthellate coral communities: an overview with implications for the fossil record. Palaios 3, 233–242. ( 10.2307/3514534) [DOI] [Google Scholar]
- 54.Warner ME, Fitt WK, Schmidt GW. 1999. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl Acad. Sci. USA 96, 8007–8012. ( 10.1073/pnas.96.14.8007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained by mailing at enriquez@cmarl.unam.mx.